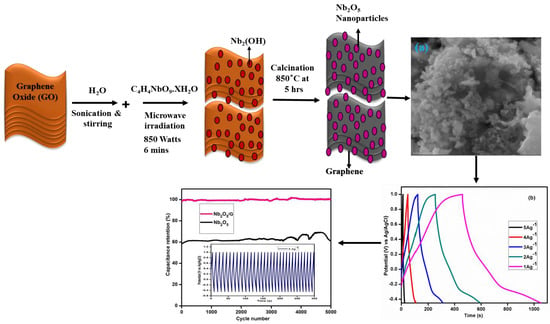
Surfactant-Free Synthesis of Nb2O5 Nanoparticles Anchored Graphene Nanocomposites with Enhanced Electrochemical Performance for Supercapacitor Electrodes
Abstract
:1. Introduction
2. Experimental Section
2.1. Material Synthesis
2.2. Characterization
2.3. Fabrication of Working Electrode
3. Results and Discussion
3.1. X-ray Diffraction Analysis
3.2. Scanning Electron Microscopy Investigations
3.3. TEM Analysis
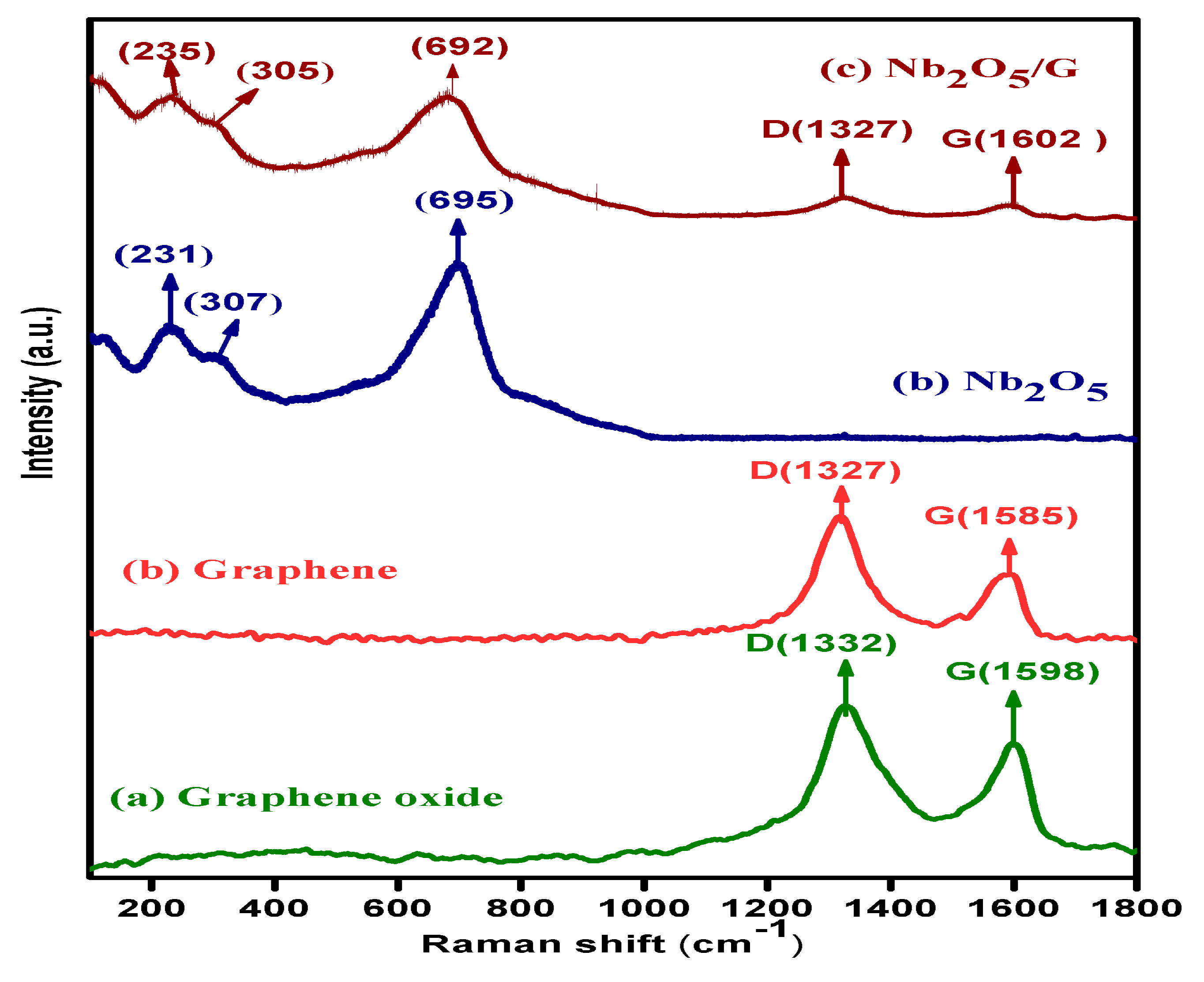
3.4. Analysis of Functional Groups
3.5. Optical Properties
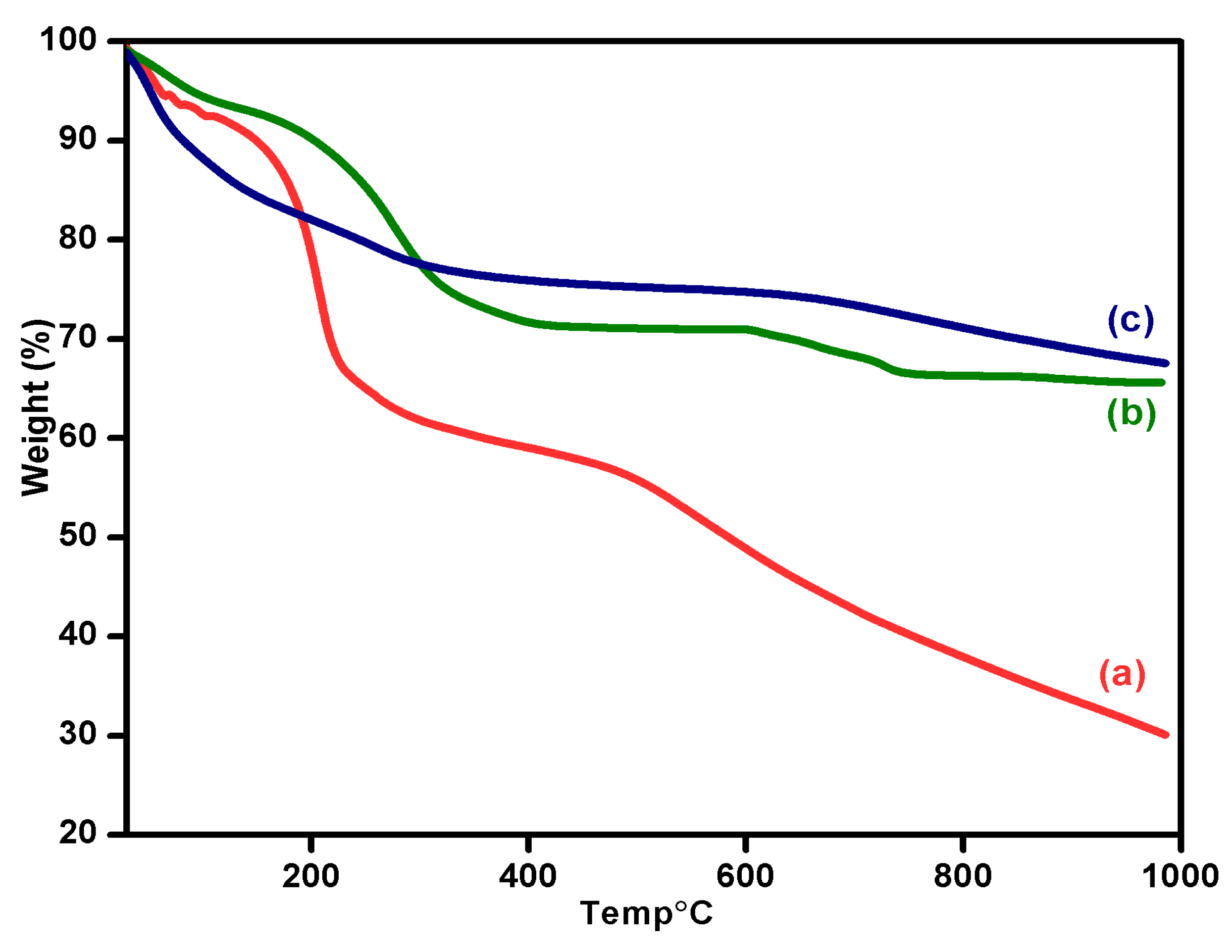
3.6. Thermal Behavior
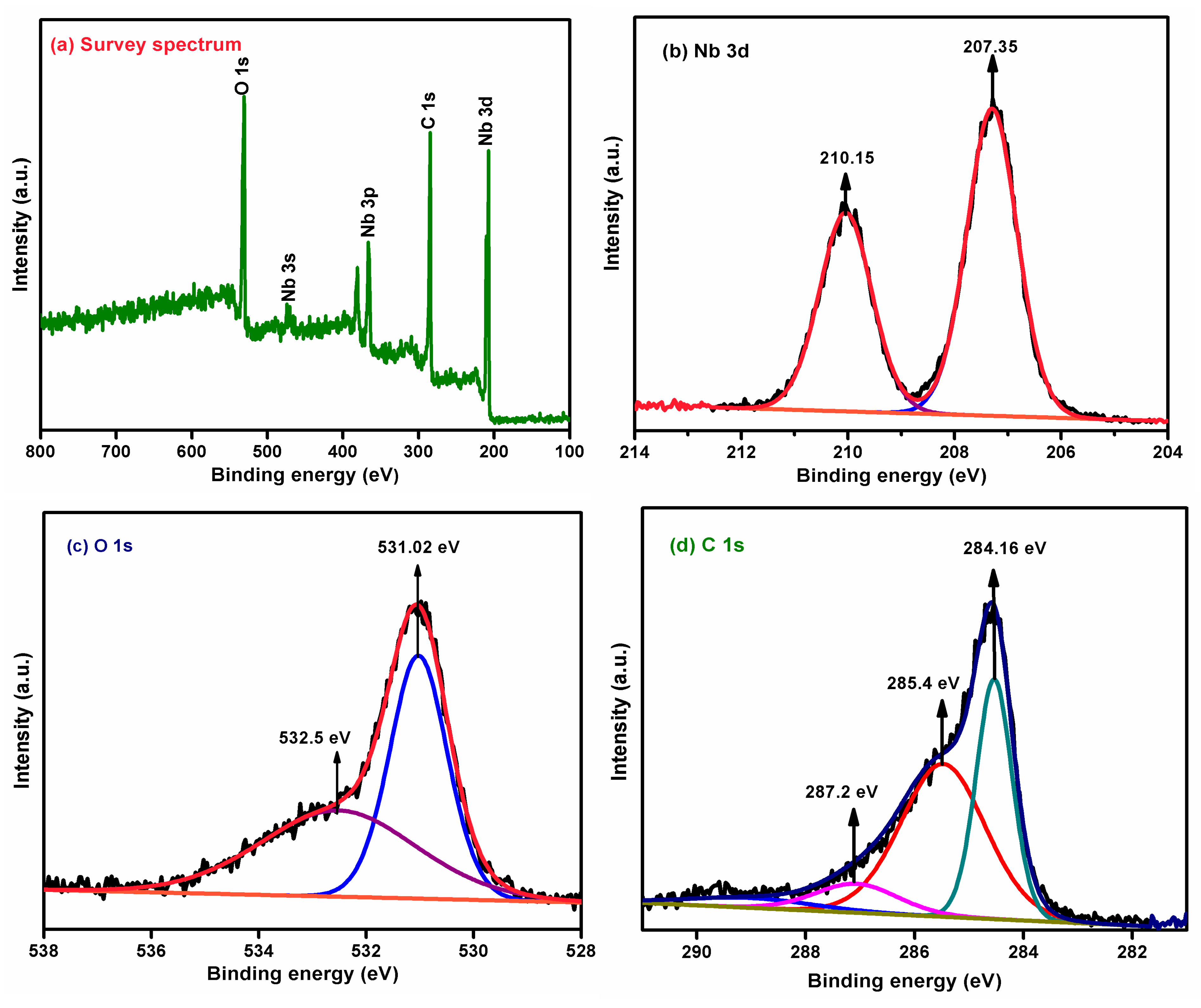
3.7. Binding Energy States
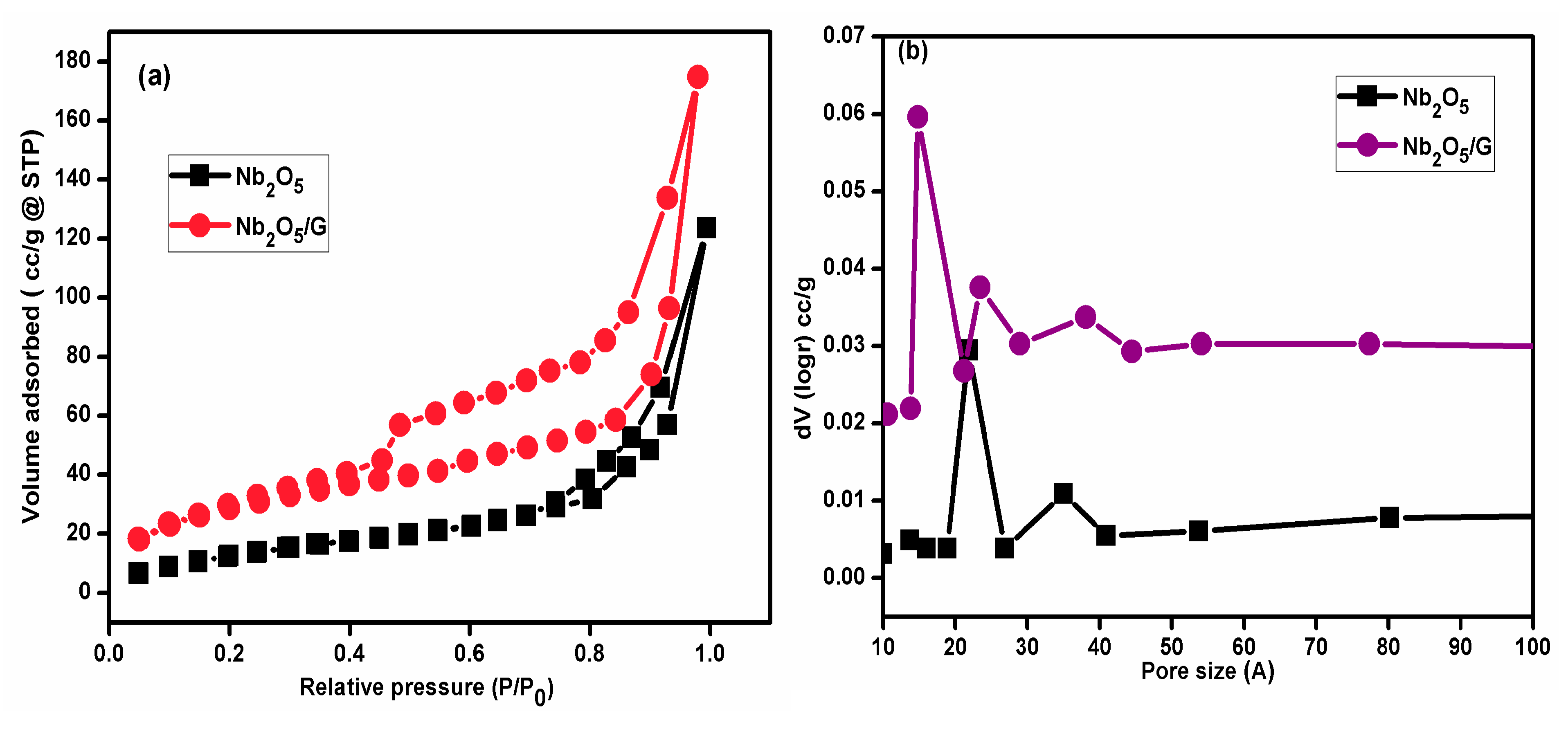
3.8. Surface Area Analysis
3.9. Electrochemical Evaluation
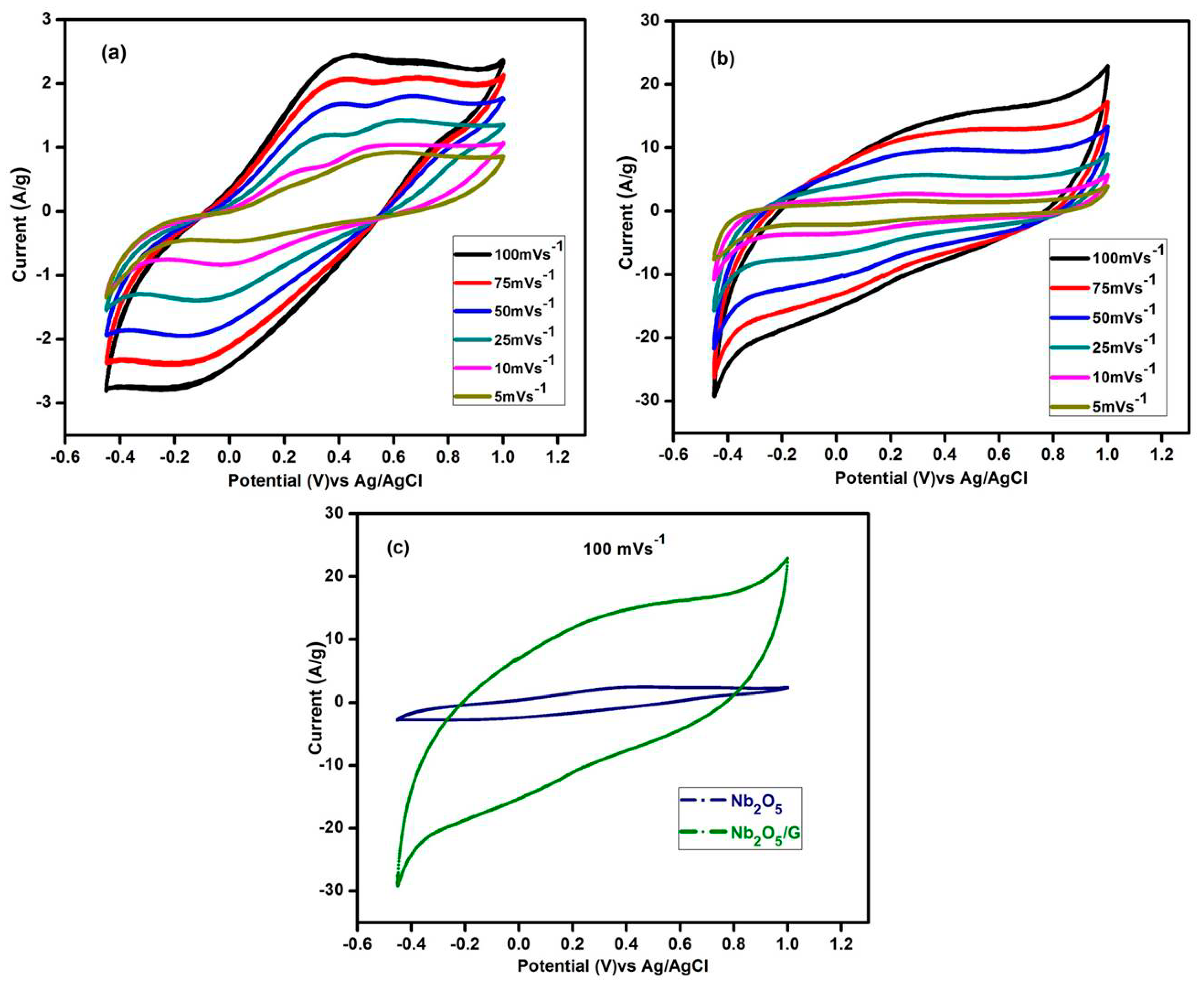
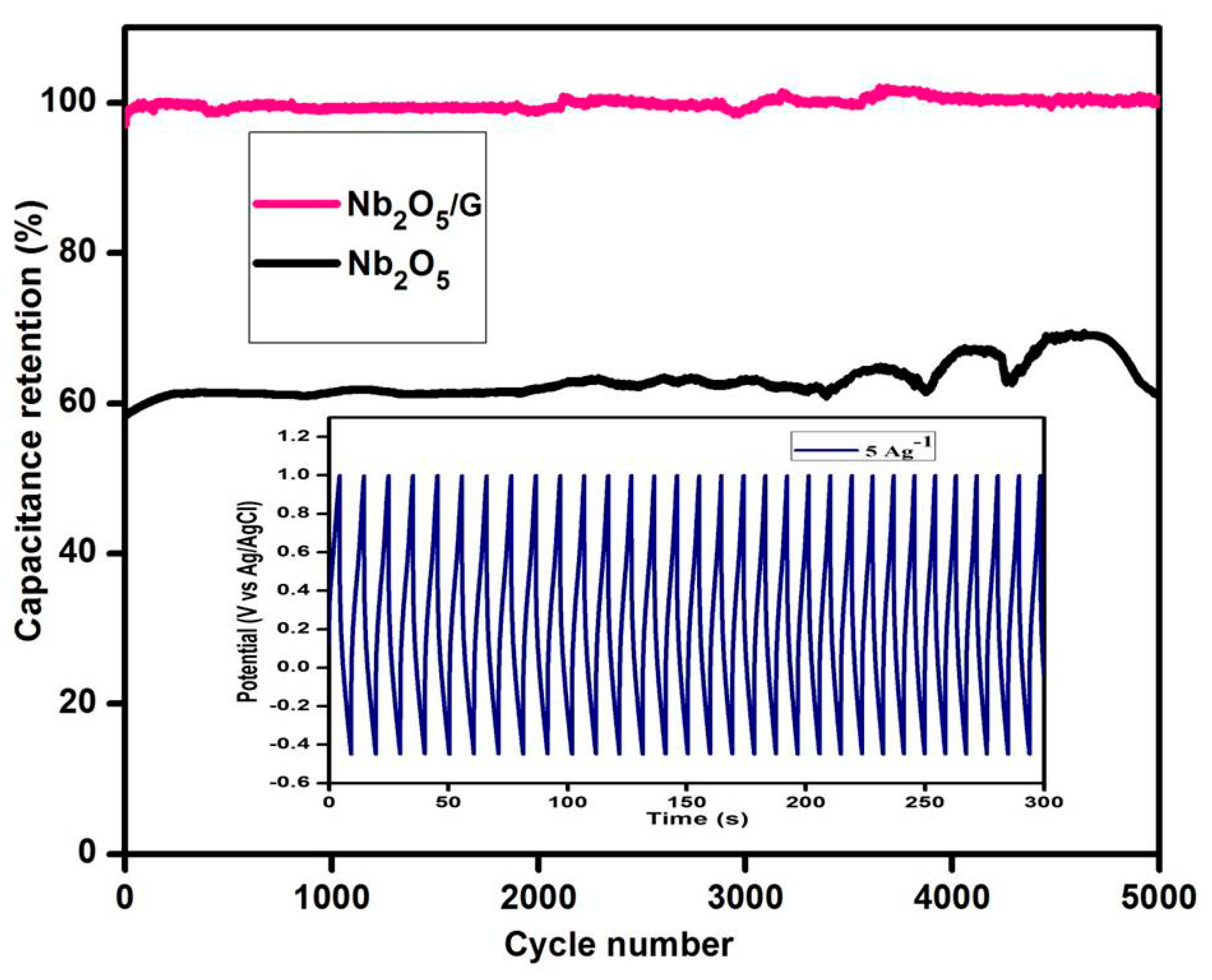
3.9.1. Cyclic Voltammogram
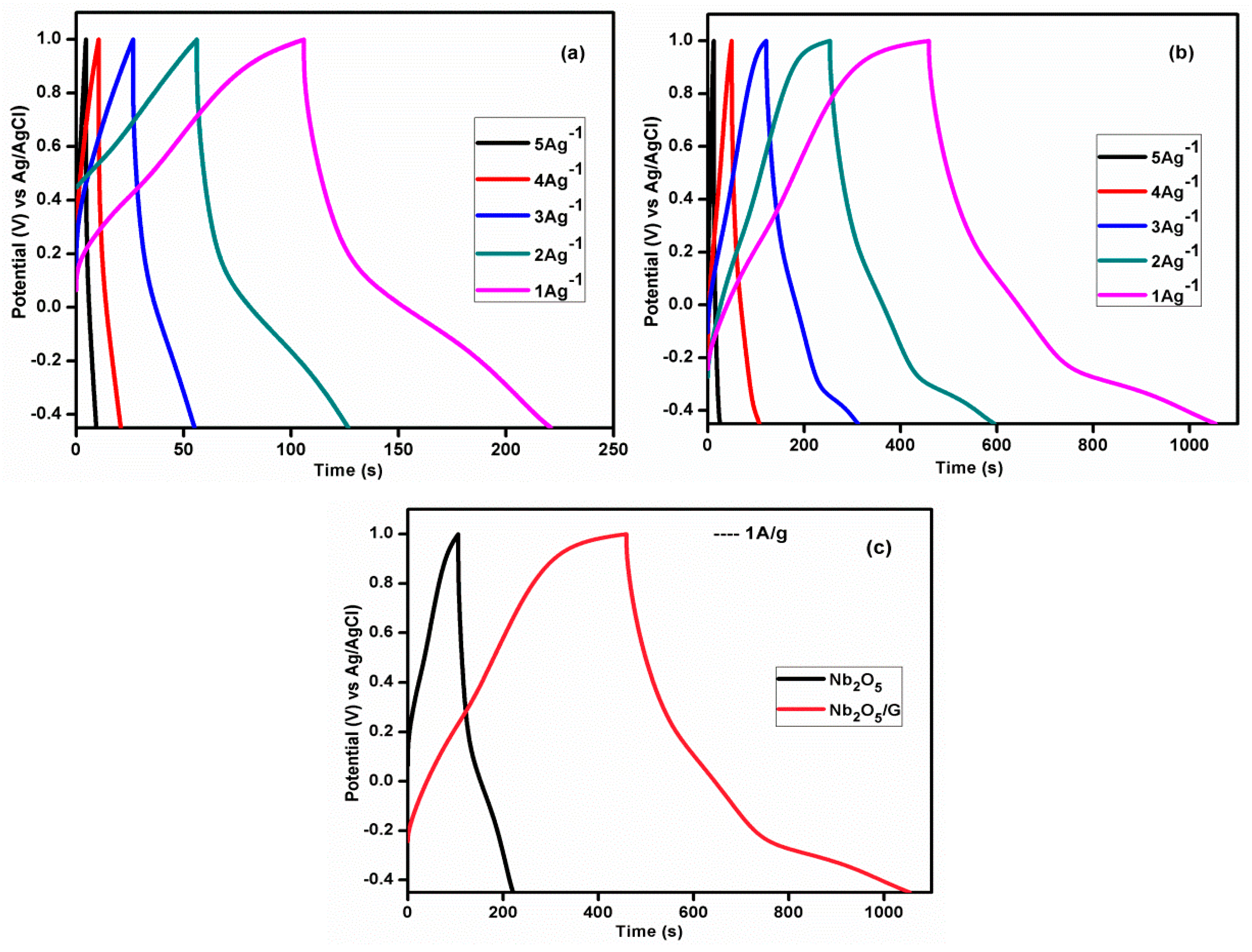
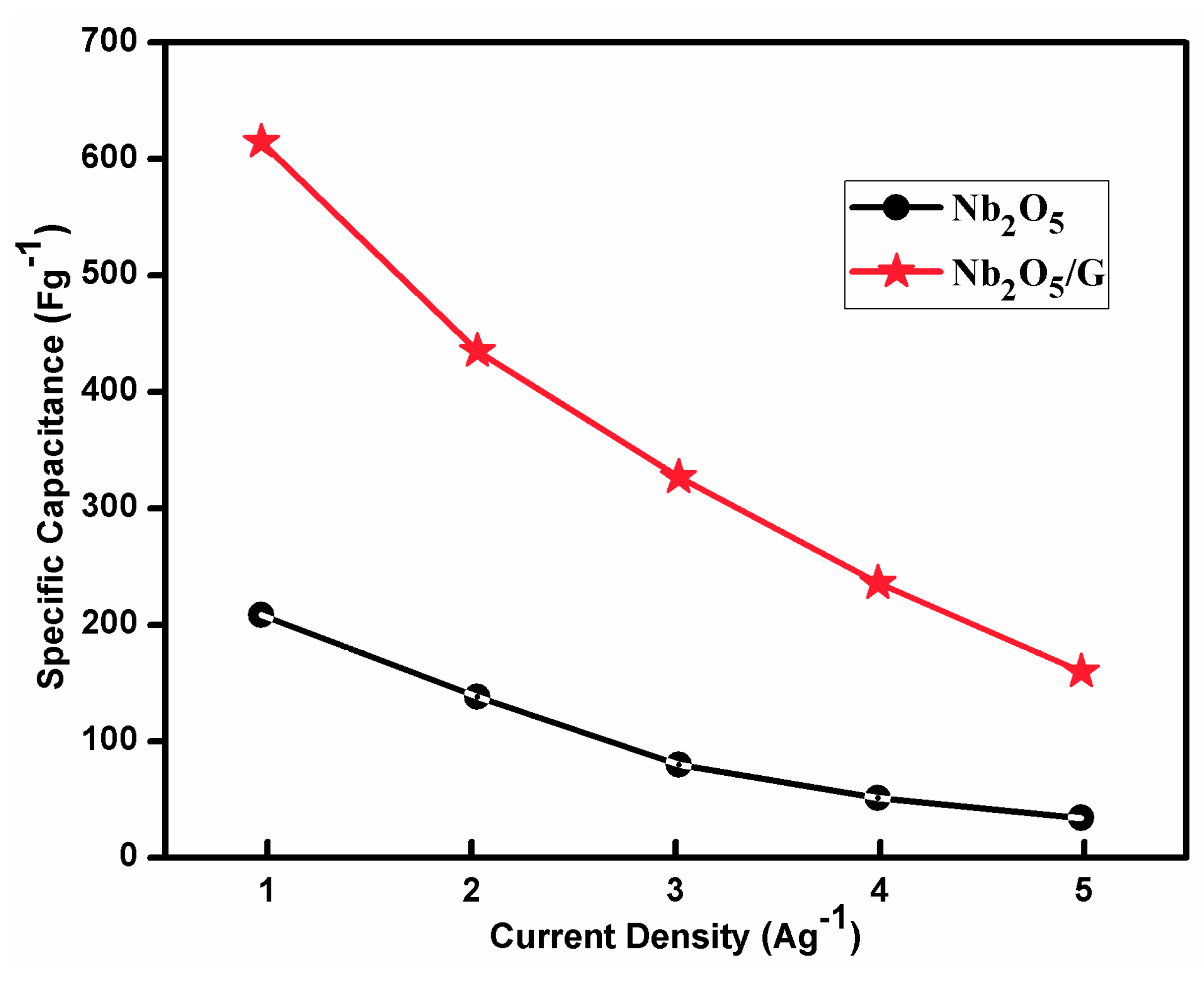
3.9.2. Chronopotentiometry Measurements
3.9.3. Electrochemical Impedance Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Xia, Y. Recent Progress in Supercapacitors: From Materials Design to System Construction. Adv. Mater. 2013, 25, 5336–5342. [Google Scholar] [CrossRef]
