Silicon Nanowires for Gas Sensing: A Review
Abstract
:1. Introduction
2. Fabrication of SiNWs
2.1. Top-Down Fabrication Methods
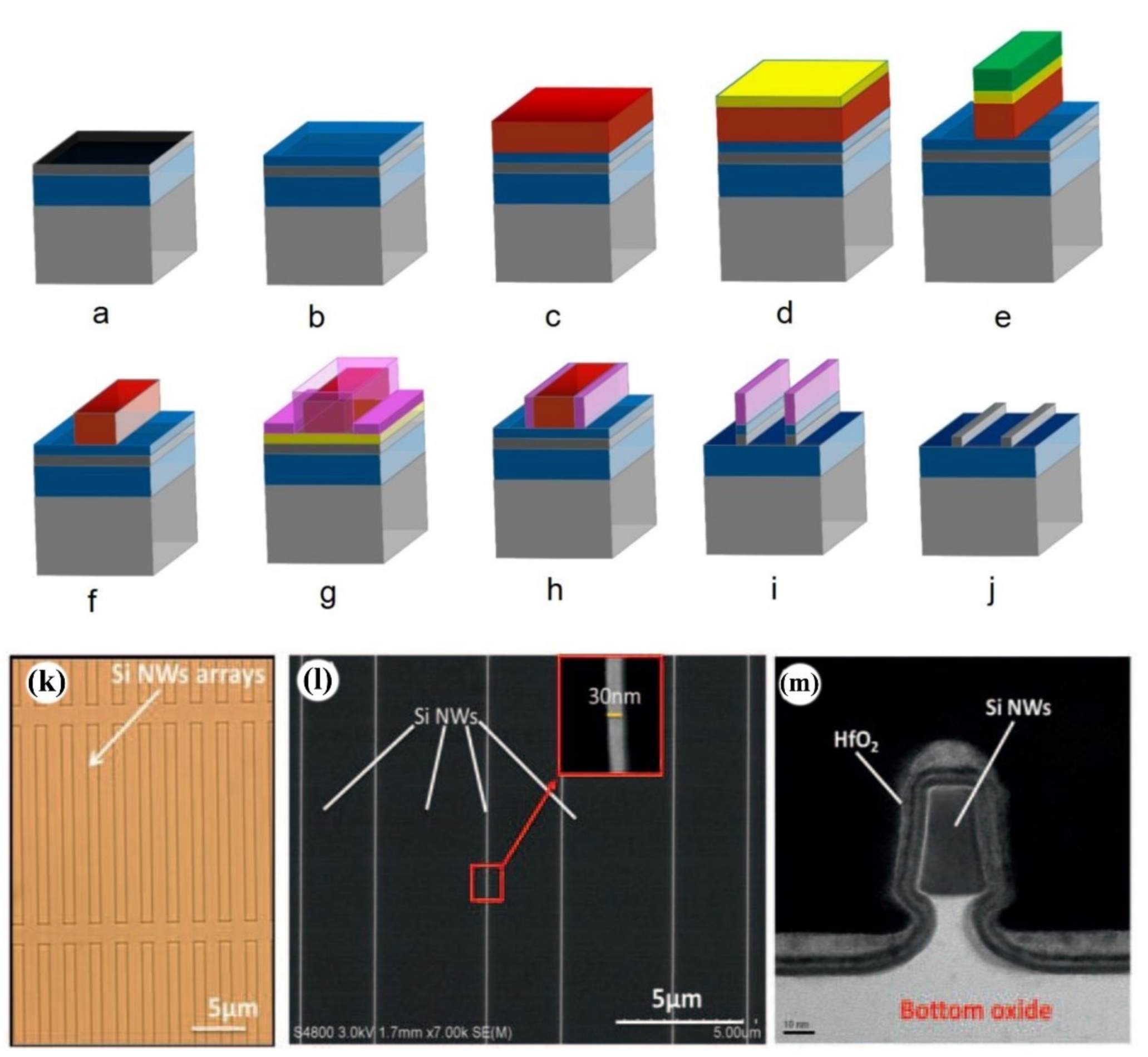
2.1.1. Lithography
Photolithography
E-Beam Lithography
Side Wall Transfer Lithography (STL)
