Hierarchical Cluster Analysis of Medical Chemicals Detected by a Bacteriophage-Based Colorimetric Sensor Array
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sensor Analysis Analytes
2.2. Development of Functional Bioreporter Materials
2.3. Phage Colorimetric Sensor-Based Multi-Array Chip
2.4. Colorimetric Signal Analysis and Data Processing
2.5. Analytical Data Statistics
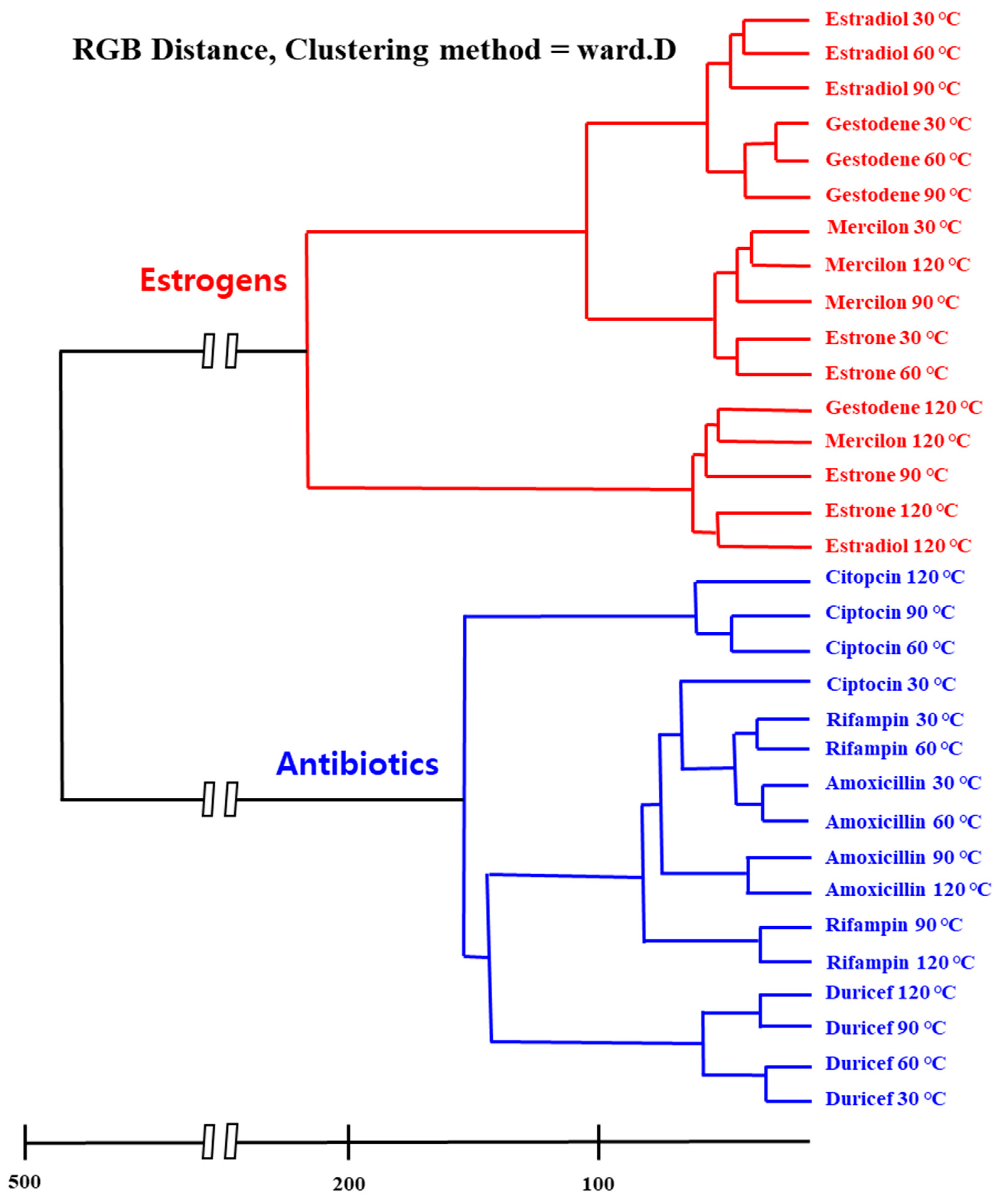
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Petrenko, V.A.; Vodyanoy, V.J. Phage display for detection of biological threat agents. J. Microbiol. Methods 2003, 53, 253. [Google Scholar] [CrossRef]
- Goldman, E.R.; Pazirandeh, M.P.; Mauro, J.M.; King, K.D.; Frey, J.C.; Anderson, G.P. Phage-displayed peptides as biosensor reagents. J. Mol. Recognit. 2000, 13, 382. [Google Scholar] [CrossRef]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P.; Petrenko, V.A. Phage display. Chem. Rev. 1997, 97, 391. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, V.A.; Smith, G.P. Phages from landscape libraries as substitute antibodies. Protein Eng. Des. Sel. 1996, 9, 797. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, S.-Y.; Kim, W.-G.; Oh, J.-W. High sensitive and selective virus based structural colorimetric sensor. Mol. Cryst. Liq. Cryst. 2014, 598, 171. [Google Scholar] [CrossRef]
- Moon, J.-S.; Lee, Y.; Shin, D.M.; Kim, C.; Kim, W.G.; Park, M.; Han, J.; Song, H.; Kim, K.; Oh, J.W. Identification of endocrine disrupting chemicals using a virus-based colorimetric sensor. Chem. Asian. J. 2016, 11, 3097. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-S.; Kim, W.-G.; Shin, D.-M.; Lee, S.-Y.; Kim, C.; Lee, Y.; Han, J.; Kim, K.; Yoo, S.Y.; Oh, J.-W. Bioinspired M-13 bacteriophage-based photonic nose for differential cell recognition. Chem. Sci. 2017, 8, 921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, W.-J.; Oh, J.-W.; Kwak, K.; Lee, B.Y.; Meyer, J.; Wang, E.; Hexemer, A.; Lee, S.-W. Biomimetic self-templating supramolecular structures. Nature 2011, 478, 364. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-W.; Chung, W.-J.; Heo, K.; Jin, H.-E.; Lee, B.Y.; Wang, E.; Zueger, C.; Wong, W.; Meyer, J.; Kim, C.; et al. Biomimetic virus-based colourimetric sensors. Nat. Commun. 2014, 5, 3043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, A.G.; Cissell, R.; Liamthong, S. Antibiotic resistance in bacteria associated with food animals: A united states perspective of livestock production. Foodborne Pathog. Dis. 2007, 4, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, L.J.; Chichester, C. Review of evidence: Are endocrine-disrupting chemicals in the aquatic environment impacting fish populations? Sic. Total Environ. 2005, 343, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamid, H.; Eskicioglu, C. Fate of estrogenic hormones in wastewater and sludge treatment: A review of properties and analytical detection techniques in sludge matrix. Water Res. 2012, 46, 5813. [Google Scholar] [CrossRef] [PubMed]
