Morpho-Structural, Thermal and Mechanical Properties of PLA/PHB/Cellulose Biodegradable Nanocomposites Obtained by Compression Molding, Extrusion, and 3D Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of Cellulose Nanocrystals from Plum Seed Shells
2.3. Preparation of PLA/PHB/NC Nanocomposites
2.4. Characterization Methods
3. Results and Discussion
3.1. Morphology of Cellulose Nanocrystals
3.2. Morphology of Nanocomposites as Films and Filaments
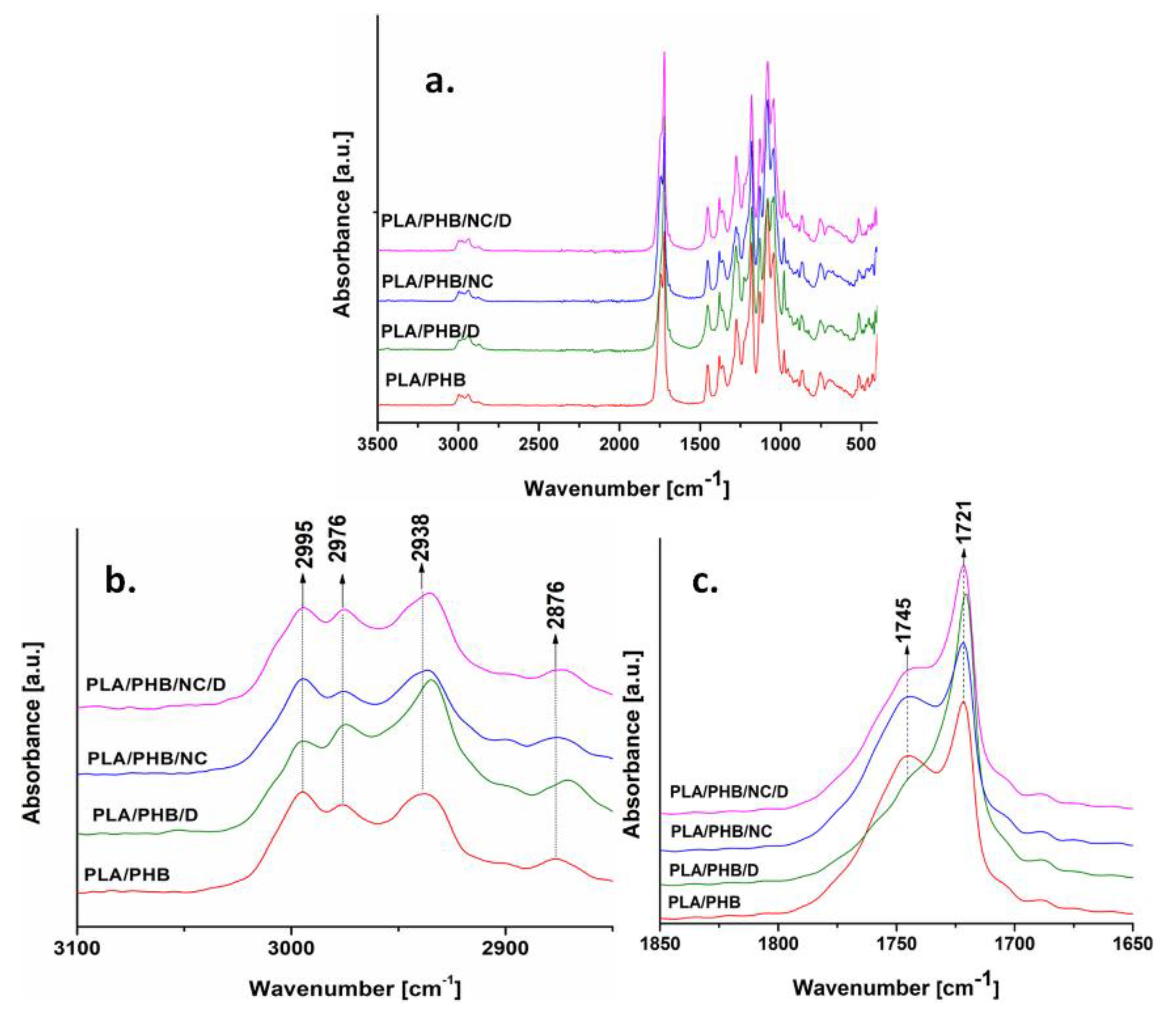
3.3. Fourier Transform Infrared Analysis
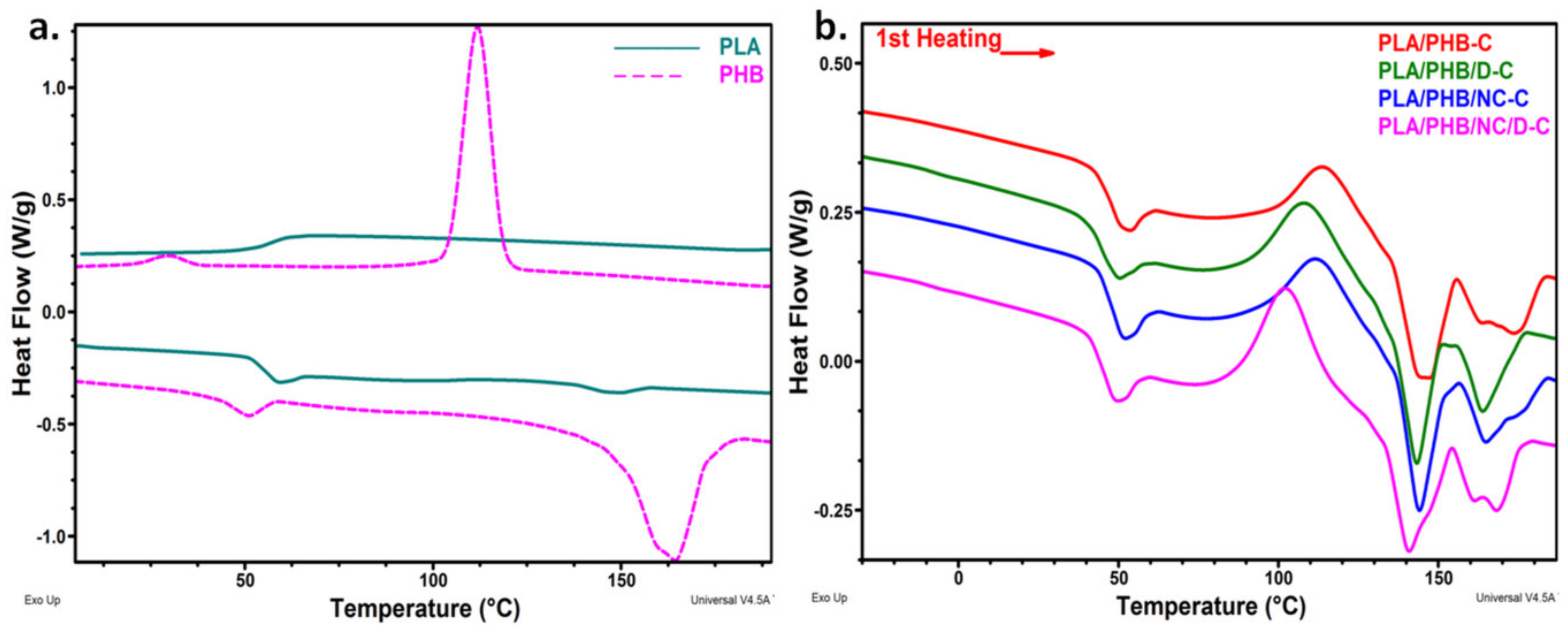
3.4. Differential Scanning Calorimetry
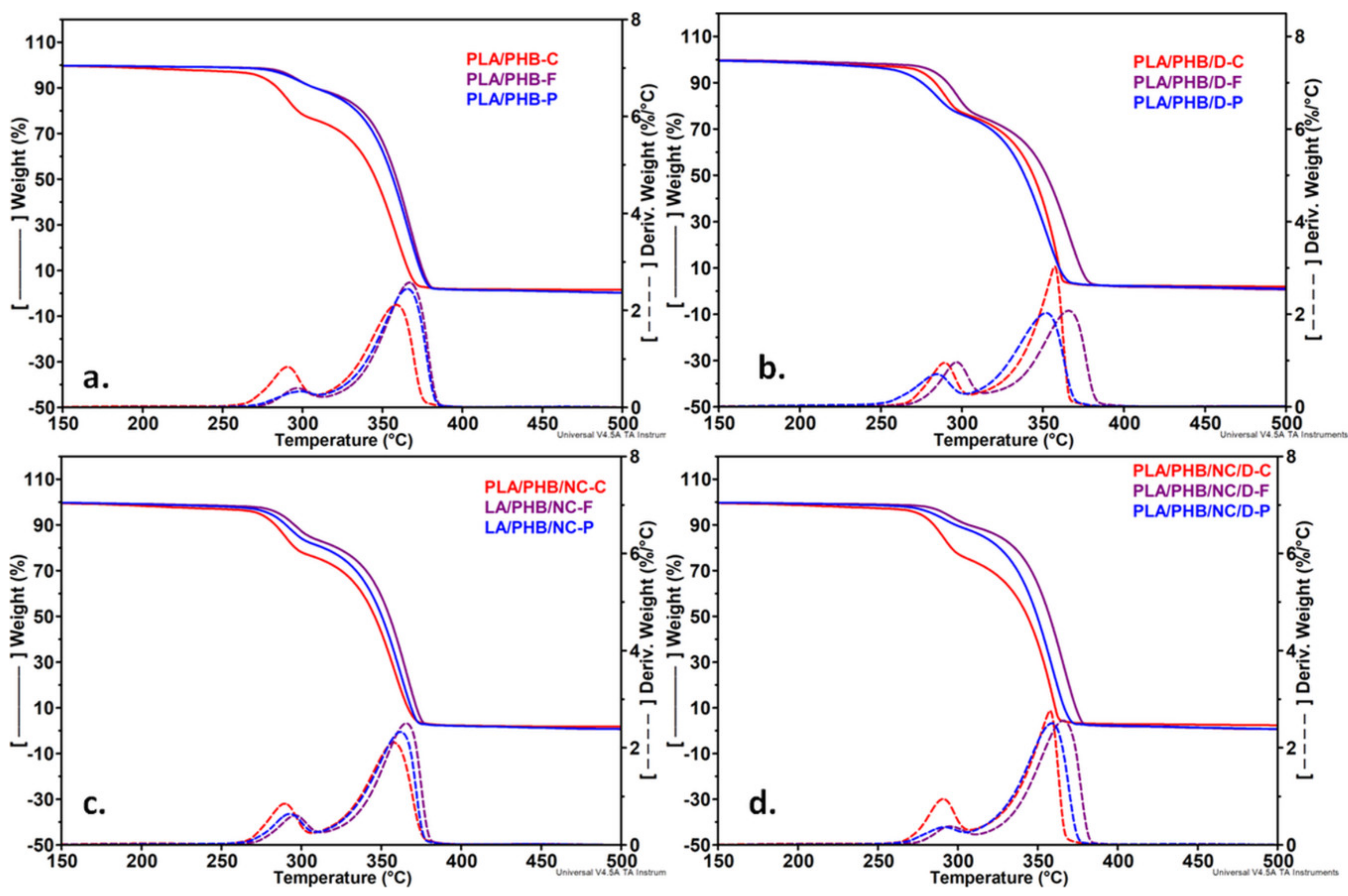
3.5. Thermogravimetric Analysis
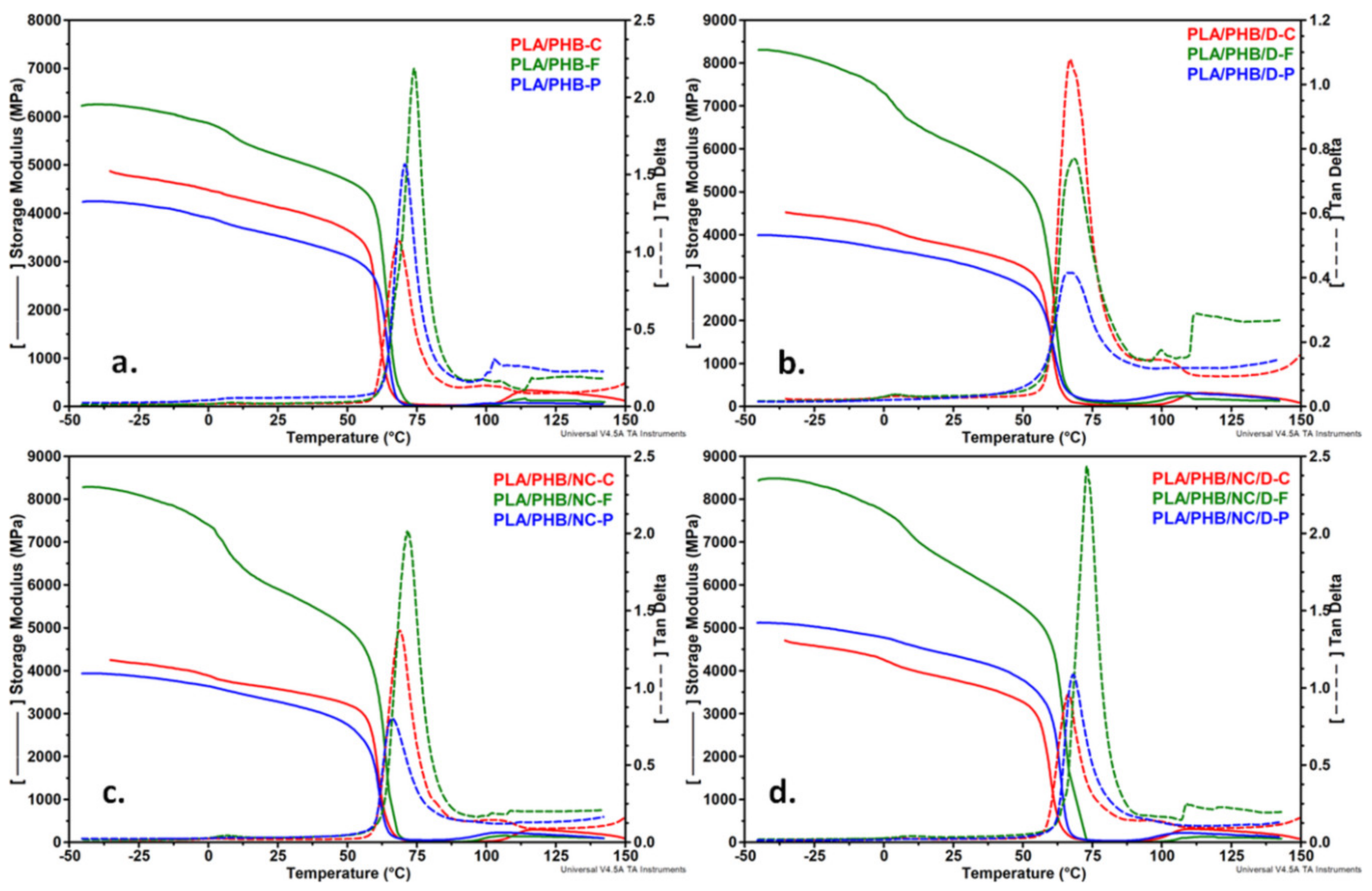
3.6. Dynamic Mechanical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salapare, H.S.; Amigoni, S.; Guittard, F. Bioinspired and Biobased Materials. Macromol.Chem. Phys. 2019, 220, 1900241. [Google Scholar] [CrossRef] [Green Version]
- Moustafa, H.; Youssef, A.M.; Darwish, N.A.; Abou-Kandil, A.I. Eco-friendly polymer composites for green packaging: Future vision and challenges. Compos. Part B Eng. 2019, 172, 16–25. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Frone, A.N.; Chiulan, I. Nanostructured biocomposites from aliphatic polyesters and bacterial cellulose. Ind. Crop. Prod. 2016, 93, 251–266. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, L.K. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on Polylactic Acid (PLA) based Sustainable Materials for Durable Applications: Focus on Toughness and Heat Resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jiménez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Processing and characterization of plasticized PLA/PHB blends for biodegradable multiphase systems. Express Polym. Lett. 2015, 9, 583–596. [Google Scholar] [CrossRef]