2.1.2. Etching Methods
2.1.3. Contact Resistance of SiNW
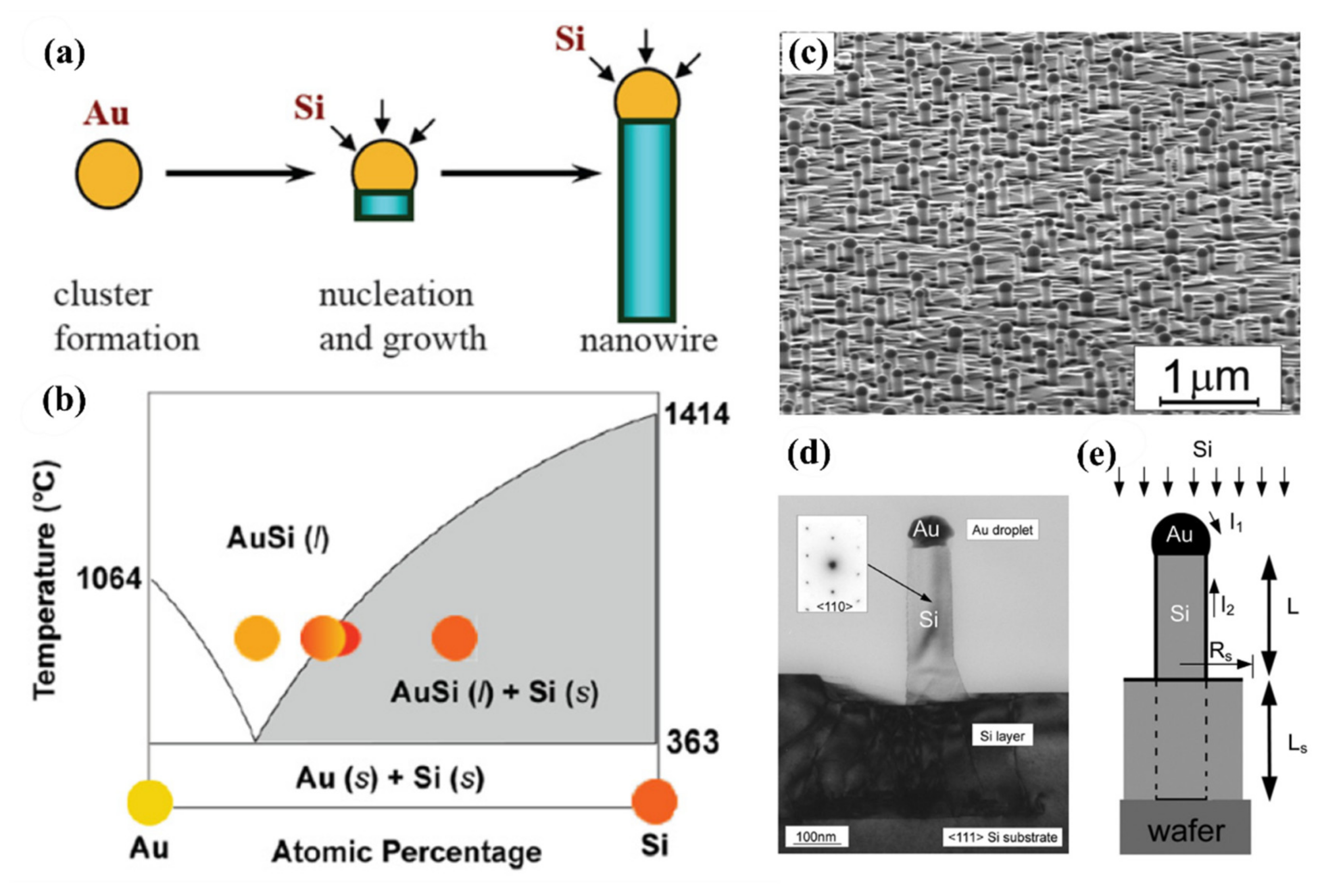
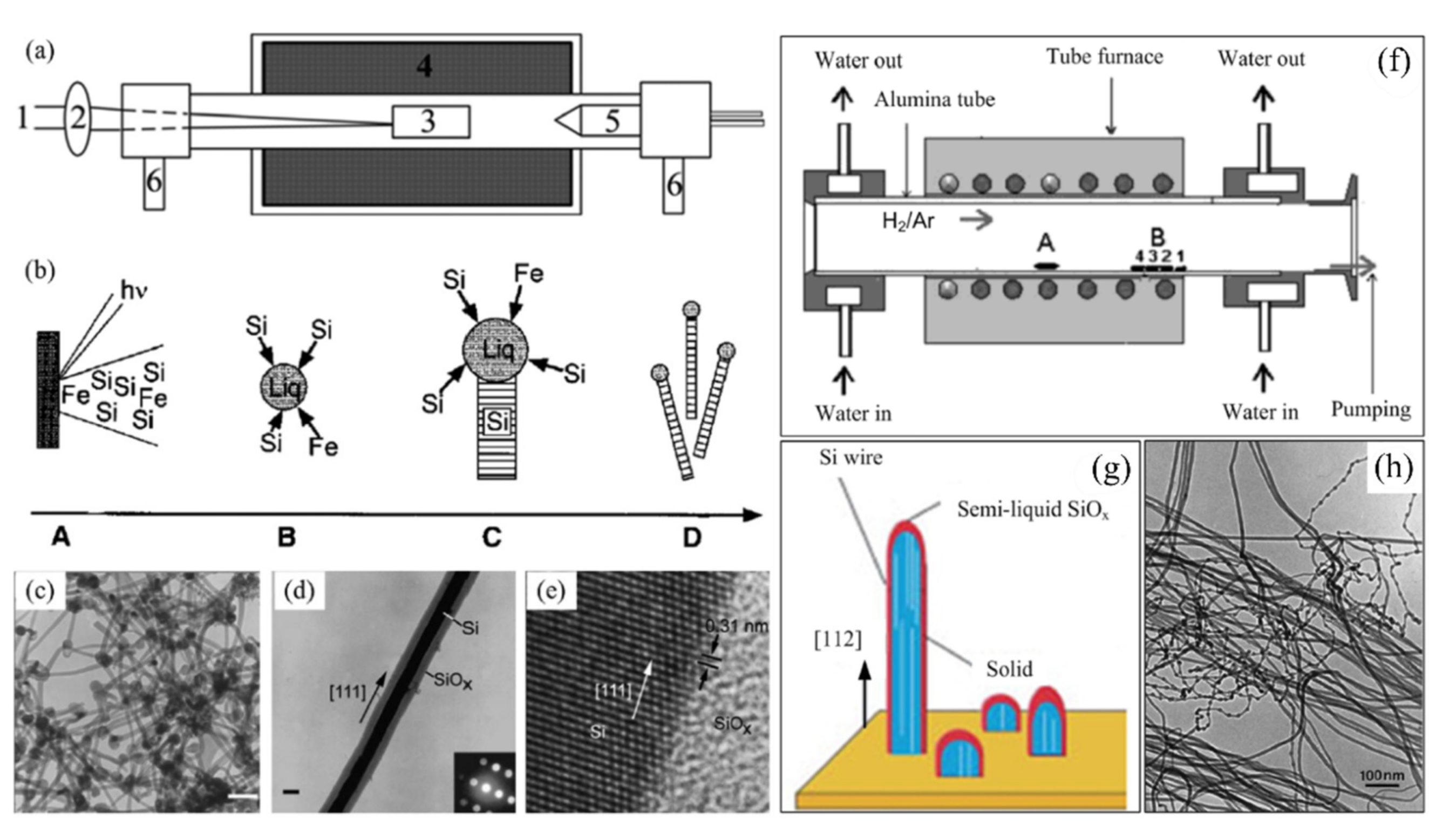
