Characterization and Electrochemical Behaviour of Nanoscale Hydrotalcite-Like Compounds toward the Reduction of Nitrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of HTLCs
2.3. Characterization of HTLCs
2.4. Electrochemical Measurement
2.5. Analytical Methods
3. Results and Discussion
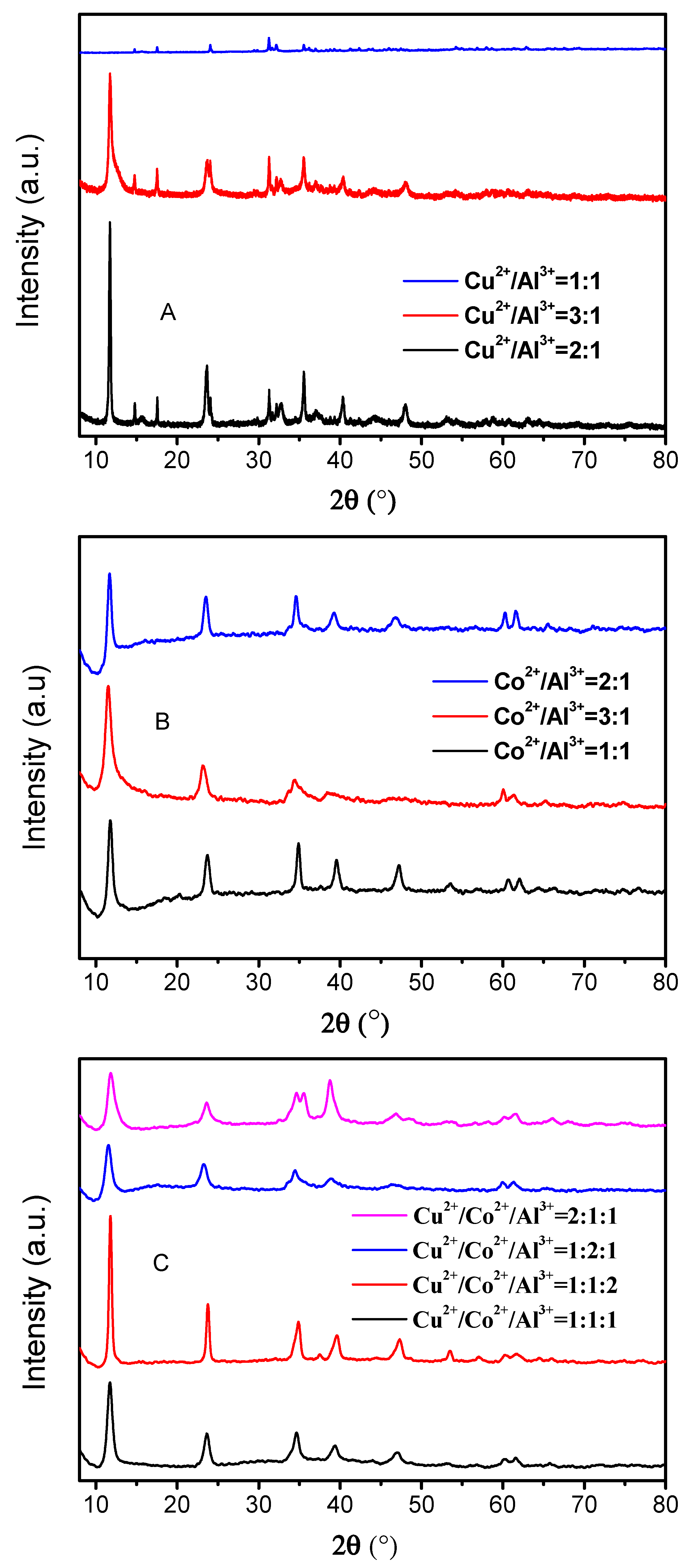
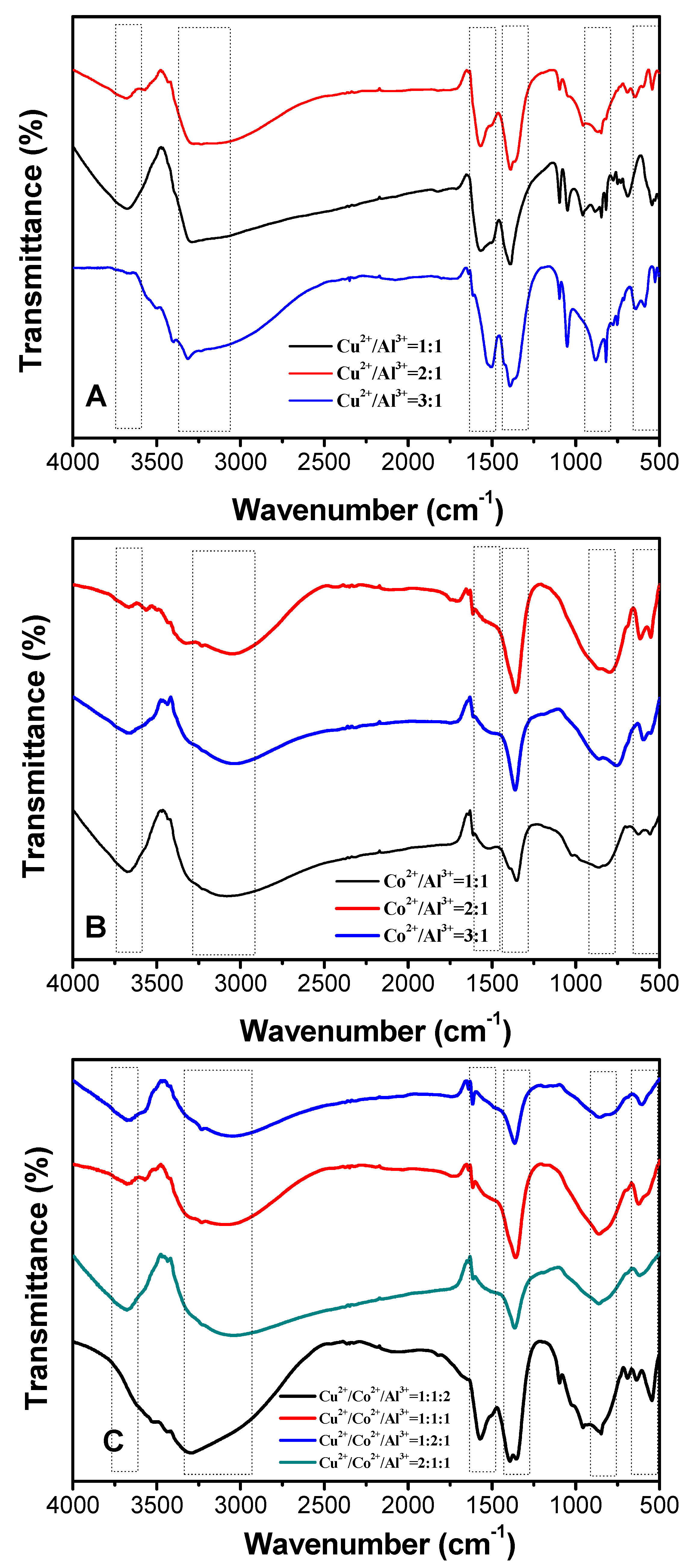
3.1. Characterization of The Prepared HTLCs
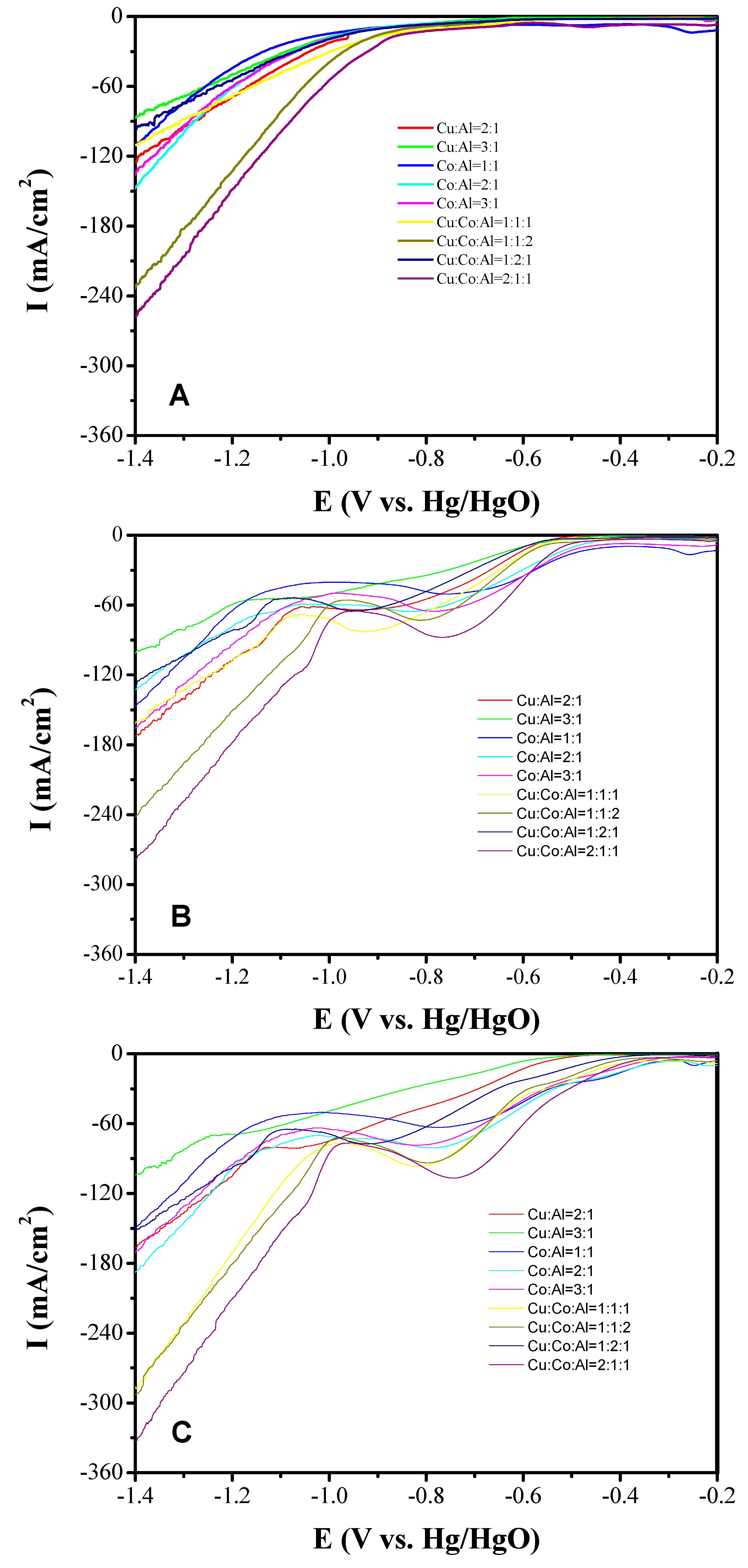
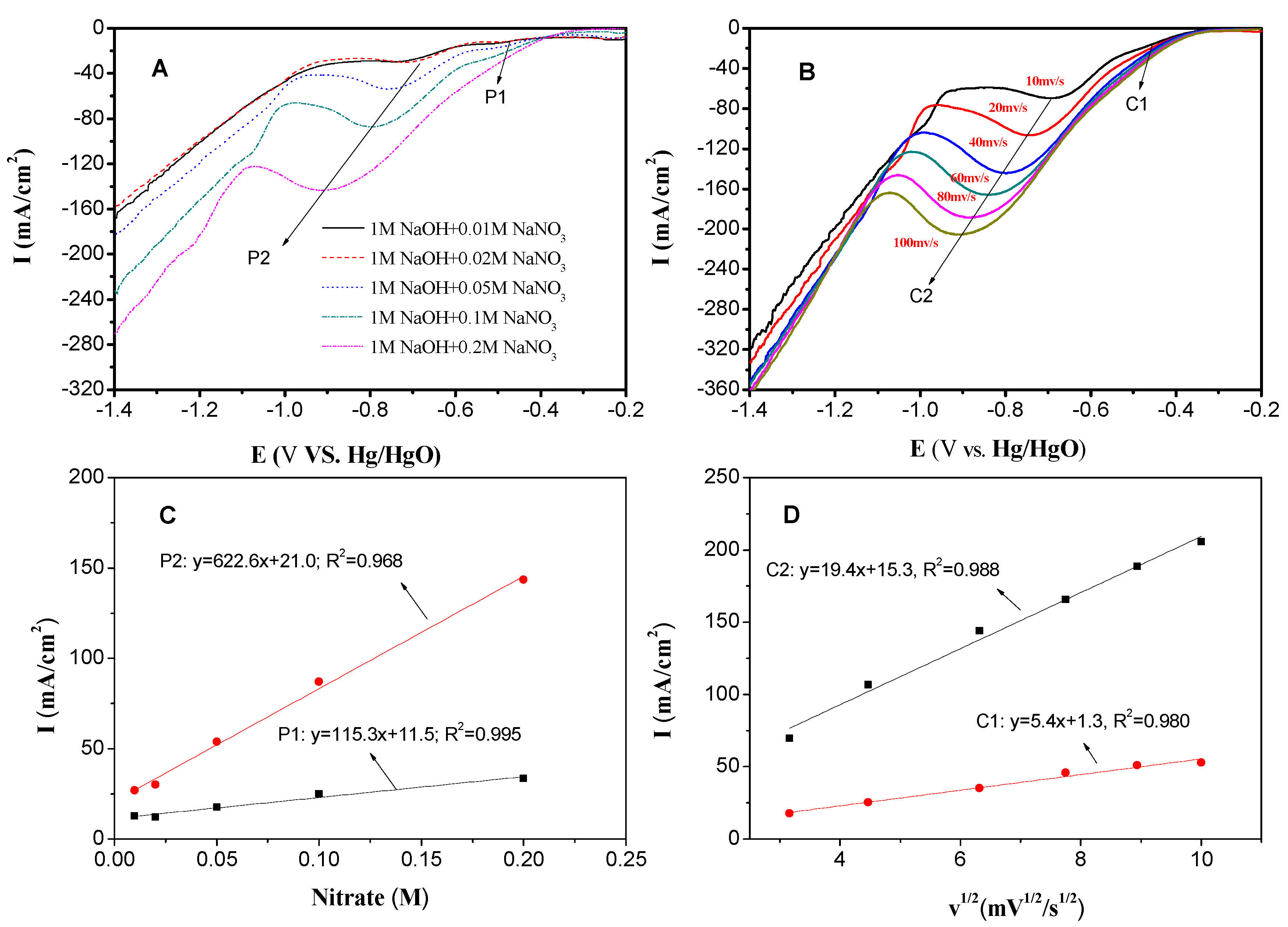
3.2. Electrocatalytic Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Impact Statement
References
- Chan, T.Y.K. Vegetable-borne nitrate and nitrite and the risk of methaemoglobinaemia. Toxicol. Lett. 2011, 200, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.; Deschamps, J.; Martin, M.H.; Bertin, E.; Reyter, D.; Garbarino, S.; Roué, L.; Guay, D. Identification of Cu surface active sites for a complete nitrate-to-nitrite conversion with nanostructured catalysts. Appl. Catal. B Environ. 2016, 187, 399–407. [Google Scholar] [CrossRef]
- Calderon, R.L.; Craun, G.F. Rolling Revision of the WHO Guidelines for Drinking-Water Quality; A review of epidemiological studies 1957–1979; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Pérez-Gallent, E.; Figueiredo, M.C.; Katsounaros, I.; Koper, M.T.M. Electrocatalytic reduction of nitrate on copper single crystals in acidic and alkaline solutions. Electrochim. Acta 2017, 227, 77–84. [Google Scholar] [CrossRef]