- Xiong, P.; Zhu, J.; Wang, X. Recent advances on multi-component hybrid nanostructures for electrochemical capacitors. J. Power Sources 2015, 294, 31–50. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Liu, B.; Chen, D.; Tong, Y.; Shen, G. Flexible Energy-Storage Devices: Design Consideration and Recent Progress. Adv. Mater. 2014, 26, 4763–4782. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Q.; Wei, T.; Fan, Z. Recent Advances in Design and Fabrication of Electrochemical Supercapacitors with High Energy Densities. Adv. Energy Mater. 2014, 4, 1300816. [Google Scholar] [CrossRef]
- Yang, J.; Yu, C.; Fan, X.; Zhao, C.; Qiu, J. Ultrafast Self-Assembly of Graphene Oxide-Induced Monolithic NiCo–Carbonate Hydroxide Nanowire Architectures with a Superior Volumetric Capacitance for Supercapacitors. Adv. Funct. Mater. 2015, 25, 2109–2116. [Google Scholar] [CrossRef]
- Zhao, X.; Sánchez, B.M.; Dobson, P.J.; Grant, P.S. The role of nanomaterials in redox-based supercapacitors for next generation energy storage devices. Nanoscale 2011, 3, 839–855. [Google Scholar] [CrossRef]
- Guan, Q.; Cheng, J.; Wang, B.; Ni, W.; Gu, G.; Li, X.; Huang, L.; Yang, G.; Nie, F. Needle-like Co3O4 Anchored on the Graphene with Enhanced Electrochemical Performance for Aqueous Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 7626–7632. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Wu, H.B.; Madhavi, S.; Lou, X.W. Controlled Growth of NiMoO4 Nanosheet and Nanorod Arrays on Various Conductive Substrates as Advanced Electrodes for Asymmetric Supercapacitors. Adv. Energy Mater. 2015, 5, 1401172. [Google Scholar] [CrossRef]