- Chiulan, I.; Frone, A.N.; Brandabur, C.; Panaitescu, D.M. Recent Advances in 3D Printing of Aliphatic Polyesters. Bioengineering 2018, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Frone, A.N.; Panaitescu, D.M.; Chiulan, I.; Gabor, A.R.; Nicolae, C.A.; Oprea, M.; Ghiurea, M.; Gavrilescu, D.; Puitel, A.C. Thermal and mechanical behavior of biodegradable polyester films containing cellulose nanofibers. J. Therm. Anal. Calorim. 2019, 138(4), 2387–2398. [Google Scholar] [CrossRef]
- Frone, A.N.; Panaitescu, D.M.; Chiulan, I.; Nicolae, C.A.; Vuluga, Z.; Vitelaru, C.; Damian, C.M. The effect of cellulose nanofibers on the crystallinity and nanostructure of poly (lactic acid) composites. J. Mater. Sci. 2016, 51, 9771–9791. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; Lopez, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Ollier, R.P.; D’Amico, D.A.; Schroeder, W.F.; Cyras, V.P.; Alvarez, V.A. Effect of clay treatment on the thermal degradation of PHB based nanocomposites. Appl. Clay Sci. 2018, 163, 146–152. [Google Scholar] [CrossRef]
- Furukawa, T.; Sato, H.; Murakami, R.; Zhang, J.; Duan, Y.X.; Noda, I.; Ochiai, S.; Ozaki, Y. Structure, Dispersibility, and Crystallinity of Poly (hydroxybutyrate)/Poly (l-lactic acid) Blends Studied by FT-IR Microspectroscopy and Differential Scanning Calorimetry. Macromolecules 2005, 38, 6445–6454. [Google Scholar] [CrossRef]
- Formela, K.; Zedler, L.; Hejna, A.; Tercjak, A. Reactive extrusion of bio-based polymer blends and composites—Current trends and future developments. Express Polym. Lett. 2018, 12, 24–57. [Google Scholar] [CrossRef]
- Rytlewski, P.; Zenkiewicz, M.; Malinowski, R. Influence of Dicumyl Peroxide Content on Thermal and Mechanical Properties of Polylactide. Int. Polym. Process. 2011, 26, 580–586. [Google Scholar] [CrossRef]
- Ma, P.; Cai, X.; Zhang, Y.; Wang, S.; Dong, W.; Chen, M.; Lemstra, P.J. In-situ compatibilization of poly (lactic acid) and poly(butyleneadipate-co-terephthalate) blends by using dicumyl peroxide as a free-radical initiator. Polym. Degrad. Stab. 2014, 102, 145–151. [Google Scholar] [CrossRef]
- Semba, T.; Kitagawa, K.; Ishiaku, U.S.; Hamad, H. The Effect of Crosslinking on the Mechanical Properties of Polylactic Acid/Polycaprolactone Blends. J. Appl. Polym. Sci. 2006, 101, 1816–1825. [Google Scholar] [CrossRef]
