Mesenchymal Stem Cells and Transforming Growth Factor-β3 (TGF-β3) to Enhance the Regenerative Ability of an Albumin Scaffold in Full Thickness Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Albumin Scaffolds
2.1.1. Mesenchymal Stem Cell Isolation and Characterization
2.1.2. Fluorescent Labeling of Mesenchymal Stem Cells
2.1.3. MSC Transduction and Preparation
2.2. Animal Surgery
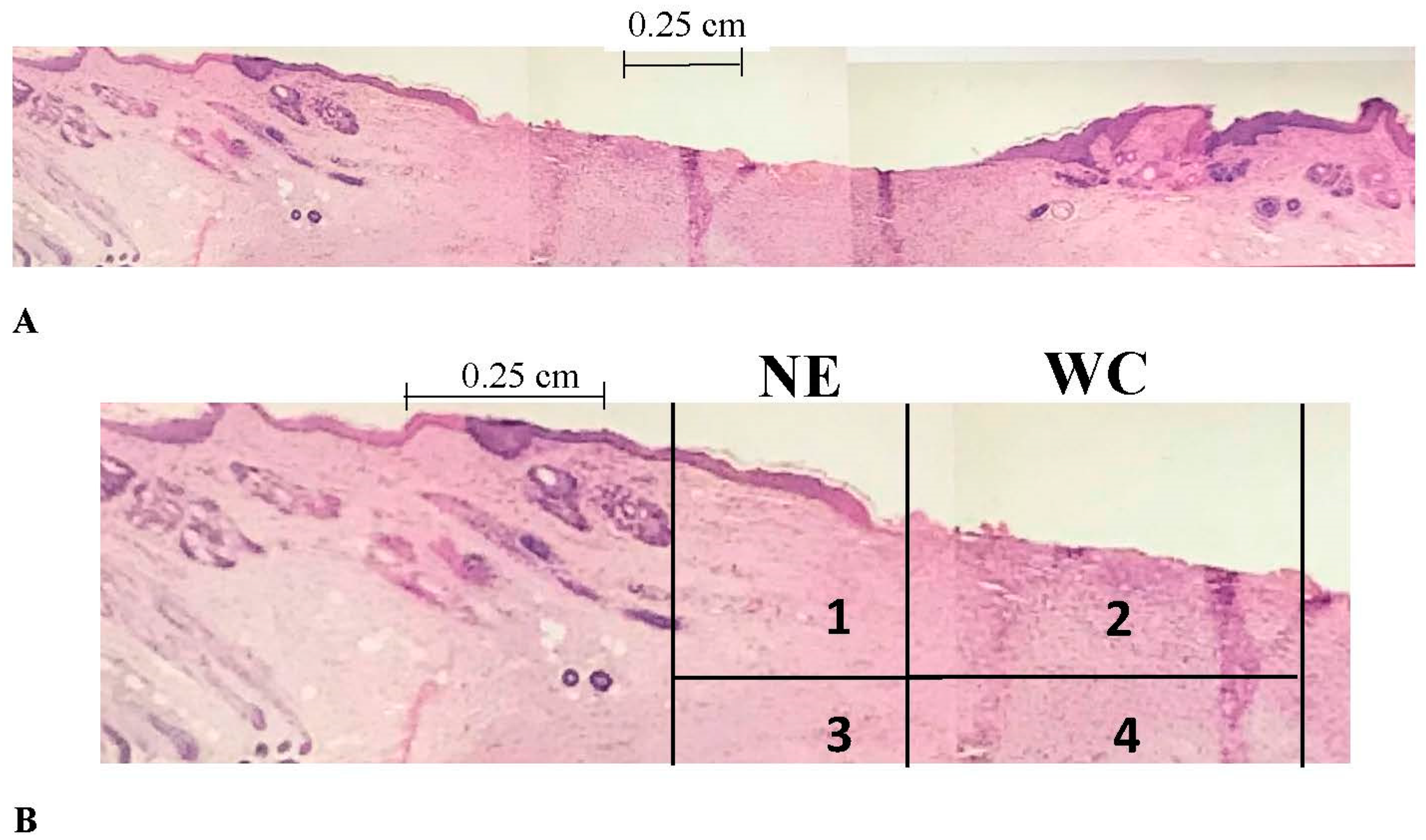
2.3. Histomorphometry
2.3.1. Epithelialization Rate
2.3.2. Contraction Rate
2.3.3. Healing Rate
2.3.4. Epithelialization Rate/Contraction Rate Ratio
2.3.5. Fluorescent Cell Volume Fraction
2.4. Statistical Analysis
3. Results
3.1. Epithelialization Rate
3.2. Contraction Rate
3.3. Healing Rate
3.4. Epithelialization Rate/Contraction Rate Ratio
3.5. Fluorescent Cell Volume Fraction
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaltenthaler, E.; Whitfield, M.D.; Walters, S.J.; Akehurst, R.L.; Paisley, S. UK, USA and Canada: How do their pressure ulcer prevalence and incidence data compare? J. Wound Care 2001, 10, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Sanders, S. Pressure ulcer, part I: Prevention strategies. J. Am. Acad. Nurse Pract. 1992, 4, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Allman, R.; Laprade, C.; Noel, L. Pressure sores among hospitalized patients. Ann. Intern. Med. 1986, 105, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.ihi,org/IHI/Programs/Campaign/Pressure Ulcers.htm (accessed on 7 January 2018).
