Metal–Organic Framework-Based Sustainable Nanocatalysts for CO Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of UiO-66
2.2. Incorporation of Metallic Species
2.3. Catalyst Characterization
2.4. Catalytic Evaluations
3. Results and Discussion
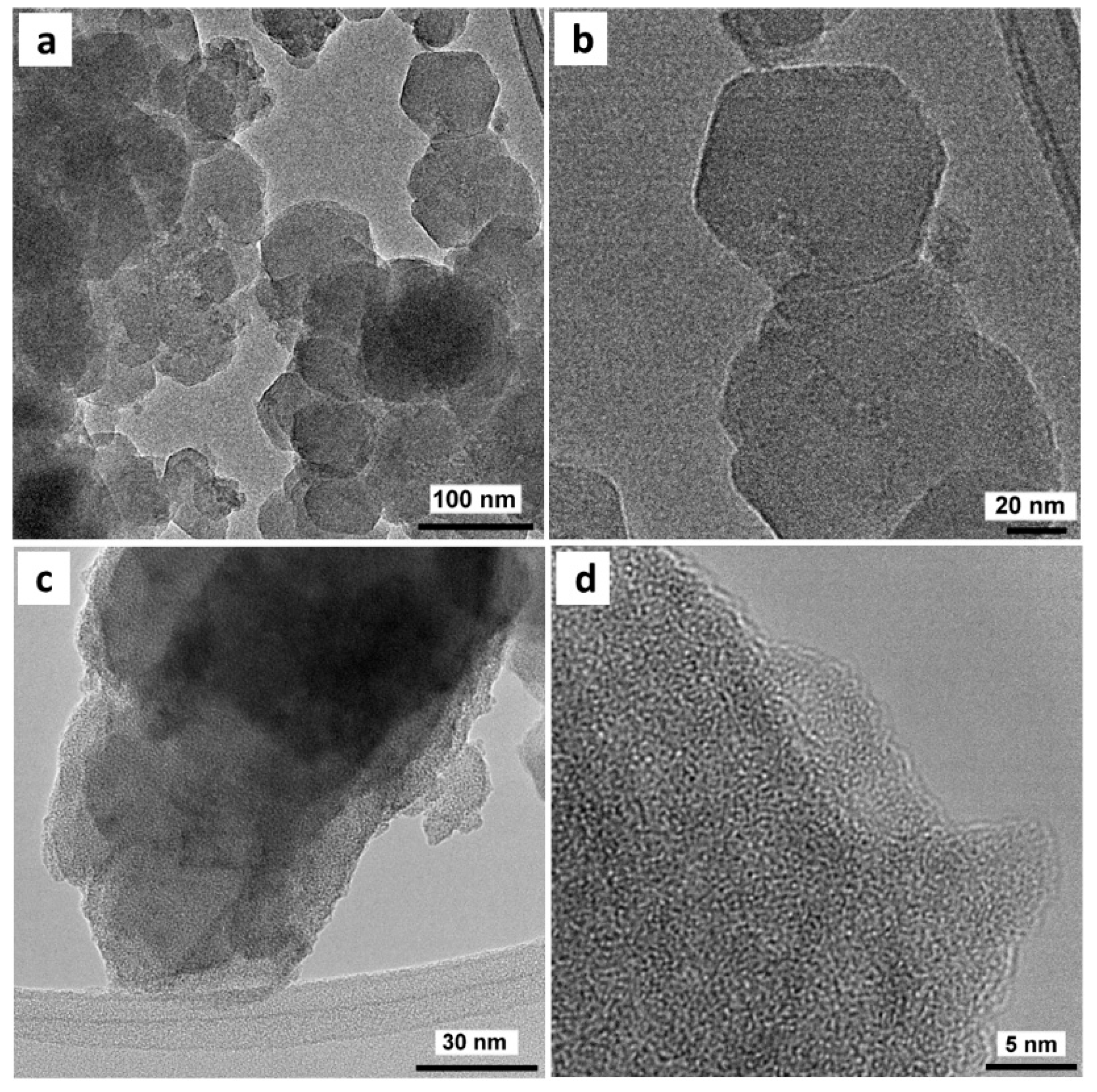
3.1. Dispersion of Copper Species in UiO-66
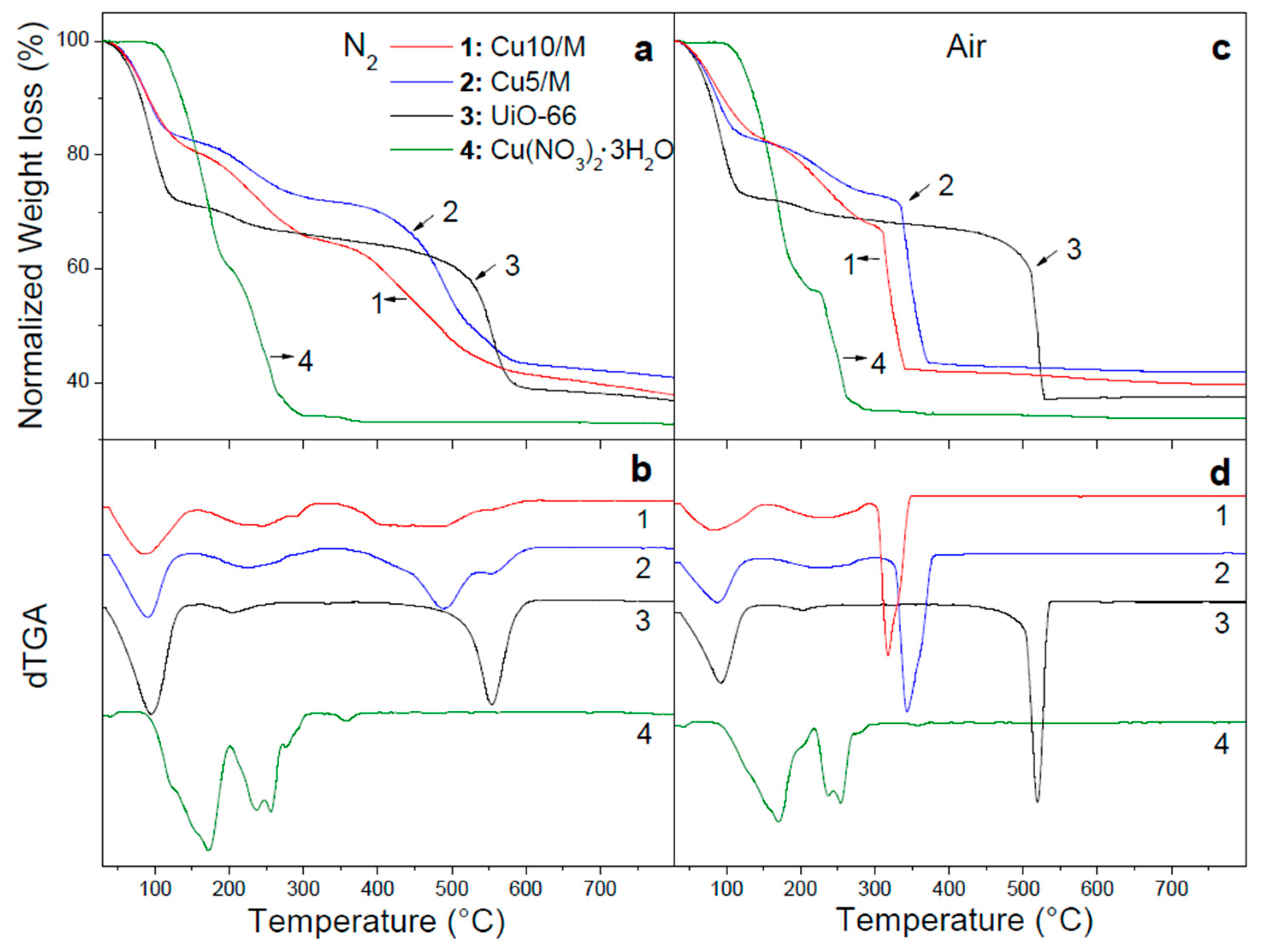
3.1.1. Study of the Precursor Decomposition
3.1.2. Copper-Based UiO-66 Catalysts
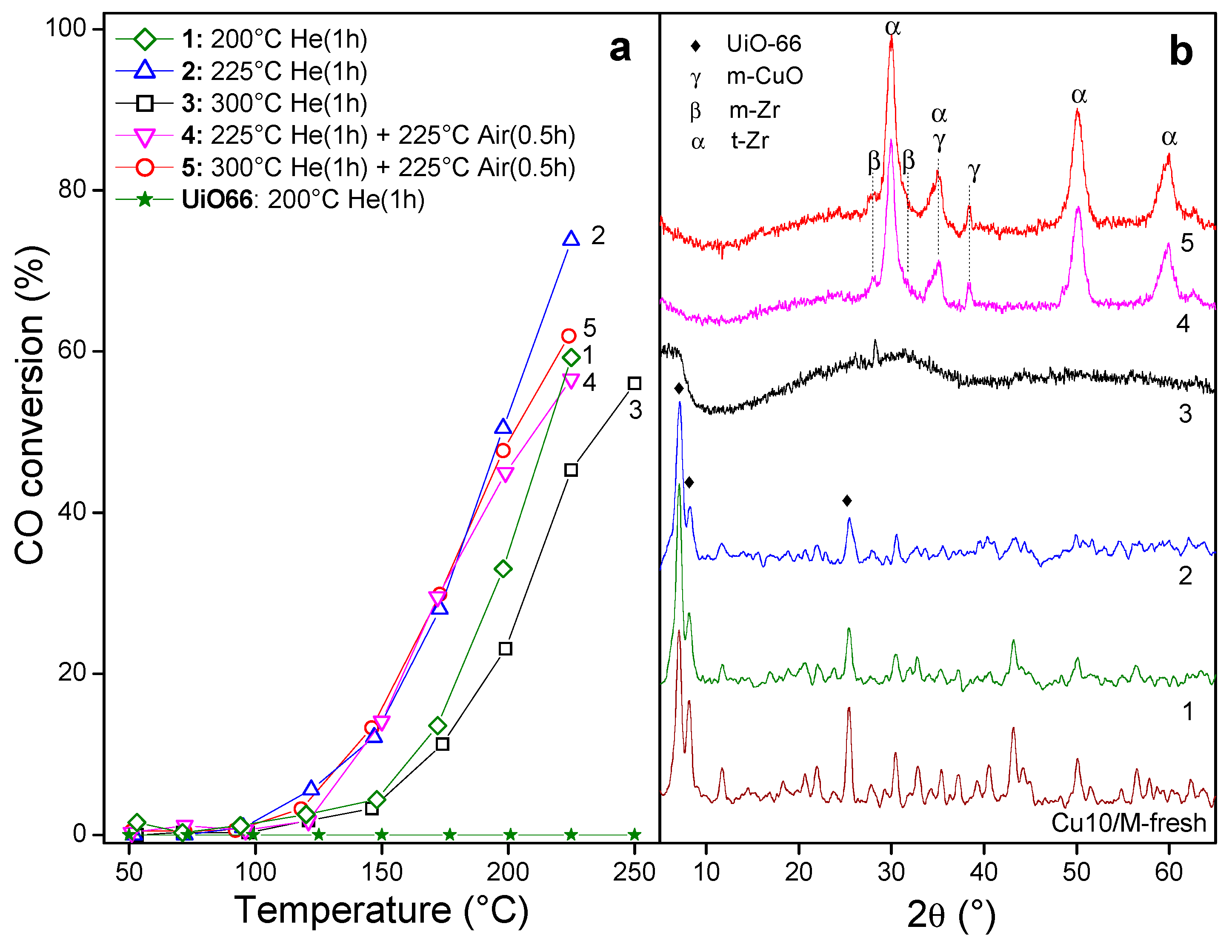
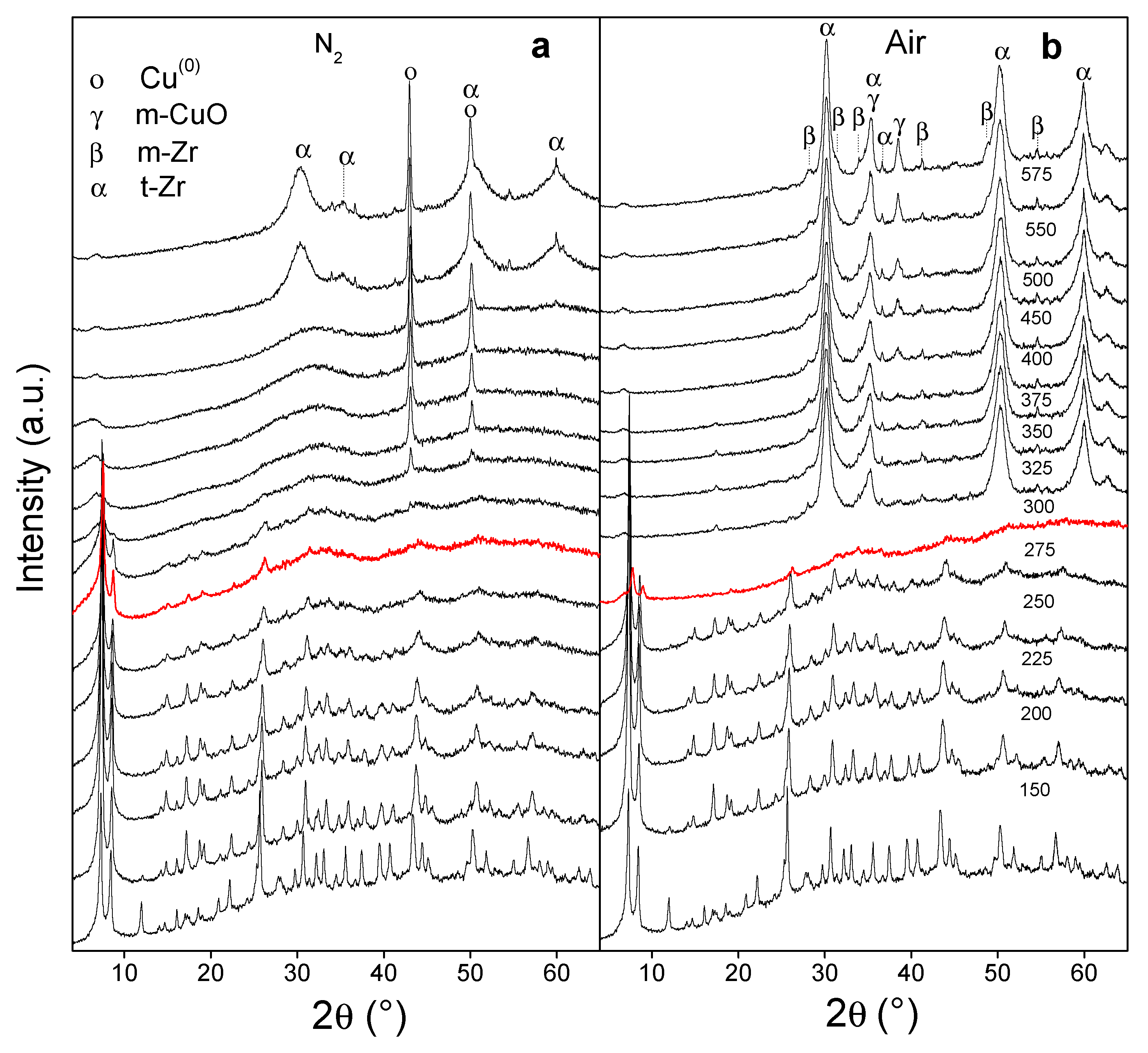
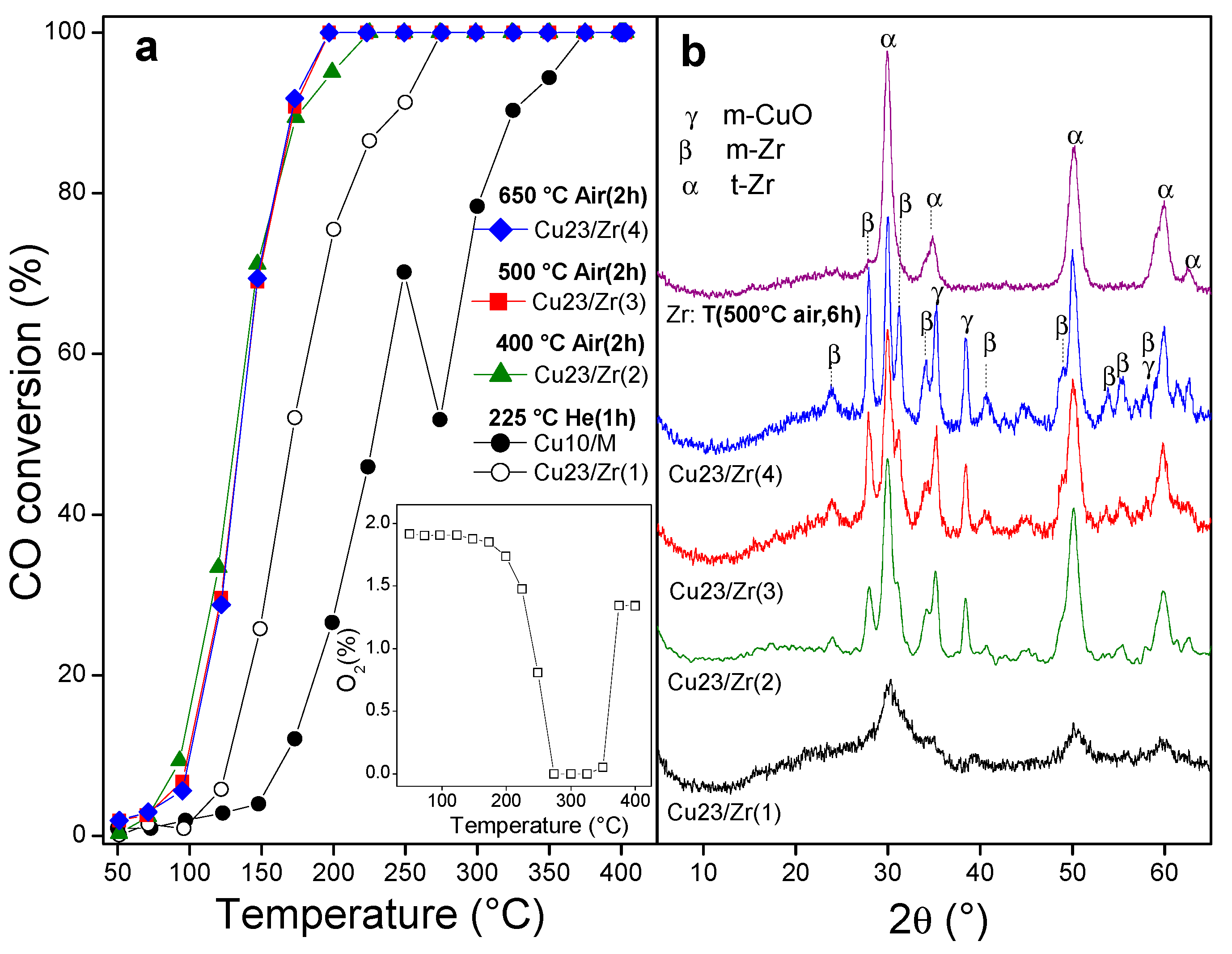
3.2. Derived Cu/UiO-66 Catalysts
3.2.1. Monitoring of the Thermal Transformation of Cu/MOF
3.2.2. Catalytic Behavior of Derived Cu/UiO-66 Catalysts
3.3. Derived Cobalt and Iron-Based UiO-66 Catalysts
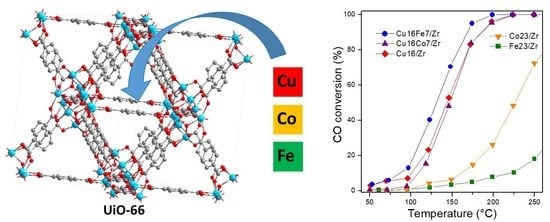
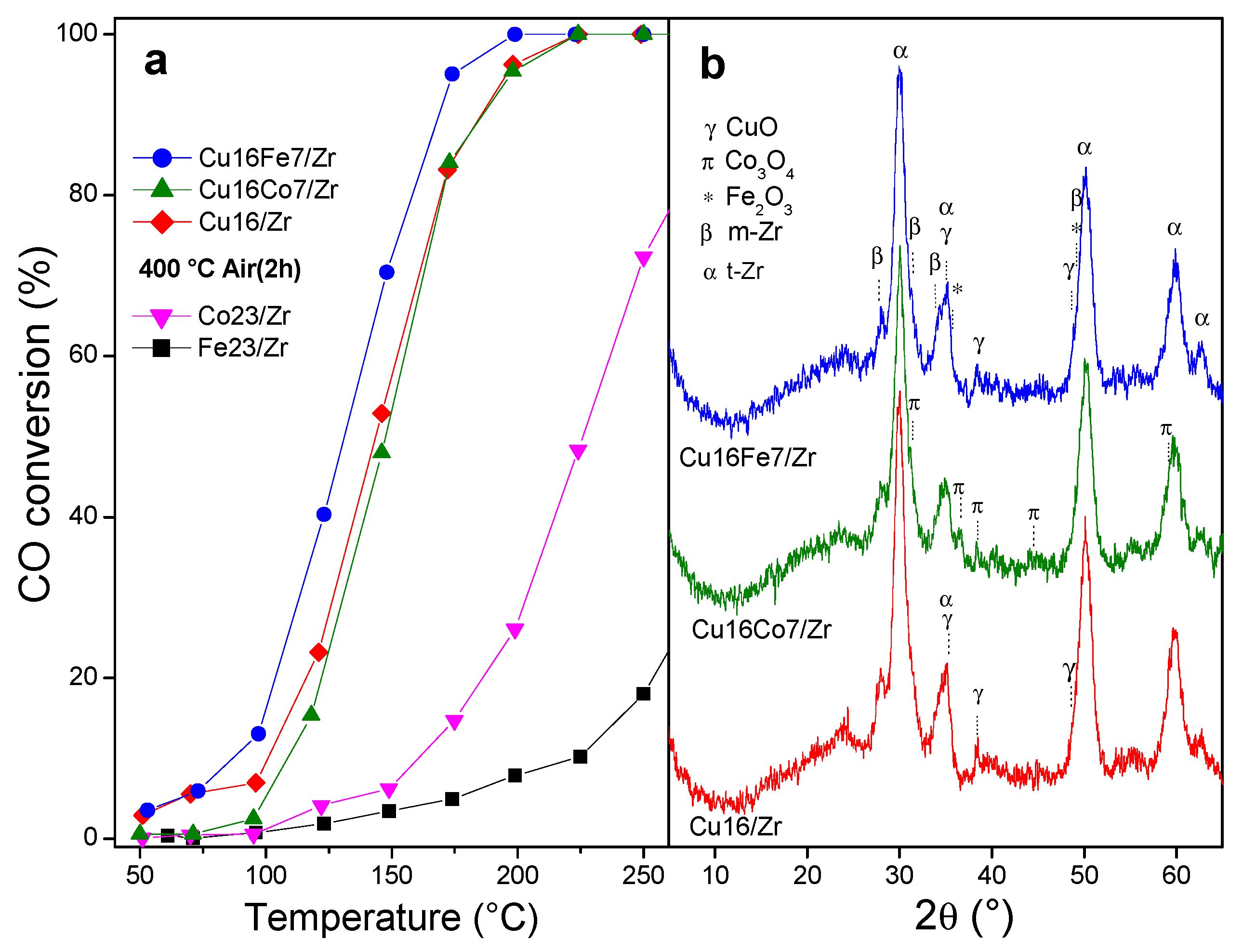
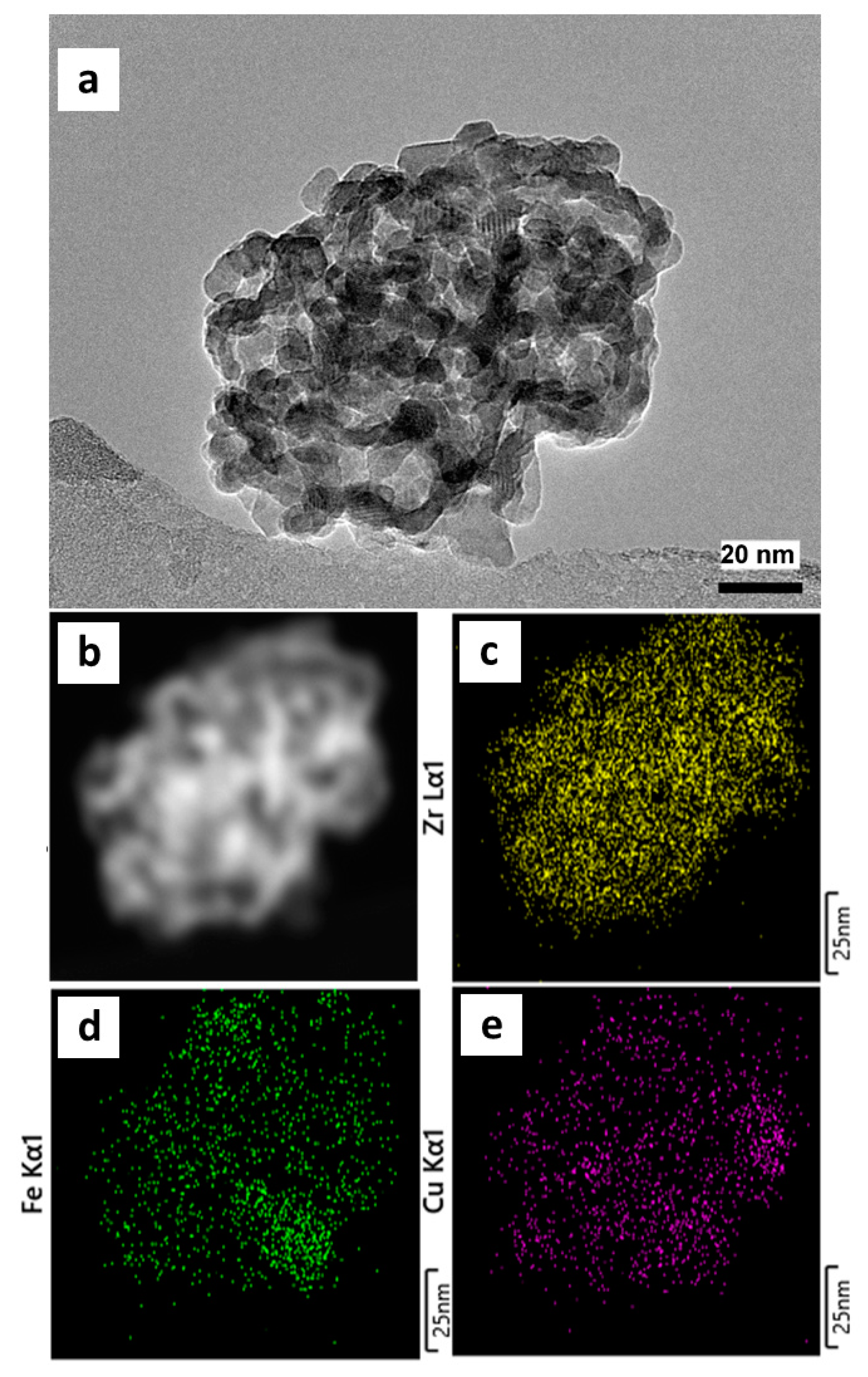
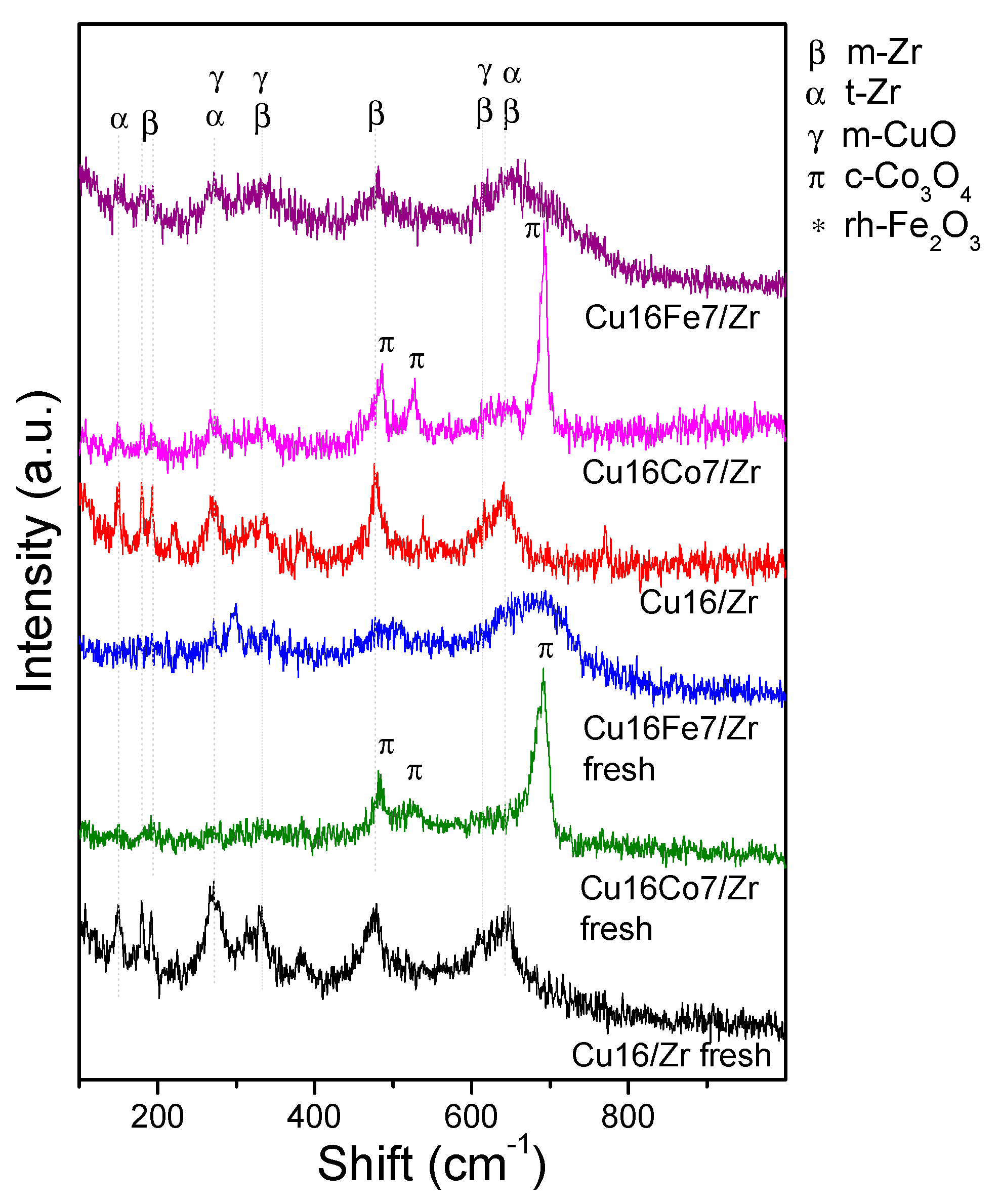
3.4. Bimetallic CuCo/UiO-66- and CuFe/UiO-66-Derived Nanocatalysts
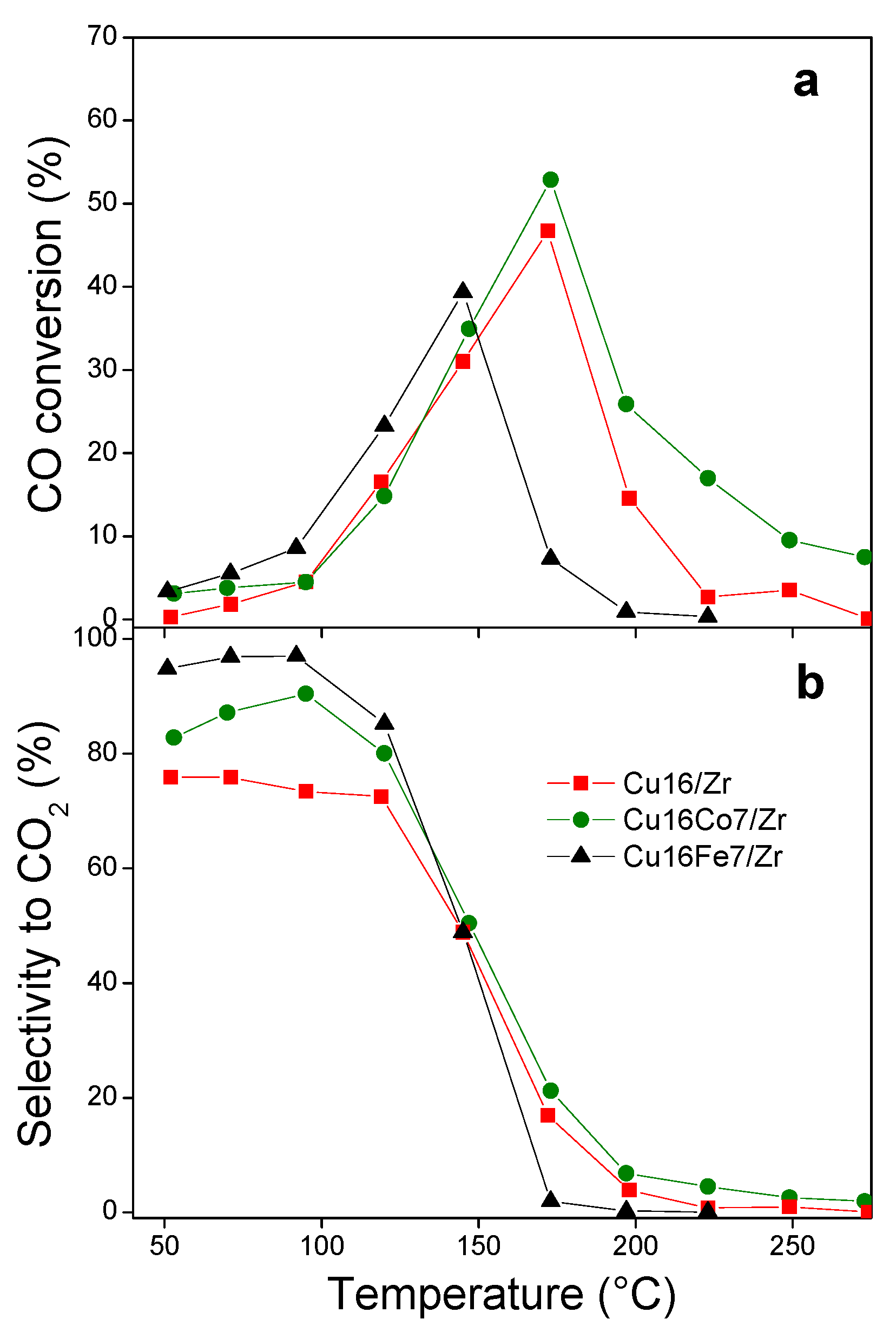
3.4.1. CO Oxidation
3.4.2. CO Oxidation in Hydrogen-Rich Stream (COProx)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal-organic frameworks: Opportunities for catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef]