2.2. Bottom-Up Fabrication Methods
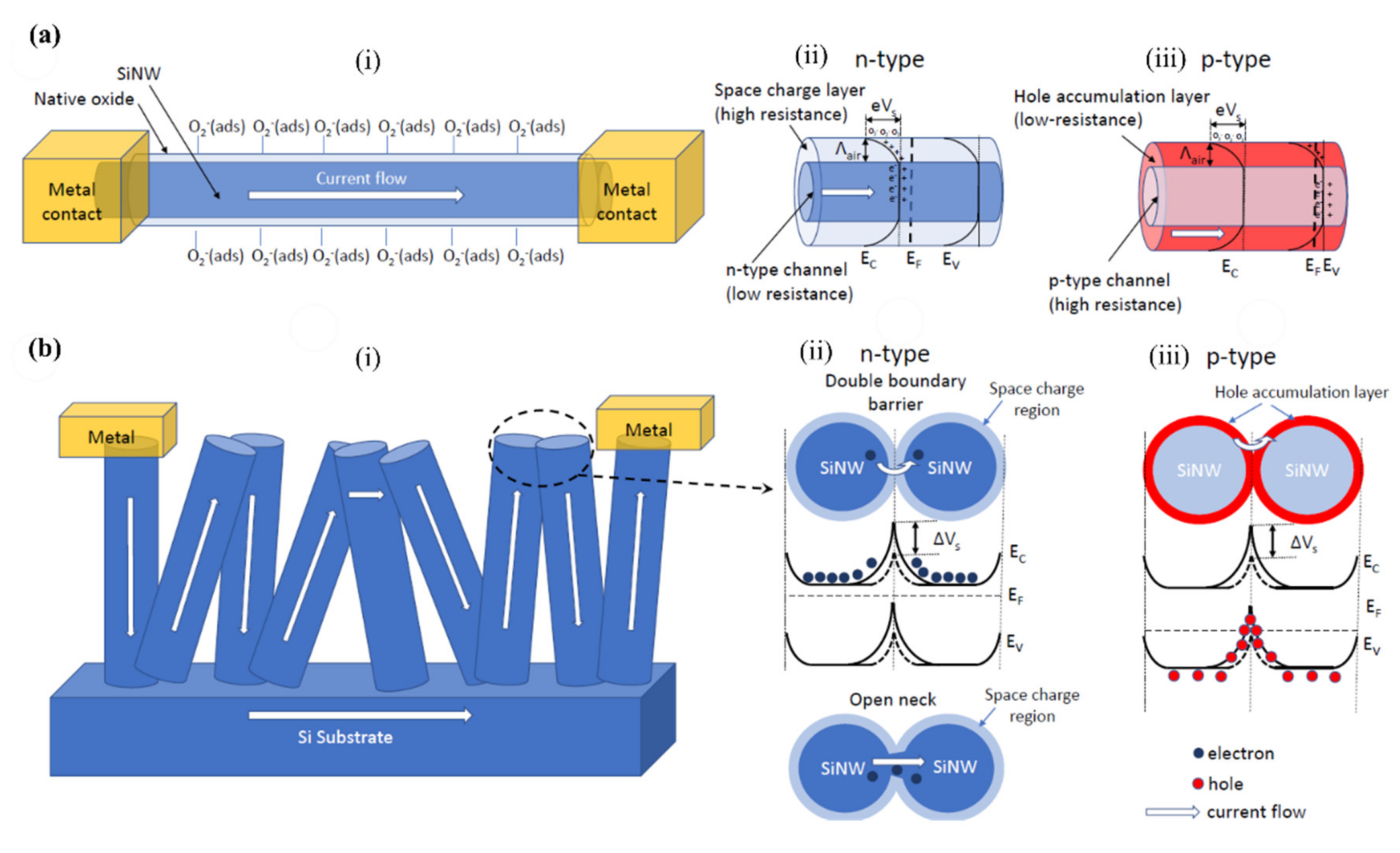
3. SiNWs Gas Sensing Mechanism