- Mattarozzi, L.; Cattarin, S.; Comisso, N.; Gerbasi, R.; Guerriero, P.; Musiani, M. Electrodeposition of cu-ni alloy electrodes with bimodal porosity and their use for nitrate reduction. ECS Electrochem. Lett. 2013, 2, D58–D60. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Brindley, G.W.; Kikkawa, S. A crystal-chemical study of magnesium, aluminum and nickel, aluminum hydroxy-perchlorates and hydroxy-carbonates. Am. Mineral. 1979, 64, 836–843. [Google Scholar]
- Du, F.; Ling, X.; Wang, Z.; Guo, S.; Zhang, Y.; He, H.; Li, G.; Jiang, C.; Zhou, Y.; Zou, Z. Strained heterointerfaces in sandwich–like NiFe layered double hydroxides/Co1-xS for highly efficient and superior long–term durable oxygen evolution reaction. J. Catal. 2020, 389, 132–139. [Google Scholar] [CrossRef]
- Cao, L.; Guo, J.; Tian, J.; Xu, Y.; Hu, M.; Wang, M.; Fan, J. Preparation of Ca/Al-Layered Double Hydroxide and the influence of their structure on early strength of cement. Constr. Build. Mater. 2018, 184, 203–214. [Google Scholar] [CrossRef]
- Elmoubarki, R.; Mahjoubi, F.Z.; Elhalil, A.; Tounsadi, H.; Abdennouri, M.; Sadiq, M.; Qourzal, S.; Zouhri, A.; Barka, N. Ni/Fe and Mg/Fe layered double hydroxides and their calcined derivatives: Preparation, characterization and application on textile dyes removal. J. Mater. Res. Technol. 2017, 6, 271–283. [Google Scholar] [CrossRef]
- Alhumaimess, M.S.; Hotan Alsohaimi, I.; Hassan, H.M.A.; El-Sayed, M.Y.; Alshammari, M.S.; Aldosari, O.F.; Alshammari, H.M.; Kamel, M.M. Synthesis of ionic liquid intercalated layered double hydroxides of magnesium and aluminum: A greener catalyst of Knoevenagel condensation. J. Saudi Chem. Soc. 2020, 24, 321–333. [Google Scholar] [CrossRef]