- Falcaro, P.; Ricco, R.; Yazdi, A.; Imaz, I.; Furukawa, S.; Maspoch, D.; Ameloot, R.; Evans, J.D.; Doonan, C.J. Application of metal and metal oxide nanoparticles@MOFs. Coord. Chem. Rev. 2016, 307, 237–254. [Google Scholar] [CrossRef]
- Vermoortele, F.; Ameloot, R.; Alaerts, L.; Matthessen, R.; Carlier, B.; Ramós Fernandez, E.V.; Gascon, J.; Kapteijn, F.; De Vos, D.E. Tuning the catalytic performance of metal–organic frameworks in fine chemistry by active site engineering. J. Mater. Chem. 2012, 22, 10313–10321. [Google Scholar] [CrossRef] [Green Version]
- Lescouet, T.; Kockrick, E.; Bergeret, G.; Pera-Titus, M.; Farrusseng, D. Engineering MIL-53(Al) flexibility by controlling amino tags. Dalton Trans. 2011, 40, 11359–11361. [Google Scholar] [CrossRef]
- Juan-Alcañiz, J.; Gascon, J.; Kapteijn, F. Metal–organic frameworks as scaffolds for the encapsulation of active species: State of the art and future perspectives. J. Mater. Chem. 2012, 22, 10102–10118. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Sun, L.; Wang, L. Metal/metal oxide nanostructures derived from metal–organic frameworks. RSC Adv. 2015, 5, 7267–7279. [Google Scholar] [CrossRef]
- Sun, J.-K.; Xu, Q. Functional materials derived from open framework templates/precursors: Synthesis and applications. Energy Environ. Sci. 2014, 7, 2071–2100. [Google Scholar] [CrossRef]