O2(ads) + e− → O2−(ads)
O2−(ads) + e− → 2O−(ads)
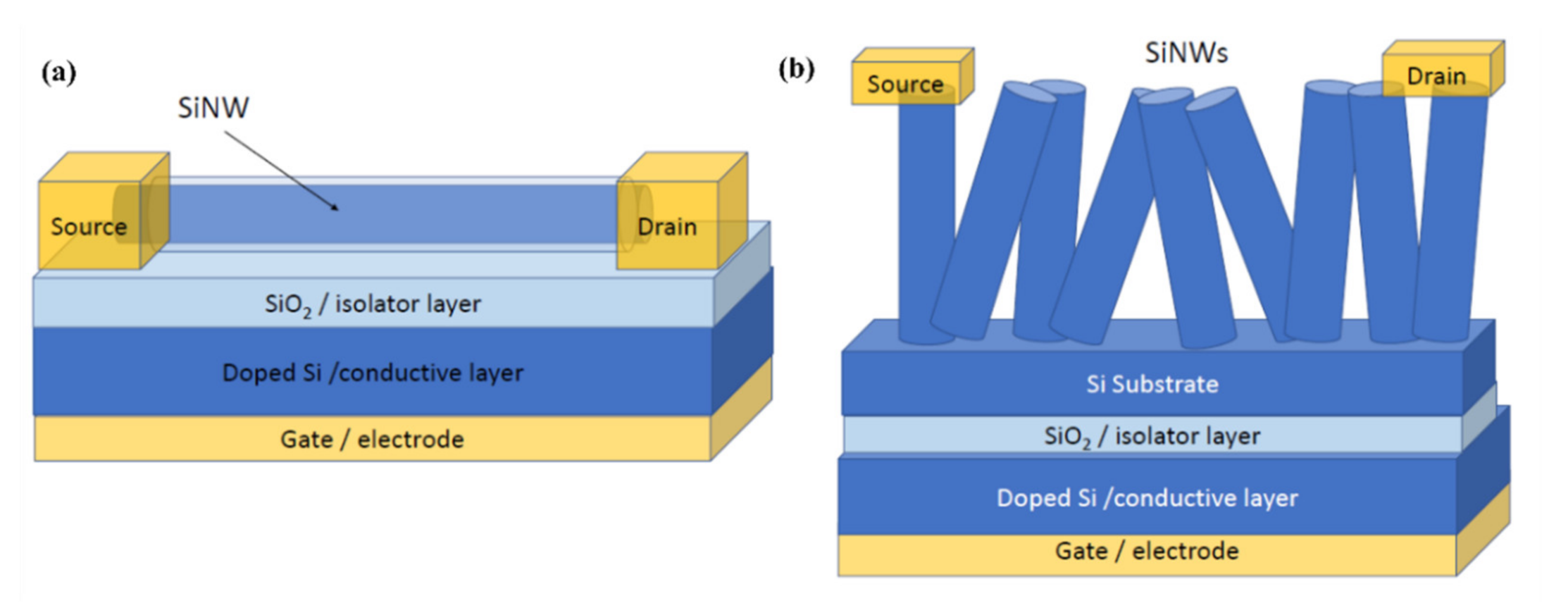
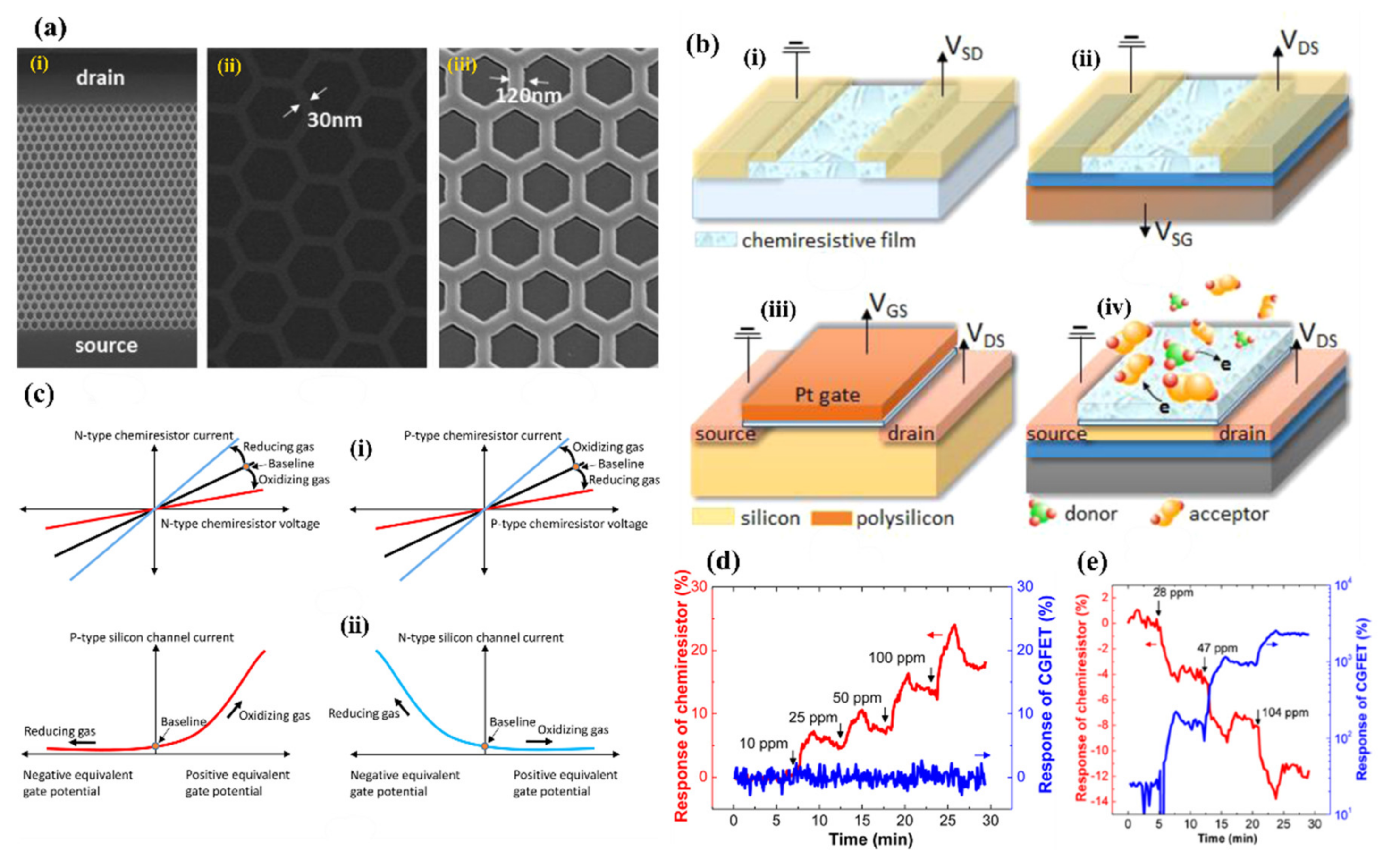