- Das, J.; Das, D.; Parida, K.M. Preparation and characterization of Mg-Al hydrotalcite-like compounds containing cerium. J. Colloid Interface Sci. 2006, 301, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Carja, G.; Nakamura, R.; Niiyama, H. Tailoring the porous properties of iron containing mixed oxides for As (V) removal from aqueous solutions. Microporous Mesoporous Mater. 2005, 83, 94–100. [Google Scholar] [CrossRef]
- Halajnia, A.; Oustan, S.; Najafi, N.; Khataee, A.R.; Lakzian, A. The adsorption characteristics of nitrate on Mg–Fe and Mg–Al layered double hydroxides in a simulated soil solution. Appl. Clay Sci. 2012, 70, 28–36. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Pan, T.; Zhao, Y.; Zhang, J.Q.; Cao, C.N. The structure and electrochemical performance of spherical Al-substituted α-Ni(OH)2 for alkaline rechargeable batteries. J. Power Sources 2005, 143, 243–255. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Zhang, X.; Fu, S. Studies on Me/Al-layered double hydroxides (Me = Ni and Co) as electrode materials for electrochemical capacitors. Electrochim. Acta 2004, 49, 3137–3141. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, R.; Evans, D.; Duan, X. Preparation of Layered Double-Hydroxide Nanomaterials with a Uniform Crystallite Size Using a New Method Involving Separate Nucleation and Aging Setp. Chem. Mater. 2002, 14. [Google Scholar] [CrossRef]