- Chen, W.; Xia, C.; Alshareef, H.N. One-Step Electrodeposited Nickel Cobalt Sulfide Nanosheet Arrays for High-Performance Asymmetric Supercapacitors. ACS Nano 2014, 8, 9531–9541. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Xuan, Y.; Wu, Y.Q.; Shen, T.; Qi, M.; Capano, M.A.; Cooper, J.A.; Ye, P.D. Atomic-layer-deposited nanostructures for graphene-based nanoelectronics. Appl. Phys. Lett. 2008, 92, 013101. [Google Scholar] [CrossRef] [Green Version]
- Machado, B.F.; Serp, P. Graphene-based materials for catalysis. Catal. Sci. Technol. 2012, 2, 54–75. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/Polymer Nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Nowak, I.; Ziolek, M. Niobium Compounds: Preparation, Characterization, and Application in Heterogeneous Catalysis. Chem. Rev. 1999, 99, 3603–3624. [Google Scholar] [CrossRef]
- Kim, J.W.; Augustyn, V.; Dunn, B. The Effect of Crystallinity on the Rapid Pseudocapacitive Response of Nb2O5. Adv. Energy Mater. 2012, 2, 141–148. [Google Scholar] [CrossRef]
- Liu, M.; Yan, C.; Zhang, Y. Fabrication of Nb2O5 nanosheets for high-rate lithium ion storage applications. Sci. Rep. 2015, 5, 8326. [Google Scholar] [CrossRef] [Green Version]