- Zamaro, J.M.; Pérez, N.C.; Miró, E.E.; Casado, C.; Seoane, B.; Téllez, C.; Coronas, J. HKUST-1 MOF: A matrix to synthesize CuO and CuO–CeO2 nanoparticle catalysts for CO oxidation. Chem. Eng. J. 2012, 195–196, 180–187. [Google Scholar] [CrossRef]
- Green, E.; Short, S.; Shuker, L.K.; Harrison, P.T.C. Carbon Monoxide Exposure in the Home Environment and the Evaluation of Risks to Health—A UK Perspective. Indoor Built Environ. 1999, 8, 168–175. [Google Scholar] [CrossRef]
- Xanthopoulou, G.G.; Novikov, V.A.; Knysh, Y.A.; Amosov, A.P. Nanocatalysts for Low-Temperature Oxidation of CO: Review. Eurasian Chem. J. 2015, 17, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Prasad, R. A Review on Preferential Oxidation of Carbon Monoxide in Hydrogen Rich Gases. Bull. Chem. React. Eng. Catal. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Bion, N.; Epron, F.; Moreno, M.; Mariño, F.; Duprez, D. Preferential oxidation of carbon monoxide in the presence of hydrogen (PROX) over noble metal and transition metal oxides: Advantages and drawbacks. Top. Catal. 2008, 51, 76–88. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D. Catalytic Oxidation of Carbon Monoxide over Transition Metal Oxides. ChemCatChem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Firsova, A.A.; Khomenko, T.I.; Sil’chenkova, O.N.; Korchak, V.N. Oxidation of Carbon Monoxide in the Presence of Hydrogen on the CuO, CoO, and Fe2O3 oxides supported on ZrO2. Kinet. Catal. 