- Dong, W.; Ma, P.; Wang, S.; Chen, M.; Cai, X.; Zhang, Y. Effect of partial crosslinking on morphology and properties of the poly(β-hydroxybutyrate)/poly (d,l-lactic acid) blends. Polym. Degrad. Stab. 2013, 98, 1549–1555. [Google Scholar] [CrossRef]
- Wei, L.; McDonald, A.G. Peroxide induced cross-linking by reactive melt processing of two biopolyesters: Poly(3-hydroxybutyrate) and poly (l-lactic acid) to improve their melting processability. J. Appl. Polym. Sci. 2015, 132, 41724. [Google Scholar] [CrossRef]
- Arrieta, M.P.; García, A.D.; López, D.; Fiori, S.; Peponi, L. Antioxidant Bilayers Based on PHBV and Plasticized Electrospun PLA-PHB Fibers Encapsulating Catechin. Nanomaterials 2019, 9, 346. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, W.; Mirzadeh, A.; Tan, V.; Kamal, M.R. Influence of Nanoparticle Pretreatment on the Thermal, Rheological and Mechanical Properties of PLA-PBSA Nanocomposites Incorporating Cellulose Nanocrystals or Montmorillonite. Nanomaterials 2019, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Dufresne, A. Cellulose nanomaterial reinforced polymer nanocomposites. Curr. Opin. Colloid Interfaces Sci. 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Frone, A.N.; Chiulan, I.; Nicolae, C.A.; Trusca, R.; Ghiurea, M.; Gabor, A.R.; Mihailescu, M.; Casarica, A.; Lupescu, I. Role of bacterial cellulose and poly (3-hydroxyhexanoate-co-3-hydroxyoctanoate) in poly (3-hydroxybutyrate) blends and composites. Cellulose 2018, 25, 5569–5591. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Lupescu, I.; Frone, A.N.; Chiulan, I.; Nicolae, C.A.; Tofan, V.; Stefaniu, A.; Somoghi, R.; Trusca, R. Medium Chain-Length Polyhydroxyalkanoate Copolymer Modified by Bacterial Cellulose for Medical Devices. Biomacromolecules 2017, 18, 3222–3232. [Google Scholar] [CrossRef]
- Frone, A.N.; Berlioz, S.; Chailan, J.F.; Panaitescu, D.M. Morphology and thermal properties of PLA–cellulose nanofibers composites. Carbohydr. Polym. 2013, 91, 377–384. [Google Scholar] [CrossRef]