- El Hassani, K.; Kalnina, D.; Turks, M.; Beakou, B.H.; Anouar, A. Enhanced degradation of an azo dye by catalytic ozonation over Ni-containing layered double hydroxide nanocatalyst. Sep. Purif. Technol. 2019, 210, 764–774. [Google Scholar] [CrossRef]
- Singh, P.; Singh, M.K.; Beg, Y.R.; Nishad, G.R. A review on spectroscopic methods for determination of nitrite and nitrate in environmental samples. Talanta 2019, 191, 364–381. [Google Scholar] [CrossRef]
- Zhou, L.; Boyd, C.E. Comparison of Nessler, phenate, salicylate and ion selective electrode procedures for determination of total ammonia nitrogen in aquaculture. Aquaculture 2016, 450, 187–193. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Pan, Y.F.; Feng, M.; Cui, X.; Xu, X.-F. Catalytic activity of alkali metal doped Cu-Al mixed oxides for N2O decomposition in the presence of oxygen. J. Fuel Chem. Technol. 2012, 40, 601–607. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.V. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid State Chem. 2019, 269, 494–506. [Google Scholar] [CrossRef]
- Aider, N.; Touahra, F.; Bali, F.; Djebarri, B.; Lerari, D.; Bachari, K.; Halliche, D. Improvement of catalytic stability and carbon resistance in the process of CO2 reforming of methane by CoAl and CoFe hydrotalcite-derived catalysts. Int. J. Hydrog. Energy 2018, 43, 8256–8266. [Google Scholar] [CrossRef]
- Qu, J.; He, X.; Chen, M.; Hu, H.; Zhang, Q.; Liu, X. Mechanochemical synthesis of Cu-Al and methyl orange intercalated Cu-Al layered double hydroxides. Mater. Chem. Phys. 2017, 191, 173–180. [Google Scholar] [CrossRef]