2010, 51, 299–311. [Google Scholar] [CrossRef]
- Zhao, Z.; Yung, M.M.; Ozkan, U.S. Effect of support on the preferential oxidation of CO over cobalt catalysts. Catal. Commun. 2008, 9, 1465–1471. [Google Scholar] [CrossRef]
- Liang, Q.; Zhao, Z.; Liu, J.; Wei, Y.C.; Jiang, G.Y.; Duan, A.J. Pd nanoparticles deposited on metal-organic framework of MIL-53(AI): An active catalyst for CO oxidation. Acta Phys.-Chim. Sin. 2014, 30, 129–134. [Google Scholar]
- Jiang, H.; Liu, B.; Akita, T.; Haruta, M.; Sakurai, H.; Xu, Q. Au@ ZIF-8: CO oxidation over gold nanoparticles deposited to metal-organic framework. J. Am. Chem. Soc. 2009, 131, 11302–11303. [Google Scholar] [CrossRef] [PubMed]
- Morris Bullock, R. Reaction: Earth-Abundant Metal Catalysts for Energy Conversions. Chem 2017, 2, 444–446. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, J.R.; Schindler, C.S. Catalyst: Sustainable Catalysis. Chem 2017, 2, 313–316. [Google Scholar] [CrossRef] [Green Version]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new Zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Rungtaweevoranit, B.; Parlinska-Wojtan, M.; Xiaokun, P.; Yaghi, O.M.; Behm, R.J. Highly