- Frone, A.N.; Berlioz, S.; Chailan, J.F.; Panaitescu, D.M.; Donescu, D. Cellulose Fiber-Reinforced Polylactic Acid. Polym. Compos. 2011, 32, 976–985. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayon, E.; Lopez, J.; Kenny, J.M. PLA-PHB/cellulose based films: Mechanical, barrier and disintegration properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Bhat, A.H.; Ahmad, F. Polymer blend of PLA/PHBV based bionanocomposites reinforced with nanocrystalline cellulose for potential application as packaging material. Carbohydr. Polym. 2017, 157, 1323–1332. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, Z.; Shukla, S.; Agnihotri, S.; Clemons, C.M.; Pilla, S. PHBV-graft-GMA via reactive extrusion and its use in PHBV/nanocellulose crystal composites. Carbohydr. Polym. 2019, 205, 27–34. [Google Scholar] [CrossRef]
- Liu, C.; Chan, K.W.; Shen, J.; Wong, H.M.; Yeung, K.; Tjong, S.C. Melt-compounded polylactic acid composite hybrids with hydroxyapatite nanorods and silver nanoparticles: Biodegradation, antibacterial ability, bioactivity and cytotoxicity. RSC Adv. 2015, 5, 72288–72299. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Sefadi, J.S.; Sadiku, E.R.; John, M.J.; Mochane, M.J.; Mtibe, A. Thermoplastic Processing of PLA/Cellulose Nanomaterials Composites. Polymers 2018, 10, 1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiziltas, A.; Nazari, B.; ErbasKiziltas, E.; Gardner, D.J.; Han, Y.; Rushing, T.S. Method to reinforce polylactic acid with cellulose nanofibers via a polyhydroxybutyrate carrier system. Carbohydr. Polym. 2016, 140, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Montero, M.; Roundy, S.; Odell, D.; Ahn, S.H.; Wright, P.K. Material characterization of fused deposition modeling (FDM) ABS by designed experiments. In Proceedings of the Society of Manufacturing Engineer’s Rapid Prototyping and Manufacturing Conference, Amsterdam, The Netherlands, 28–30 May 2011; pp. 15–17. [Google Scholar]
