The Effect of Platelet-Rich Fibrin, Calcium Sulfate Hemihydrate, Platelet-Rich Plasma and Resorbable Collagen on Soft Tissue Closure of Extraction Sites
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
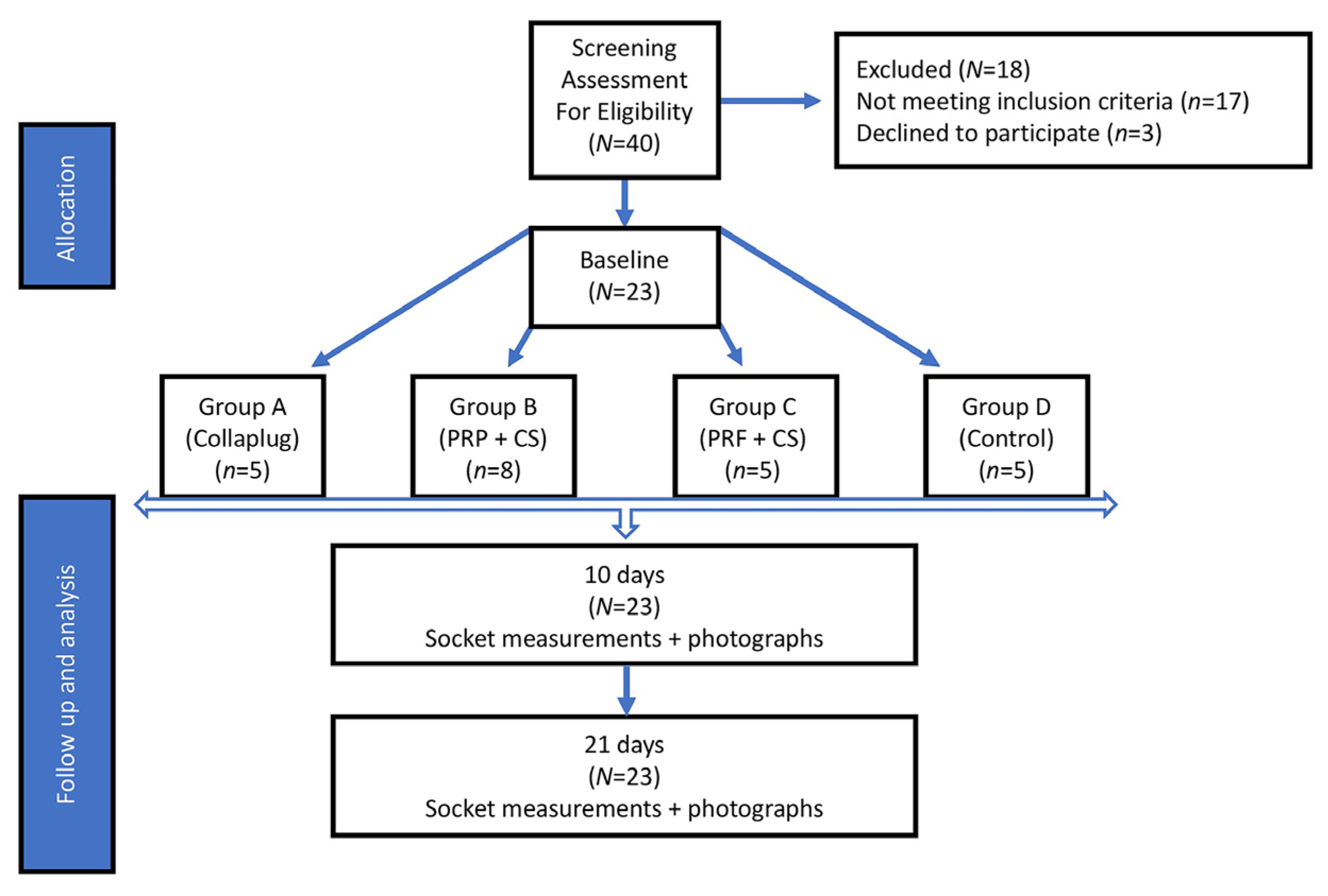
4.1. Study Design
4.2. Study Population
4.3. Extraction Procedures
- Group A: Five extraction sites were treated with RCD alone (Collaplug®; Zimmer Dental, Carlsbad, CA, USA) and served as a positive control. The RCD was placed in the socket apical to the free gingival margin and secured in place by a 4-0 silk horizontal mattress suture.