- Lu, Y.; Hou, W.; Yang, D.; Chen, Y. CoP nanosheets in-situ grown on N-doped graphene as an efficient and stable bifunctional electrocatalyst for hydrogen and oxygen evolution reactions. Electrochim. Acta. 2019, 307, 543–552. [Google Scholar] [CrossRef]
- Peng, X.; Wang, M.; Hu, F.; Qiu, F.; Zhang, T.; Dai, H.; Cao, Z. Multipath fabrication of hierarchical CuAl layered double hydroxide/carbon fiber composites for the degradation of ammonia nitrogen. J. Environ. Manag. 2018, 220, 173–182. [Google Scholar] [CrossRef]
- Nickell, R.A.; Zhu, W.H.; Payne, R.U.; Cahela, D.R.; Tatarchuk, B.J. Hg/HgO electrode and hydrogen evolution potentials in aqueous sodium hydroxide. J. Power Sources 2006, 161, 1217–1224. [Google Scholar] [CrossRef]
- Polatides, C.; Kyriacou, G. Electrochemical reduction of nitrate ion on various cathodes–reaction kinetics on bronze cathode. J. Appl. Electrochem. 2005, 35, 421–427. [Google Scholar] [CrossRef]
- Reyter, D.; Bélanger, D.; Roué, L. Optimization of the cathode material for nitrate removal by a paired electrolysis process. J. Hazard. Mater. 2011, 192, 507–513. [Google Scholar] [CrossRef]
- Reyter, D.; Belanger, D.; Roue, L. Study of the electroreduction of nitrate on copper in alkaline solution. Electrochim. Acta 2008, 53, 5977–5984. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Y.; Zhang, Y.; Huang, Y. Enhanced electrolytic nitrate reduction utilizing a three-dimensional electrolysis reactor packed with activated carbon and foamed copper. Environ. Eng. Sci. 2016, 33, 525–535. [Google Scholar] [CrossRef]