- Ahn, S.H.; And, S.R.; Wright, P.K.; Montero, M.; Odell, D.; Roundy, S. Anisotropic material properties of fused deposition modeling ABS. Rapid Prototyp. J. 2002, 8, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Zein, I.; Hutmacher, D.W.; Tan, L.C.; Teoh, S.W. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Kalita, S.J.; Bose, S.; Hosick, H.L.; Bandyopadhyay, A. Development of controlled porosity polymer-ceramic composite scaffolds via fused deposition modeling. Mater. Sci. Eng. C 2003, 23, 611–620. [Google Scholar] [CrossRef]
- Ausejo, G.J.; Rydz, J.; Musioł, M.; Sikorska, W.; Janeczek, H.; Sobota, M.; Włodarczyk, J.; Szeluga, U.; Hercog, A.; Kowalczuk, M. Three-dimensional printing of PLA and PLA/PHA dumbbell-shaped specimens of crisscross and transverse patterns as promising materials in emerging application areas: Prediction study. Polym. Degrad. Stab. 2018, 156, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Balogová, A.F.; Hudák, R.; Tóth, T.; Schnitzer, M.; Feranc, J.; Bakoš, D.; Živčák, J. Determination of geometrical and viscoelastic properties of PLA/PHB samples made by additive manufacturing for urethral substitution. J. Biotechnol. 2018, 284, 123–130. [Google Scholar] [CrossRef]
- Mencík, P.; Prikryl, R.; Stehnová, I.; Melcová, V.; Kontárová, S.; Figalla, S.; Alexy, P.; Bockaj, J. Effect of Selected Commercial Plasticizers on Mechanical, Thermal, and Morphological Properties of Poly(3-hydroxybutyrate)/Poly (lactic acid)/Plasticizer Biodegradable Blends for Three-Dimensional (3D) Print. Materials 2018, 11, 1893. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Daelemans, L.; Fiorio, R.; Maling, G.; D’hooge, R.D.; De Clerk, K.; Cardon, L. Improving Mechanical Properties for Extrusion-Based Additive Manufacturing of Poly (Lactic Acid) by Annealing and Blending with Poly(3-Hydroxybutyrate). Polymers 2019, 11, 1529. [Google Scholar] [CrossRef] [Green Version]
- Bartczak, Z.; Galeski, A.; Kowalczuk, M.; Sobota, M.; Malinowski, R. Tough blends of poly (lactide) and amorphous poly ([R,S]-3-hydroxy butyrate)—Morphology and properties. Eur. Polym. J. 2013, 49, 3630–3641. [Google Scholar] [CrossRef]