Active and Stable Single-Atom Cu Catalysts Supported by a Metal−Organic Framework. J. Am. Chem. Soc. 2019, 141, 5201–5210. [Google Scholar] [CrossRef]
- Wang, H.L.; Yeh, H.; Chen, Y.C.; Lai, Y.C.; Lin, C.Y.; Lu, K.Y.; Ho, R.M.; Li, B.H.; Lin, C.H.; Tsai, D.H. Thermal Stability of Metal−Organic Frameworks and Encapsulation of CuO Nanocrystals for Highly Active Catalysis. ACS Appl. Mater. Interfaces 2018, 10, 9332–9341. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Ding, T.; Gao, W.; Ma, K.; Tian, Y.; Li, X. CuO/CeO2 catalysts synthesized from Ce-UiO-66 metal-organic framework for preferential CO oxidation. Int. J. Hydrog. Energy 2017, 42, 17457–17465. [Google Scholar] [CrossRef]
- Yu, J.; Yu, J.; Wei, Z.; Guo, X.; Mao, H.; Mao, D. Preparation and Characterization of UiO-66-Supported Cu–Ce Bimetal Catalysts for Low-Temperature CO Oxidation. Catal. Lett. 2019, 149, 496–506. [Google Scholar] [CrossRef]
- Lozano, L.A.; Iglesias, C.M.; Faroldi, B.M.C.; Ulla, M.A.; Zamaro, J.M. Efficient solvothermal synthesis of highly porous UiO-66 nanocrystals in dimethylformamide-free media. J. Mater. Sci. 2018, 53, 1862–1873. [Google Scholar] [CrossRef]