- Badea, G.E. Electrocatalytic reduction of nitrate on copper electrode in alkaline solution. Electrochim. Acta 2009, 54, 996–1001. [Google Scholar] [CrossRef]
- Abdallah, R.; Geneste, F.; Labasque, T.; Djelal, H.; Fourcade, F.; Amrane, A.; Taha, S.; Floner, D. Selective and quantitative nitrate electroreduction to ammonium using a porous copper electrode in an electrochemical flow cell. J. Electroanal. Chem. 2014, 727, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.S.; Menzel, R.; Wang, Y.; Garcia-Gallastegui, A.; Bawaked, S.M.; Obaid, A.Y.; Basahel, S.N.; Mokhtar, M. Graphene-oxide-supported CuAl and CoAl layered double hydroxides as enhanced catalysts for carbon-carbon coupling via Ullmann reaction. J. Solid State Chem. 2017, 246, 130–137. [Google Scholar] [CrossRef]
- Othman, M.R.; Rasid, N.M.; Fernando, W.J.N. Mg–Al hydrotalcite coating on zeolites for improved carbon dioxide adsorption. Chem. Eng. Sci. 2006, 61, 1555–1560. [Google Scholar] [CrossRef]
- Frost, R.L.; Palmer, S.J.; Tai, N. Synthesis and Raman Spectroscopic Characterisation of Hydrotalcite with CO2 and VO3 Anions in the Interlayer. J. Raman Spectrosc. 2010, 38, 1602–1608. [Google Scholar]
- José Dos Reis, M.; Silvério, F.; Tronto, J.; Valim, J.B. Effects of pH, temperature, and ionic strength on adsorption of sodium dodecylbenzenesulfonate into Mg–Al–CO3 layered double hydroxides. J. Phys. Chem. Solids 2004, 65, 487–492. [Google Scholar] [CrossRef]