- Group B: Eight extraction sites treated with 1 g CSH (DentoGen®, Orthogen Corporation, Springfield, NJ, USA) mixed with 240 µL of the subject’s PRP. The viscous mix was placed in the apical two-thirds of the socket and covered with a 15 mm × 20 mm resorbable collagen membrane (OraMem®, Salvin, Charlotte, NC, USA) that was shaped to cover the extraction site [12]. The membrane was used to cover the mixture and protect it from oral debris. It was secured in place under the free gingival margin with a silk 4-0 horizontal mattress suture.
- Group C: Five extraction sites were treated with 1 g CSH mixed with a PRF membrane that was cut into small pieces (approximately 2 mm × 2 mm) and applied into the socket [20]. The materials were then covered with another PRF membrane that was adapted to cover the extraction site and placed just apical to the gingival margin, then secured with a silk 4-0 horizontal mattress suture.
- Group D: Five extraction sites did not receive any grafting material after extraction (Group D) and served as a negative control. A horizontal mattress suture using 4-0 silk was used to close the site and readapt the tissues.
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bartee, B.K. Extraction site reconstruction for alveolar ridge preservation. Part 1: Rationale and materials selection. J. Oral Implantol. 2001, 27, 187–193. [Google Scholar] [CrossRef]
- Al-Hezaimi, K.; Rudek, I.; Al-Hamdan, K.S.; Javed, F.; Nooh, N.; Wang, H.L. Efficacy of using a dual layer of membrane (dPTFE placed over collagen) for ridge preservation in fresh extraction sites: A micro-computed tomographic study in dogs. Clin. Oral Implant. Res. 2013, 24, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Ten Heggeler, J.M.; Slot, D.E.; Van der Weijden, G.A. Effect of socket preservation therapies following tooth extraction in non-molar regions in humans: A systematic review. Clin. Oral Implant. Res. 2011, 22, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Darby, I. Periodontal materials. Aust. Dent. J. 2011, 56 (Suppl. 1), 107–118. [Google Scholar] [CrossRef] [PubMed]
- Torres-Lagares, D.; Bonilla-Mejias, C.; Garcia-Calderon, M.; Gallego-Romero, D.; Serrera-Figallo, M.A.; Gutierrez-Perez, J.L. Prospective assessment of post-extraction gingival closure with bone substitute and calcium sulphate. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e774–e778. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-rich fibrin and soft tissue wound healing: A systematic review. Tissue Eng. Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.S.; Park, J.W.; Kweon, H.; Lee, K.G.; Kang, S.W.; Baek, D.H.; Choi, J.Y.; Kim, S.G. Restoration of peri-implant defects in immediate implant installations by Choukroun platelet-rich fibrin and silk fibroin powder combination graft. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pradeep, A.R. Autologous platelet-rich fibrin in the treatment of mandibular degree II furcation defects: A randomized clinical trial. J. Periodontol. 2011, 82, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Suttapreyasri, S.; Leepong, N. Influence of platelet-rich fibrin on alveolar ridge preservation. J. Craniofac. Surg. 2013, 24, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.R.; Passi, D.; Singh, P.; Sharma, S.; Singh, M.; Srivastava, D. A randomized comparative prospective study of platelet-rich plasma, platelet-rich fibrin, and hydroxyapatite as a graft material for mandibular third molar extraction socket healing. Natl. J. Maxillofac. Surg. 2016, 7, 45–51. [Google Scholar] [PubMed]
- Pagni, G.; Pellegrini, G.; Giannobile, W.V.; Rasperini, G. Postextraction alveolar ridge preservation: Biological basis and treatments. Int. J. Dent. 2012, 2012, 151030. [Google Scholar] [CrossRef] [PubMed]
- Intini, G.; Andreana, S.; Intini, F.E.; Buhite, R.J.; Bobek, L.A. Calcium sulfate and platelet-rich plasma make a novel osteoinductive biomaterial for bone regeneration. J. Transl. Med. 2007, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Kutkut, A.; Andreana, S.; Kim, H.L.; Monaco, E., Jr. Extraction socket preservation graft before implant placement with calcium sulfate hemihydrate and platelet-rich plasma: A clinical and histomorphometric study in humans. J. Periodontol. 2012, 83, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Aimetti, M.; Romano, F.; Griga, F.B.; Godio, L. Clinical and histologic healing of human extraction sockets filled with calcium sulfate. Int. J. Oral Maxillofac. Implant. 2009, 24, 902–909. [Google Scholar]