- Wu, H.; Chua, Y.S.; Krungleviciute, V.; Tyagi, M.; Chen, P.; Yildirim, T.; Zhou, W. Unusual and highly tunable missing-linker defects in zirconium metal–organic framework UiO-66 and their important effects on gas adsorption. J. Am. Chem. Soc. 2013, 135, 10525–10532. [Google Scholar] [CrossRef]
- Yan, X.; Lu, N.; Fan, B.; Bao, J.; Pan, D.; Wang, M.; Li, R. Synthesis of mesoporous and tetragonal zirconia with inherited morphology from metal–organic frameworks. CrystEngComm 2015, 17, 6426–6433. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.; Hermes, S.; Kähler, K.; Van den Berg, M.W.E.; Muhler, M.; Fischer, R.A. Loading of MOF-5 with Cu and ZnO Nanoparticles by Gas-Phase Infiltration with Organometallic Precursors: Properties of Cu/ZnO@MOF-5 as Catalyst for Methanol Synthesis. Chem. Mater. 2008, 20, 4576–4587. [Google Scholar] [CrossRef]
- Yang, Z.; Mao, D.; Guo, X.; Lu, G. CO oxidation over CuO catalysts supported on CeO2-ZrO2 prepared by microwave assisted co-precipitation: The influence of CuO content. J. Rare Earths 2014, 32, 117–123. [Google Scholar] [CrossRef]
- Morales, F.; Viniegra, M.; Arroyo, R.; Córdoba, G.; Zepeda, T.A. CO oxidation over CuO/ZrO2 catalysts: Effect of loading and incorporation procedure of CuO. Mater. Res. Innnov. 2010, 14, 183–188. [Google Scholar] [CrossRef]