- Kuila, T.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Recent advances in the efficient reduction of graphene oxide and its application as energy storage electrode materials. Nanoscale 2013, 5, 52–71. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ramaprabhu, S. Functionalized Graphene-Based Nanocomposites for Supercapacitor Application. J. Phys. Chem. C 2011, 115, 14006–14013. [Google Scholar] [CrossRef]
- Wang, L.P.; Yu, L.; Satish, R.; Zhu, J.; Yan, Q.; Srinivasan, M.; Xu, Z. High-performance hybrid electrochemical capacitor with binder-free Nb2O5@graphene. RSC Adv. 2014, 4, 37389–37394. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, C.; Zhang, S.; Wang, J.; Cai, R.; Lv, C.; Qiao, W.; Ling, L.; Long, D. High-power and high-energy asymmetric supercapacitors based on Li+-intercalation into a T-Nb2O5/graphene pseudocapacitive electrode. J. Mater. Chem. A 2014, 2, 17962–17970. [Google Scholar] [CrossRef]
- Murugan, M.; Kumar, R.M.; Alsalme, A.; Alghamdi, A.; Jayavel, R. Facile hydrothermal preparation of niobium pentaoxide decorated reduced graphene oxide nanocomposites for supercapacitor applications. Chem. Phys. Lett. 2016, 650, 35–40. [Google Scholar] [CrossRef]