- Makelbust, J.; Sieggreen, M. Pressure Ulcers: Guidelines for Prevention and Management; Springhouse Corporation: Springhouse, PA, USA, 2001; pp. 110–111. [Google Scholar]
- Curtin, L. Wound management: Care and cost—An overview. Nurs. Manag. 1985, 15, 22–25. [Google Scholar]
- Braden, B. Costs of Pressure Ulcer Prevention, National Pressure Ulcer Advisory Panel. 2012. Available online: http://www.npuap.org (accessed on 7 January 2018).
- Tenenhaus, M.; Rennekamoff, H.O. Current Concepts in Tissue Engineering: Skin Wound Healing. Plast. Reconstr. Surg. 2016, 138, 42S–50S. [Google Scholar] [CrossRef] [PubMed]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [PubMed]
- Baranoski, S.; Ayello, E.A. Wound Care Essentials: Practice Principles; Lippincott, Williams, and Wilkins: Philadelphia, PA, USA, 2004; pp. 62–70. [Google Scholar]
- Marx, G. Evolution of Fibrin Glue Applicators. Transfus. Med. Rev. 2003, 17, 287–298. [Google Scholar] [CrossRef]
- Lawrence, W.T.; Diegelmann, R.T. Growth factors in wound healing. Clin. Dermatol. 1994, 12, 157–169. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.W.J.; O’Kane, S. Scar-free healing. Philos. Trans. R. Soc. Lond. B 2004, 359, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.W.J.; Duncan, J.; Bond, J.; Bush, J.; Durani, P.; So, K.; Taylor, L.; Chantrey, J.; Mason, T.; James, G.; et al. Prophylactic administration of avotermin for improvement of skin scarring: Three double-blind, placebo-controlled, phase I/II studies. Lancet 2009, 373, 1264–1274. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D. Quantification and modeling of biological processes for tissue engineering and regenerative medicine. J. Environ. Biol. Res. 2018, in press. [Google Scholar]
- Nakagawa, H.; Akita, S.; Fukui, M.; Fujii, T.; Akino, K. Human mesenchymal stem cells successfully improve skin- substitute wound healing. Br. J. Dermatol. 2005, 153, 29–36. [Google Scholar] [CrossRef] [PubMed]
- McFarlin, K.; Gao, X.; Liu, Y.B.; Dulchavsky, D.S.; Kwon, D.; Arbab, A.S.; Bansal, M.; Li, Y.; Chopp, M.; Dulchavsky, S.A.; et al. Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen. 2006, 14, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Badiavas, E.V.; Falanga, V. Treatment of chronic wounds with bone marrow-derived cells. Arch. Dermatol. 2003, 139, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Foreman, D.M.; Ferguson, M.W.J. Neutralisation of TGF- β1 and TGF-β2 or exogenous addition of TGF-β3 to cutaneous rat wounds reduces scarring. J. Cell Sci. 1995, 108, 985–1002. [Google Scholar] [PubMed]
- Jennings, A.; Andino, R.; Feldman, D. Clinical evaluation of an electrical stimulation bandage (Posifect Dressing). Wound Repair Regen. 2005, 13, A13. [Google Scholar]
- McCullars, J.; Moore, S.; Jennings, A.; Feldman, D. Local versus systemic delivery of endothelial progenitor cells for a tissue scaffold. Trans. Soc. Biomater. 2006, 31, 588. [Google Scholar]
- Overby, R.; Feldman, D. Investigating how molecular weight of a PEG cross-linker affects the stability of an albumin tissue scaffold system. Trans. Soc. Biomater. 2003, 29, 294. [Google Scholar]
- Overby, R.; Feldman, D. Compositional effects on albumin system properties. Trans. Biomed. Eng. Soc. 2002, 34, 148. [Google Scholar]
- Blum, B.; Huang, S.; Eberhardt, A.; Overby, R.; Feldman, D. Effect of composition and porosity on mechanical strength of an adhesive albumin. Trans. Soc. Biomater. 2002, 28, 334. [Google Scholar]
- Feldman, D.; Blum, B.; Huang, S.; Boyd, N.; Barker, T. Effect of an adhesive albumin scaffold on the healing of open skin wounds in rabbits. Trans. Soc. Biomater. 2001, 27, 11. [Google Scholar]