- Ribeiro, N.F.P.; Souza, M.V.M.; Schmal, M. Combustion synthesis of copper catalysts for selective CO oxidation. J. Power Sources 2008, 179, 329–334. [Google Scholar] [CrossRef]
- Pokrovski, K.; Jung, K.T.; Bell, A.T. Investigation of CO and CO2 Adsorption on Tetragonal and Monoclinic Zirconia. Langmuir 2001, 17, 4297–4303. [Google Scholar] [CrossRef]
- Soliman, N.K. Factors affecting CO oxidation reaction over nanosized materials: A review. J. Mater. Res. Technol. 2019, 8, 2395–2407. [Google Scholar] [CrossRef]
- Li, D.; Liu, X.; Zhang, Q.; Wang, Y.; Wan, H. Cobalt and Copper Composite Oxides as Efficient Catalysts for Preferential Oxidation of CO in H2-Rich Stream. Catal. Lett. 2009, 127, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Yeste, M.P.; Vidal, H.; García-Cabeza, A.L.; Hernández-Garrido, J.C.; Guerra, F.M.; Cifredo, G.A.; González-Leal, J.M.; Gatica, J.M. Low temperature prepared copper-iron mixed oxides for the selective CO oxidation in the presence of hydrogen. Appl. Catal. A Gen. 2018, 552, 58–69. [Google Scholar] [CrossRef]
- Said, A.E.A.A.; Abd El-Wahab, M.M.M.; Goda, M.N. Synthesis and characterization of pure and (Ce, Zr, Ag) doped mesoporous CuO-Fe2O3 as highly efficient and stable nanocatalystsfor CO oxidation at low temperature. Appl. Surf. Sci. 2016, 390, 649–665. [Google Scholar] [CrossRef]