- Nagaraju, P.; Alsalme, A.; Alswieleh, A.; Jayavel, R. Facile in-situ microwave irradiation synthesis of TiO2/graphene nanocomposite for high-performance supercapacitor applications. J. Electroanal. Chem. 2018, 808, 90–100. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Joo, J.B.; Ko, J.W.; Yi, J. Preparation of coral-like porous gold for metal ion detection. Microporous Mesoporous Mater. 2009, 122, 283–287. [Google Scholar] [CrossRef]
- Yue, Z.; Chu, D.; Huang, H.; Huang, J.; Yang, P.; Du, Y.; Zhu, M.; Lu, C. A novel heterogeneous hybrid by incorporation of Nb2O5 microspheres and reduced graphene oxide for photocatalytic H2 evolution under visible light irradiation. RSC Adv. 2015, 5, 47117–47124. [Google Scholar] [CrossRef]
- Castro, D.C.; Cavalcante, R.P.; Jorge, J.; Martines, M.A.U.; Oliveira, L.C.S.; Casagrande, G.A.; Machulek, A., Jr. Synthesis and Characterization of Mesoporous Nb2O5 and Its Application for Photocatalytic Degradation of the Herbicide Methylviologen. J. Braz. Chem. Soc. 2016, 27, 303–313. [Google Scholar]
- Kong, L.; Zhang, C.; Wang, J.; Qiao, W.; Ling, L.; Long, D. Nanoarchitectured Nb2O5 hollow, Nb2O5@carbon and NbO2@carbon Core-Shell Microspheres for Ultrahigh-Rate Intercalation Pseudocapacitors. Sci. Rep. 2016, 6, 21177. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Xu, Q.; Uchaker, E.; Cao, X.; Cao, G. Comparison of amorphous, pseudohexagonal and orthorhombic Nb2O5 for high-rate lithium ion insertion. CrystEngComm 2016, 18, 2532–2540. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.; Dai, H.; Zhou, Y.; Liu, D.; Duan, G.; Cai, W.; Li, Y. Monodispersed Nb2O5 Microspheres: Facile Synthesis, Air/Water Interfacial Self-Assembly, Nb2O5-Based Composite Films, and Their Selective NO2 Sensing. Adv. Mater. Interfaces 2015, 2, 1500167. [Google Scholar] [CrossRef]
- Yu, J.; Jin, J.; Cheng, B.; Jaroniec, M. A noble metal-free reduced graphene oxide–CdS nanorod composite for the enhanced visible-light photocatalytic reduction of CO2 to solar fuel. J. Mater. Chem. A 2014, 2, 3407–3416. [Google Scholar] [CrossRef]
- Nagaraju, P.; Alsalme, A.; Alkathiri, A.M.; Jayavel, R. Rapid synthesis of WO3/graphene nanocomposite via in-situ microwave method with improved electrochemical properties. J. Phys. Chem. Solids 2018, 120, 250–260. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Wang, D.-W.; Ren, W.; Zhao, J.; Zhou, G.; Li, F.; Cheng, H.-M. Anchoring Hydrous RuO2 on Graphene Sheets for High-Performance Electrochemical Capacitors. Adv. Funct. Mater. 2010, 20, 3595–3602. [Google Scholar] [CrossRef]
- Yang, S.; Song, X.; Zhang, P.; Gao, L. Heating-Rate-Induced Porous α-Fe2O3 with Controllable Pore Size and Crystallinity Grown on Graphene for Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, P.; Vasudevan, R.; Arivanandhan, M.; Alsalme, A.; Jayavel, R. High-performance electrochemical capacitor based on cuprous oxide/graphene nanocomposite electrode material synthesized by microwave irradiation method. Emerg. Mater. 2019, 2, 495–504. [Google Scholar] [CrossRef]