- Kilpadi, D.; Feldman, D.; Huang, S. A comparison of the adhesive strength of albumin and fibrin glues for use with metallic implants. Trans. Soc. Biomater. 1998, 24, 120. [Google Scholar]
- Bowman, J.; Feldman, D. Tissue Adhesives for Growth Factor Delivery, Biomaterials and Bioengineering Handbook; Wise, D., Ed.; Marcel Dekker: New York, NY, USA, 2000; pp. 261–312. [Google Scholar]
- Feldman, D.; Barker, T.; Bowman, J.; Blum, B.; Kilpadi, D.; Redden, R. Biomaterial Enhanced Regeneration for Skin Wounds, Biomaterials and Bioengineering Handbook; Wise, D., Ed.; Marcel Dekker: New York, NY, USA, 2000; pp. 807–842. [Google Scholar]
- Stoff, A.; Rivera, A.A.; Sanjib Banerjee, N.; Moore, S.T.; Michael Numnum, T.; Espinosa-de-Los-Monteros, A.; Richter, D.F.; Siegal, G.P.; Chow, L.T.; Feldman, D.; et al. Promotion of incisional wound repair by human mesenchymal stem cell transplantation. Exp. Dermatol. 2009, 18, 362–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oswald, J.; Boxberger, S.; Jørgensen, B.; Feldmann, S.; Ehninger, G.; Bornhäuser, M.; Werner, C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 2004, 22, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.; Vanguri, P.; Simonetti, D.; Young, R. Adult mesenchymal stem cells: Potential for muscle and tendon regeneration and use in gene therapy. J. Muscoloskelet. Neuronal Interact. 2002, 2, 309–320. [Google Scholar]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell- based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, S.A.; Mankani, M.H.; Gronthos, S.; Satomura, K.; Bianco, P.; Robey, P.G. Circulating skeletal stem cells. J. Cell Biol. 2001, 153, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Martin, B.J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 2004, 95, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal Stem Cells Are Recruited into Wounded Skin and Contribute to Wound Repair by Transdifferentiation into Multiple Skin Cell Type. J. Immunol. 2008, 180, 2581–2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Han, B.; Cai, S.; Lei, Y.; Sun, T.; Sheng, Z. Migration of bone marrow-derived mesenchymal stem cells induced by tumor necrosis factor-α and its possible role in wound healing. Wound Repair Regen. 2009, 17, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Weigand, A.; Beier, J.P.; Hess, A.; Gerber, T.; Arkudas, A.; Horch, R.E.; Boos, A.M. Acceleration of vascularized bone tissue-engineered constructs in a large animal model combining intrinsic and extrinsic vascularization. Tissue Eng. Part A 2015, 21, 1680–1694. [Google Scholar] [CrossRef] [PubMed]
- Meinel, L.; Karageorgiou, V.; Fajardo, R.; Snyder, B.; Shinde-Patil, V.; Zichner, L.; Kaplan, D.; Langer, R.; Vunjak-Novakovic, G. Bone tissue engineering using human mesenchymal stem cells: Effects of scaffold material and medium flow. Ann. Biomed. Eng. 2004, 32, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Fathke, C.; Wilson, L.; Hutter, J.; Kapoor, V.; Smith, A.; Hocking, A.; Isik, F. Contribution of bone marrow-derived cells to skin: Collagen deposition and wound repair. Stem Cells 2004, 22, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neman, J.; Hambrecht, A.; Cadry, C.; Goodarzi, A.; Youssefzadeh, J.; Chen, M.Y.; Jandial, R. Clinical efficacy of stem cell mediated osteogenesis and bioceramics for bone tissue engineering. Adv. Exp. Med. Biol. 2012, 760, 174–187. [Google Scholar] [PubMed]