- Cabello, A.P.; Ulla, M.A.; Zamaro, J.M. CeO/CuOx nanostructured films for CO oxidation and CO oxidation in hydrogen-rich streams using a micro-structured reactor. Top. Catal. 2019, 62, 931–940. [Google Scholar] [CrossRef]
- Yung, M.M.; Zhao, Z.; Woods, M.P.; Ozkan, U.S. Preferential oxidation of carbon monoxide on CoOx/ZrO2. J. Mol. Catal. A Chem. 2008, 279, 1–9. [Google Scholar] [CrossRef]

| Tmax N2 1 | Tmax Air 2 | |
|---|---|---|
| Cu(NO3)2∙3H2O | 259 | 255 |
| UiO-66 | 555 | 520 |
| Cu5/UiO-66 | 490 | 346 |
| Cu10/UiO-66 | 460 | 318 |
| Tmax Air 1 | |
|---|---|
| Co(NO3)2∙6H2O | 263 |
| Fe(NO3)3∙9H2O | 163 |
| Co10/UiO-66 | 465 |
| Fe10/UiO-66 | 435 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano, L.A.; Faroldi, B.M.C.; Ulla, M.A.; Zamaro, J.M. Metal–Organic Framework-Based Sustainable Nanocatalysts for CO Oxidation. Nanomaterials 2020, 10, 165. https://doi.org/10.3390/nano10010165
Lozano LA, Faroldi BMC, Ulla MA, Zamaro JM. Metal–Organic Framework-Based Sustainable Nanocatalysts for CO Oxidation. Nanomaterials. 2020; 10(1):165. https://doi.org/10.3390/nano10010165
Chicago/Turabian StyleLozano, Luis A., Betina M. C. Faroldi, María A. Ulla, and Juan M. Zamaro. 2020. "Metal–Organic Framework-Based Sustainable Nanocatalysts for CO Oxidation" Nanomaterials 10, no. 1: 165. https://doi.org/10.3390/nano10010165