- Arunkumar, P.; Ashish, A.G.; Babu, B.; Sarang, S.; Suresh, A.; Sharma, C.H.; Thalakulam, M.; Shaijumon, M.M. Nb2O5/graphene nanocomposites for electrochemical energy storage. RSC Adv. 2015, 5, 59997–60004. [Google Scholar] [CrossRef]
- Gao, F.; Qu, J.; Zhao, Z.; Zhou, Q.; Li, B.; Qiu, J. A green strategy for the synthesis of graphene supported Mn3O4 nanocomposites from graphitized coal and their supercapacitor application. Carbon 2014, 80, 640–650. [Google Scholar] [CrossRef]
- Cai, G.; Wang, X.; Cui, M.; Darmawan, P.; Wang, J.; Eh, A.L.-S.; Lee, P.S. Electrochromo-supercapacitor based on direct growth of NiO nanoparticles. Nano Energy 2015, 12, 258–267. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Piner, R.D.; Jung, I.; Yang, D.; Velamakanni, A.; Nguyen, S.T.; Ruoff, R.S. Aqueous Suspension and Characterization of Chemically Modified Graphene Sheets. Chem. Mater. 2008, 20, 6592–6594. [Google Scholar] [CrossRef]






| Electro-Active Material | Synthesis Method | Specific Capacitance (F/g) | Number of Cycles | Retention | Ref. |
|---|---|---|---|---|---|
| Nb2O5/graphene | Hydrothermal | 34 | 50 | ~80 | [36] |
| Nb2O5/graphene | Hydrothermal | 58 | 50 | 91% | [21] |
| T-Nb2O5/graphene | Facile-hydrothermal | 80 | ~3000 | ~100% | [22] |