4. Resistors and Field Effect Transistors for Gas Sensing
5. Impact of Functionalization on SiNWs Gas Sensing
5.1. Morphology and Size Effect
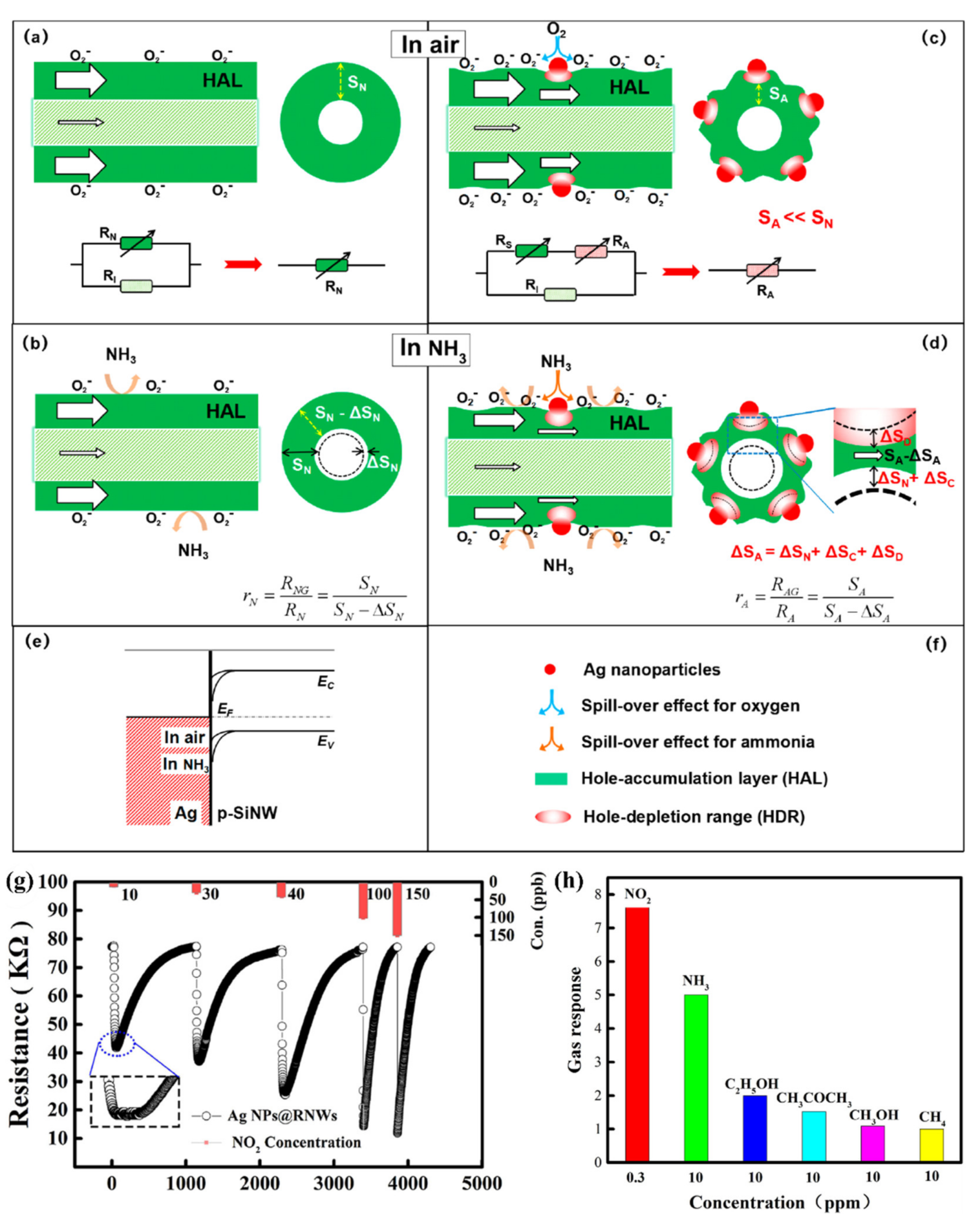
5.2. Decoration by Metal Nanoparticles
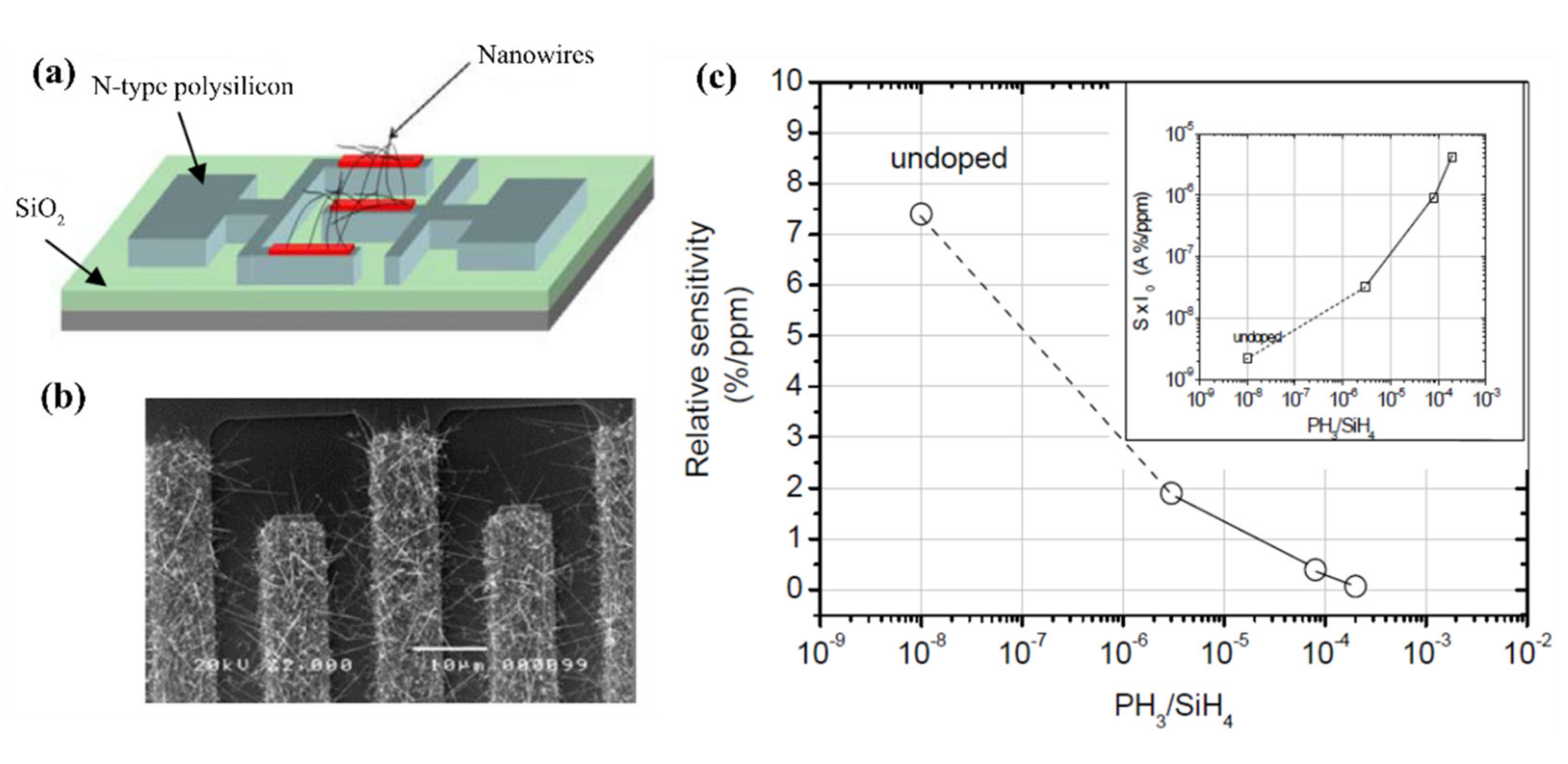