- Dai, L.; Cheng, D.; Duan, C.; Zhao, W.; Zhang, W.; Zou, X.; Aspler, J.; Ni, Y. 3D Printing Using Plant-derived Cellulose and Its Derivatives: A Review. Carbohydr. Polym. 2018, 203, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Monika, S.; Pal, A.K.; Bhasney, S.M.; Bhagabati, P.; Katiyar, V. Effect of Dicumyl Peroxide on a Poly (lactic acid) (PLA)/Poly (butylene succinate) (PBS)/Functionalized Chitosan-Based Nanobiocomposite for Packaging: A Reactive Extrusion Study. ACS Omega 2018, 3, 13298–13312. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Fortunati, E.; Dominci, F.; Rayon, E.; Lopez, J.; Kenny, J.M. Multifunctional PLA-PHB/cellulose nanocrystal films: Processing, structural and thermal properties. Carbohydr. Polym. 2014, 107, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; McDonald, A.G.; Stark, N.M. Grafting of Bacterial Polyhydroxybutyrate (PHB) onto Cellulose via In Situ Reactive Extrusion with DicumylPeroxid. Biomacromolecules 2015, 16, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Tarafder, D.; Kumar, A.; Katiyar, V. Thermally recyclable polylactic acid/cellulose nanocrystal films through reactive extrusion process. Polymer 2016, 87, 268–282. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Nicolae, C.A.; Frone, A.N.; Chiulan, I.; Stanescu, P.O.; Draghici, C.; Iorga, M.; Mihailescu, M. Plasticized poly(3-hydroxybutyrate) with improved melt processing and balanced properties. J. Appl. Polym. Sci. 2017, 134, 44810. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological characterization of plasticized PLA-PHB blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Zhengqiu, L.; Lei, L.; Yu, R.; Longchang, R.; Ting, W.; Rong, N.; De Schutter, A.; Yalin, L.; Zhenming, C. Mechanical and antibacterial properties of oriented poly (lactic acid). Polym. Eng. Sci. 2019, 59, 2121–2127. [Google Scholar]
- Yang, M.; Hu, J.; Xiong, N.; Xu, B.; Weng, Y.; Liu, Y. Preparation and properties of PLA/PHBV/PBAT blends 3D printing filament. Mater. Res. Express 2019, 6, 065401. [Google Scholar] [CrossRef]