- Cheah, C.W.; Vaithilingam, R.D.; Siar, C.H.; Swaminathan, D.; Hornbuckle, G.C. Histologic, histomorphometric, and cone-beam computerized tomography analyses of calcium sulfate and platelet-rich plasma in socket preservation: A pilot study. Implant Dent. 2014, 23, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Sammartino, G.; Tia, M.; Gentile, E.; Marenzi, G.; Claudio, P.P. Platelet-rich plasma and resorbable membrane for prevention of periodontal defects after deeply impacted lower third molar extraction. J. Oral Maxillofac. Surg. 2009, 67, 2369–2373. [Google Scholar] [CrossRef] [PubMed]
- Del Corso, M.; Vervelle, A.; Simonpieri, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: Periodontal and dentoalveolar surgery. Curr. Pharma. Biotechnol. 2012, 13, 1207–1230. [Google Scholar] [CrossRef]
- Jain, V.; Triveni, M.G.; Kumar, A.B.; Mehta, D.S. Role of platelet-rich-fibrin in enhancing palatal wound healing after free graft. Contemp. Clin. Dent. 2012, 3, S240–S243. [Google Scholar] [PubMed]
- Dohan Ehrenfest, D.M.; del Corso, M.; Diss, A.; Mouhyi, J.; Charrier, J.B. Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. J. Periodontol. 2010, 81, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part iv: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e56–e60. [Google Scholar] [CrossRef] [PubMed]
- Yelamali, T.; Saikrishna, D. Role of platelet rich fibrin and platelet rich plasma in wound healing of extracted third molar sockets: A comparative study. J. Maxillofac. Oral Surg. 2015, 14, 410–416. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, T. The periodontal screening examination. J. Periodontol. 1967, 38, 617–624. [Google Scholar] [CrossRef]
| Patient | Age | Gender | Tooth Extracted | Grafting Material | Bony Walls after Extraction |
|---|---|---|---|---|---|
| 1 | 63 | Male | 30 | Control | 4 |
| 2 | 68 | Male | 2 | Control | 3 |
| 3 | 59 | Female | 4 | Control | 4 |
| 4 | 70 | Male | 18 | Control | 3 |
| 5 | 54 | Male | 20 | Control | 4 |
| 6 | 68 | Male | 5 | Collagen Dressing | 4 |
| 7 | 70 | Male | 15 | Collagen Dressing | 4 |
| 8 | 62 | Female | 15 | Collagen Dressing | 3 |
| 9 | 63 | Female | 15 | Collagen Dressing | 4 |
| 10 | 60 | Female | 31 | Collagen Dressing | 4 |
| 11 | 70 | Male | 2 | PRP-CSH | 4 |
| 12 | 60 | Female | 5 | PRP-CSH | 4 |
| 13 | 66 | Female | 4 | PRP-CSH | 4 |
| 14 | 65 | Female | 7 | PRP-CSH | 4 |
| 15 | 68 | Male | 15 | PRP-CSH | 3 |
| 16 | 72 | Male | 14 | PRP-CSH | 3 |
| 17 | 67 | Male | 15 | PRP-CSH | 3 |
| 18 | 40 | Female | 6 | PRP-CSH | 4 |
| 19 | 46 | Female | 4 | PRF-CSH | 4 |
| 20 | 42 | Female | 4 | PRF-CSH | 4 |
| 21 | 61 | Male | 2 | PRF-CSH | 3 |
| 22 | 56 | Female | 8 | PRF-CSH | 4 |
| 23 | 67 | Female | 29 | PRF-CSH | 4 |
| Sum of Squares | Df | Mean Square | F | p-Value | |
|---|---|---|---|---|---|
| Between Groups | 853.613 | 3 | 284.538 | 0.591 | 0.628 |
| Within Groups | 9142.360 | 19 | 481.177 | ||
| Total | 9995.973 | 22 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yerke, L.M.; Jamjoom, A.; Zahid, T.M.; Cohen, R.E. The Effect of Platelet-Rich Fibrin, Calcium Sulfate Hemihydrate, Platelet-Rich Plasma and Resorbable Collagen on Soft Tissue Closure of Extraction Sites. J. Funct. Biomater. 2017, 8, 17. https://doi.org/10.3390/jfb8020017
Yerke LM, Jamjoom A, Zahid TM, Cohen RE. The Effect of Platelet-Rich Fibrin, Calcium Sulfate Hemihydrate, Platelet-Rich Plasma and Resorbable Collagen on Soft Tissue Closure of Extraction Sites. Journal of Functional Biomaterials. 2017; 8(2):17. https://doi.org/10.3390/jfb8020017
Chicago/Turabian StyleYerke, Lisa M., Amal Jamjoom, Talal M. Zahid, and Robert E. Cohen. 2017. "The Effect of Platelet-Rich Fibrin, Calcium Sulfate Hemihydrate, Platelet-Rich Plasma and Resorbable Collagen on Soft Tissue Closure of Extraction Sites" Journal of Functional Biomaterials 8, no. 2: 17. https://doi.org/10.3390/jfb8020017