- Kuch, H.M.; Ballschmiter, K. Determination of endocrine-disrupting phenolic compounds and estrogens in surface and drinking water by HRGC-(NCI)-MS in the picogram per liter range. Environ. Sci. Technol. 2001, 35, 3201. [Google Scholar] [CrossRef] [PubMed]
- Rakow, N.A.; Sen, A.; Janzen, M.C.; Ponder, J.B.; Suslick, K.S. Molecular recognition and discrimination of amines with a colorimetric array. Angew. Chem. Int. Ed. 2005, 44, 4528. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.S.; Jin, H.-E.; Yoo, S.Y.; Lee, S.-W. Cyclic RGD peptide incorporation on phage major coat proteins for improved internalization by HeLa Cells. Bioconjug. Chem. 2014, 25, 216. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Zhang, J.; Zueger, C.; Chung, W.-J.; Yoo, S.Y.; Wang, E.; Meyer, J.; Ramesh, R.; Lee, S.-W. Virus-based piezoelectric energy generation. Nat. Nanotechnol. 2012, 7, 351. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-G.; Kim, K.; Ha, S.-H.; Song, H.; Yu, H.-W.; Kim, C.; Kim, J.-M.; Oh, J.-W. Virus-based full colour pixels using a microheater. Sci. Rep. 2015, 5, 13757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anton, H. Elementary Linear Algebra, Binder Ready Version; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Ward, J.H., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.; Lee, H.; Devaraj, V.; Kim, W.-G.; Lee, Y.; Kim, Y.; Jeong, N.-N.; Choi, E.J.; Baek, S.H.; Han, D.-W.; et al. Hierarchical Cluster Analysis of Medical Chemicals Detected by a Bacteriophage-Based Colorimetric Sensor Array. Nanomaterials 2020, 10, 121. https://doi.org/10.3390/nano10010121
Kim C, Lee H, Devaraj V, Kim W-G, Lee Y, Kim Y, Jeong N-N, Choi EJ, Baek SH, Han D-W, et al. Hierarchical Cluster Analysis of Medical Chemicals Detected by a Bacteriophage-Based Colorimetric Sensor Array. Nanomaterials. 2020; 10(1):121. https://doi.org/10.3390/nano10010121
Chicago/Turabian StyleKim, Chuntae, Hansong Lee, Vasanthan Devaraj, Won-Geun Kim, Yujin Lee, Yeji Kim, Na-Na Jeong, Eun Jung Choi, Sang Hong Baek, Dong-Wook Han, and et al. 2020. "Hierarchical Cluster Analysis of Medical Chemicals Detected by a Bacteriophage-Based Colorimetric Sensor Array" Nanomaterials 10, no. 1: 121. https://doi.org/10.3390/nano10010121