- Morreale, M.; Liga, A.; Mistretta, M.C.; Ascione, L.; La Manti, F.P. Mechanical, Thermomechanical and Reprocessing Behavior of Green Composites from Biodegradable Polymer and Wood Flour. Materials 2015, 8, 7536–7548. [Google Scholar] [CrossRef] [Green Version]






| Sample Code | Composition (wt.%) | |||||
|---|---|---|---|---|---|---|
| Compression | Extrusion | 3D Printing | PLA | PHB | NC | DCP |
| PLA/PHB-C | PLA/PHB-F | PLA/PHB-P | 75 | 25 | - | - |
| PLA/PHB/D-C | PLA/PHB/D-F | PLA/PHB/D-P | 74.25 | 24.75 | - | 1 |
| PLA/PHB/NC-C | PLA/PHB/NC-F | PLA/PHB/NC-P | 74.25 | 24.75 | 1 | - |
| PLA/PHB/NC/D-C | PLA/PHB/NC/D-F | PLA/PHB/NC/D-P | 73.50 | 24.50 | 1 | 1 |
| Sample | TgPHB (°C) | TgPLA (°C) | Tcc (°C) | ΔHcc (J/g) | TmPLA (°C) | ΔHmPLA (J/g) | TmPHB1/TmPHB2 (°C) | ΔHmPHB1/ΔHmPHB2 (J/g) | Xc (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| XcPLA | XcPHB | |||||||||
| PLA | - | 56.7 | 120.8 | 1.31 | 149.5 | 2.11 | - | - | 0.9 | 0 |
| PHB | −10.0 | - | - | - | - | - | 161.0/164.4 | 27.2/30.8 | 0 | 39.8 |
| PLA/PHB-C | 6.7 | 48.6 | 114.2 | 17.0 | 144.7 | 16.0 | 163.5/173.7 | 4.6/7.8 | 16.4 | 34.0 |
| PLA/PHB/D-C | −8.7 | 44.5 | 108.5 | 22.0 | 143.2 | 15.3 | 163.7/- | 11.17/- | 37.9 | 30.6 |
| PLA/PHB/NC-C | 19.8 | 49.2 | 112.1 | 19.7 | 144.1 | 16.2 | 164.8/172.0 | 8.2/3.4 | 40.1 | 31.9 |
| PLA/PHB/NC/D-C | −7.6 | 46.5 | 102.3 | 23.8 | 140.8 | 18.0 | 161.3/168.1 | 5.0/6.6 | 42.6 | 31.9 |
| Sample | PLA/PHB | PLA/PHB/D | PLA/PHB/NC | PLA/PHB/NC/D | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Processing Form | C | F | P | C | F | P | C | F | P | C | F | P |
| Ton, °C | 267 | 277 | 272 | 268 | 272 | 255 | 269 | 273 | 271 | 270 | 276 | 267 |
| T10%, °C | 284 | 308 | 308 | 284 | 291 | 275 | 284 | 296 | 290 | 285 | 307 | 299 |
| Wloss200, % | 1.34 | 0.53 | 0.55 | 1.48 | 0.81 | 1.48 | 1.64 | 0.79 | 0.91 | 1.32 | 0.56 | 0.66 |
| Tmax1,°C | 290 | 296 | 297 | 289 | 296 | 284 | 289 | 296 | 292 | 291 | 294 | 291 |