| Graphene/Nb2O5 | In situ hydrothermal method | 321 | 500 | 91% | [23] |
| Nb2O5/graphene | In situ microwave method | 633 | 5000 | 100% | Present work |
| Sample | RS (Ω) | Rct (Ω) | CPE1-T (F) | CPE1-p (F) | CPE2-T (F) | CPE1-p (F) |
|---|---|---|---|---|---|---|
| Nb2O5/G | 0.61 | 20.05 | 0.0009 | 1.05 | 0.30 | 0.905 |
| Nb2O5 | 2.2 | 58.53 | 0.0009 | 1.05 | 0.39 | 0.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagaraju, P.; Vasudevan, R.; Alsalme, A.; Alghamdi, A.; Arivanandhan, M.; Jayavel, R. Surfactant-Free Synthesis of Nb2O5 Nanoparticles Anchored Graphene Nanocomposites with Enhanced Electrochemical Performance for Supercapacitor Electrodes. Nanomaterials 2020, 10, 160. https://doi.org/10.3390/nano10010160
Nagaraju P, Vasudevan R, Alsalme A, Alghamdi A, Arivanandhan M, Jayavel R. Surfactant-Free Synthesis of Nb2O5 Nanoparticles Anchored Graphene Nanocomposites with Enhanced Electrochemical Performance for Supercapacitor Electrodes. Nanomaterials. 2020; 10(1):160. https://doi.org/10.3390/nano10010160
Chicago/Turabian StyleNagaraju, P., R. Vasudevan, A. Alsalme, A. Alghamdi, M. Arivanandhan, and R. Jayavel. 2020. "Surfactant-Free Synthesis of Nb2O5 Nanoparticles Anchored Graphene Nanocomposites with Enhanced Electrochemical Performance for Supercapacitor Electrodes" Nanomaterials 10, no. 1: 160. https://doi.org/10.3390/nano10010160