- Durivault, L.; Brylev, O.; Reyter, D.; Sarrazin, M.; Bélanger, D.; Roué, L. Cu–Ni materials prepared by mechanical milling: Their properties and electrocatalytic activity towards nitrate reduction in alkaline medium. J. Alloys Compd. 2007, 432, 323–332. [Google Scholar] [CrossRef]
- Mácová, Z.; Bouzek, K.; Šerák, J. Electrocatalytic activity of copper alloys for NO3− reduction in a weakly alkaline solution. J. Appl. Electrochem. 2007, 37, 557–566. [Google Scholar] [CrossRef]



| Sample | CuAl–LDH | CoAl–LDH | CuCoAl–LDH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n(Cu2+): n(Al3+): | n(Co2+): n(Al3+) | n(Cu2+): n(Co2+): n(Al3+) | ||||||||
| Theoretical molar ratio | 1:1 | 2:1 | 3:1 | 1:1 | 2:1 | 3:1 | 1:1:1 | 2:1:1 | 1:2:1 | 1:1:2 |
| Measured molar ratio | 1.1:1 | 2.1:1 | 3.1:1 | 1.2:1 | 2.1:1 | 3.3:1 | 1:1.1:1.1 | 1.9:1:1.1 | 1:2.2:1.3 | 1:1.1:2.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Yang, J.; Yun, Y.; Hu, S.; Huang, Y. Characterization and Electrochemical Behaviour of Nanoscale Hydrotalcite-Like Compounds toward the Reduction of Nitrate. Nanomaterials 2020, 10, 1926. https://doi.org/10.3390/nano10101926
Li L, Yang J, Yun Y, Hu S, Huang Y. Characterization and Electrochemical Behaviour of Nanoscale Hydrotalcite-Like Compounds toward the Reduction of Nitrate. Nanomaterials. 2020; 10(10):1926. https://doi.org/10.3390/nano10101926
Chicago/Turabian StyleLi, Liang, Junhao Yang, Yafeng Yun, Shouxun Hu, and Yuanxing Huang. 2020. "Characterization and Electrochemical Behaviour of Nanoscale Hydrotalcite-Like Compounds toward the Reduction of Nitrate" Nanomaterials 10, no. 10: 1926. https://doi.org/10.3390/nano10101926