| Tmax2, °C | 359 | 367 | 366 | 357 | 366 | 352 | 358 | 366 | 362 | 358 | 366 | 359 |
| R700°C, % | 1.21 | 0.22 | 0.21 | 1.42 | 0.46 | 0.61 | 1.45 | 0.61 | 0.66 | 1.92 | 0.27 | 0.44 |
| Sample | TgPHB (°C) | TgPLA (°C) | E’ (MPa) −10 °C | E’ (MPa) 25 °C | E’ (MPa) 95°C |
|---|---|---|---|---|---|
| PLA/PHB-C | 3.4 | 67.1 | 4934 | 4319 | 19 |
| PLA/PHB-F | 9.0 | 73.9 | 6004 | 5202 | 6 |
| PLA/PHB-P | 9.3 | 70.72 | 4047 | 3532 | 39 |
| PLA/PHB/D-C | 5.4 | 66.9 | 4312 | 3734 | 32 |
| PLA/PHB/D-F | 4.2 | 68.2 | 7724 | 6126 | 91 |
| PLA/PHB/D-P | - | 66.0 | 3789 | 3375 | 214 |
| PLA/PHB/NC-C | 1.8 | 68.8 | 4033 | 3574 | 18 |
| PLA/PHB/NC-F | 6.7 | 71.9 | 7773 | 5903 | 35 |
| PLA/PHB/NC-P | 6.3 | 66.0 | 3742 | 3280 | 133 |
| PLA/PHB/NC/D-C | 3.2 | 66.5 | 4431 | 3792 | 48 |
| PLA/PHB/NC/D-F | 9.2 | 72.9 | 8025 | 6482 | 30 |
| PLA/PHB/NC/D-P | 7.0 | 68.0 | 4884 | 4358 | 89 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frone, A.N.; Batalu, D.; Chiulan, I.; Oprea, M.; Gabor, A.R.; Nicolae, C.-A.; Raditoiu, V.; Trusca, R.; Panaitescu, D.M. Morpho-Structural, Thermal and Mechanical Properties of PLA/PHB/Cellulose Biodegradable Nanocomposites Obtained by Compression Molding, Extrusion, and 3D Printing. Nanomaterials 2020, 10, 51. https://doi.org/10.3390/nano10010051
Frone AN, Batalu D, Chiulan I, Oprea M, Gabor AR, Nicolae C-A, Raditoiu V, Trusca R, Panaitescu DM. Morpho-Structural, Thermal and Mechanical Properties of PLA/PHB/Cellulose Biodegradable Nanocomposites Obtained by Compression Molding, Extrusion, and 3D Printing. Nanomaterials. 2020; 10(1):51. https://doi.org/10.3390/nano10010051
Chicago/Turabian StyleFrone, Adriana Nicoleta, Dan Batalu, Ioana Chiulan, Madalina Oprea, Augusta Raluca Gabor, Cristian-Andi Nicolae, Valentin Raditoiu, Roxana Trusca, and Denis Mihaela Panaitescu. 2020. "Morpho-Structural, Thermal and Mechanical Properties of PLA/PHB/Cellulose Biodegradable Nanocomposites Obtained by Compression Molding, Extrusion, and 3D Printing" Nanomaterials 10, no. 1: 51. https://doi.org/10.3390/nano10010051