- Neman, J.; Hambrecht, A.; Cadry, C.; Jandial, R. Stem cell-mediated osteogenesis: Therapeutic potential for bone tissue engineering. Biologics 2012, 6, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.M.; Martina, M.; Hutmacher, D.W.; Hui, J.H.; Lee, E.H.; Lim, B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells 2007, 25, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, O.; Katsube, Y.; Hirose, M.; Ohgushi, H.; Ito, H. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif. Tissue Int. 2008, 82, 238–247. [Google Scholar] [CrossRef] [PubMed]
- White, R.; McIntosh, C. A review of the literature on topical therapies for diabetic foot ulcers. Part 2: Advanced treatments. J. Wound Care 2009, 18, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.W.J.; Whitby, D.J.; Shah, M.; Armstrong, J.; Siebert, J.W.; Longaker, M.T. Scar formation: The spectral nature of fetal and adult wound repair. Plast. Reconstr. Surg. 1996, 97, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Whitby, D.J.; Ferguson, M.W.J. The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development 1991, 112, 651–668. [Google Scholar] [PubMed]
- Zou, Z.; Sun, P.D. An improved recombinant mammalian cell expression system for human transforming growth factor-β2 and -β3 preparations. Protein Expr. Purif. 2006, 50, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Stoff, A.; Rivera, A.A.; Mathis, J.M.; Moore, S.T.; Banerjee, N.S.; Everts, M.; Espinosa-de-Los-Monteros, A.; Novak, Z.; Vasconez, L.O.; Broker, T.R.; et al. Effect of adenoviral mediated overexpression of fibromodulin on human dermal fibroblasts and scar formation in full-thickness incisional wounds. J. Mol. Med. 2007, 85, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; McCauley, M. Mesenchymal stem cells, TGF-β3, and an albumin scaffold to promote full-thickness wound healing. Trans. Biomed. Eng. Soc. 2012, 44, 152. [Google Scholar]
- Weinand, C.; Xu, J.W.; Peretti, G.M.; Bonassar, L.J.; Gill, T.J. Conditions affecting cell seeding onto three-dimensional scaffolds for cellular-based biodegradable implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 80–87. [Google Scholar] [CrossRef] [PubMed]










| 1 Week | 2 Weeks | |||
|---|---|---|---|---|
| Avg CR (mm/wk) | SD CR | Avg CR (mm/wk) | SD CR | |
| Control | 3.37 | 0.43 | 1.42 | 0.45 |
| A | 3.47 | 0.92 | 1.25 | 0.25 |
| MSC-TGFβ3-A | 4.89 | 1.79 | 2.23 | 1.38 |
| MSC-A | 3.07 | 1.99 | 1.34 | 0.81 |
| MSC-TGFβ3 | 3.11 | 1.10 | 1.62 | 0.55 |
| MSC | 2.31 | 1.19 | 1.58 | 0.18 |
| 2 Weeks | ||
|---|---|---|
| ER/CR | SD ER/CR | |
| Control | 0.77 | 0.38 |
| A | 1.24 | 0.31 |
| MSC-TGFβ3-A | 1.50 | 1.31 |
| MSC-A | 1.45 | 0.47 |
| MSC-TGFβ3 | 1.23 | 0.63 |
| MSC | 1.00 | 0.14 |
| 1 Week | ||
|---|---|---|
| Outer Fluor Volume Fraction | SD | |
| MSC-TGFβ3-A | 0.057 | 0.016 |
| MSC-A | 0.056 | 0.018 |
| MSC-TGFβ3 | 0.050 | 0.035 |
| MSC | 0.061 | 0.012 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feldman, D.S.; McCauley, J.F. Mesenchymal Stem Cells and Transforming Growth Factor-β3 (TGF-β3) to Enhance the Regenerative Ability of an Albumin Scaffold in Full Thickness Wound Healing. J. Funct. Biomater. 2018, 9, 65. https://doi.org/10.3390/jfb9040065
Feldman DS, McCauley JF. Mesenchymal Stem Cells and Transforming Growth Factor-β3 (TGF-β3) to Enhance the Regenerative Ability of an Albumin Scaffold in Full Thickness Wound Healing. Journal of Functional Biomaterials. 2018; 9(4):65. https://doi.org/10.3390/jfb9040065
Chicago/Turabian StyleFeldman, Dale S., and John F. McCauley. 2018. "Mesenchymal Stem Cells and Transforming Growth Factor-β3 (TGF-β3) to Enhance the Regenerative Ability of an Albumin Scaffold in Full Thickness Wound Healing" Journal of Functional Biomaterials 9, no. 4: 65. https://doi.org/10.3390/jfb9040065