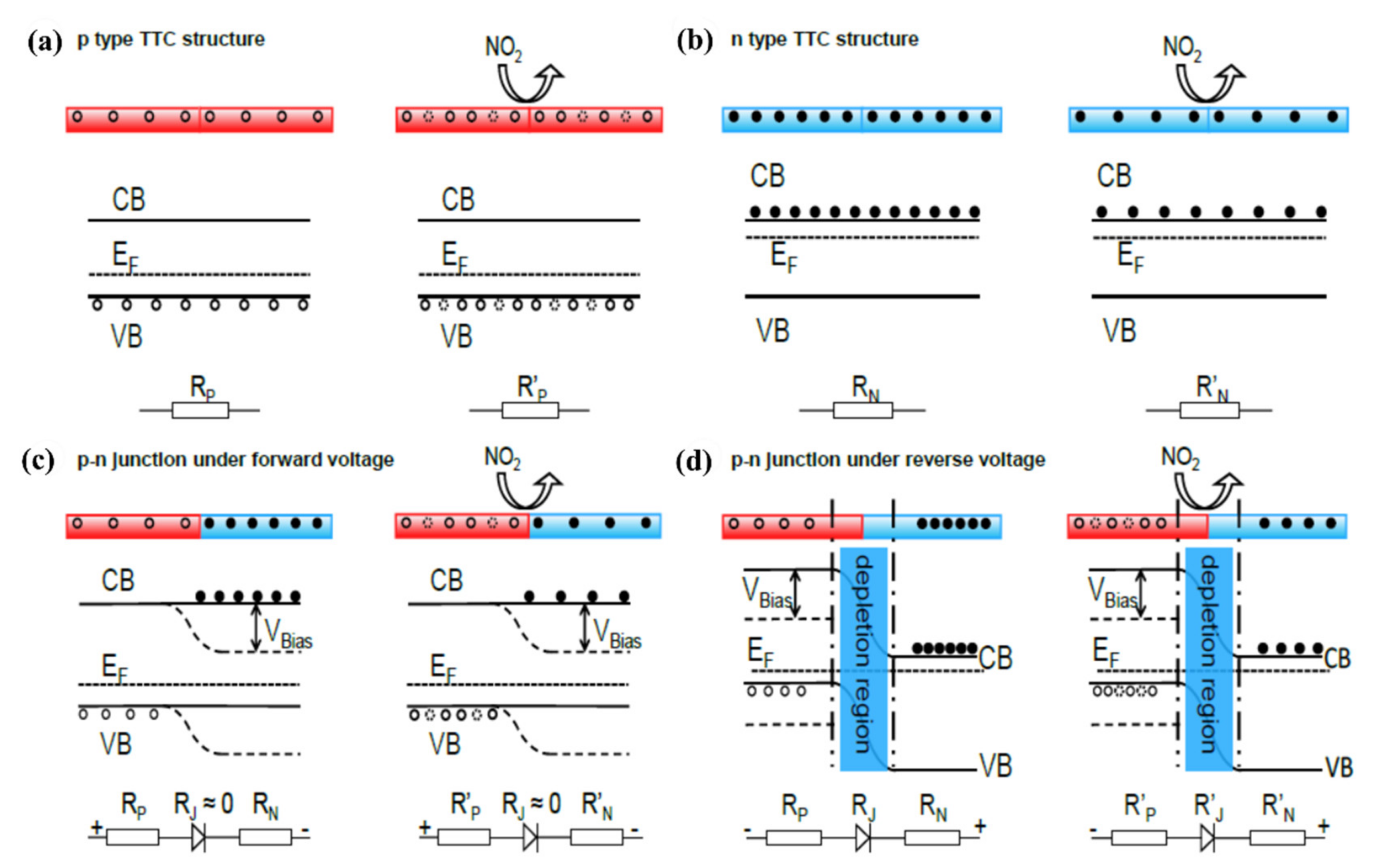
5.3. Doped Junctions
5.3.1. Homojunctions
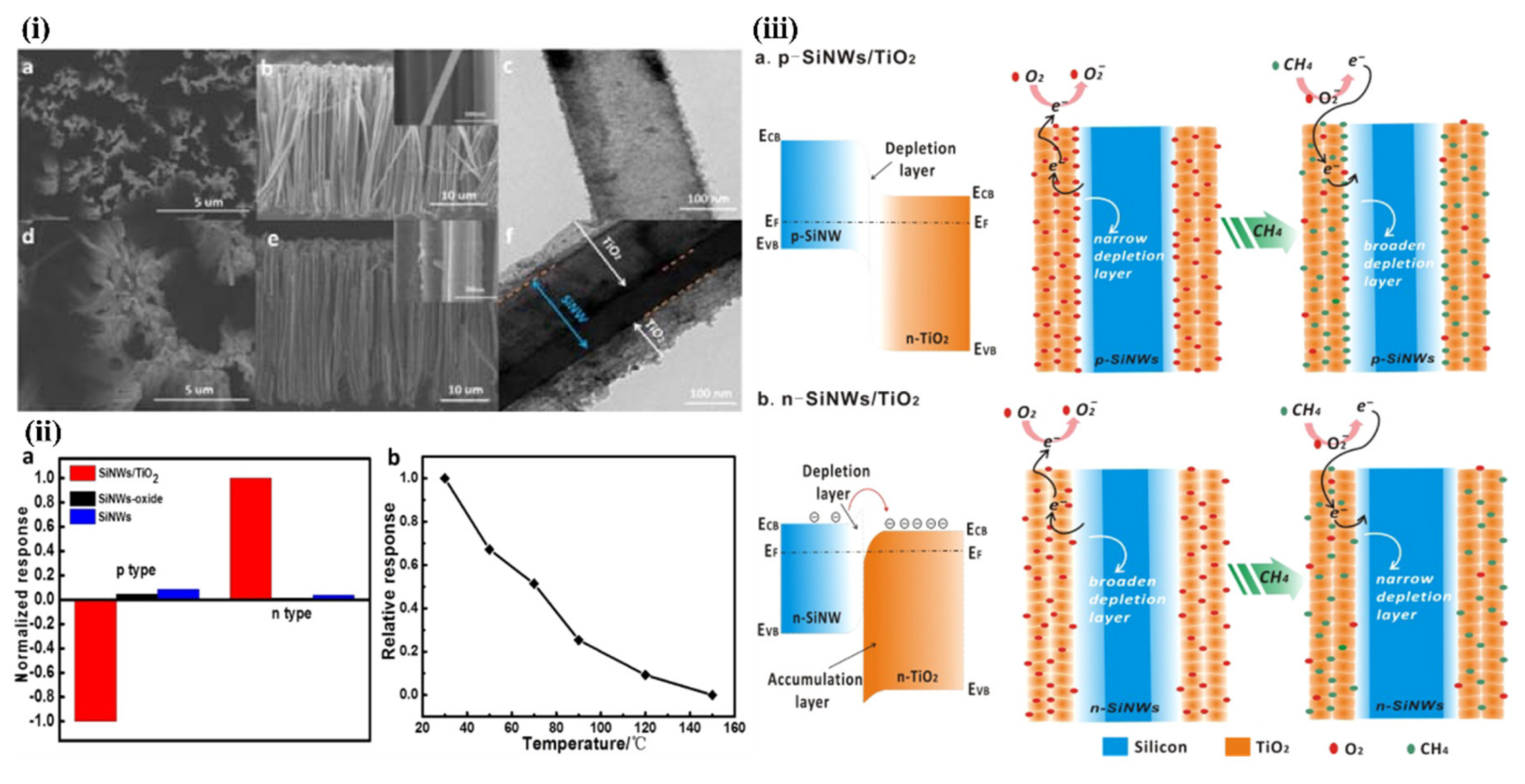
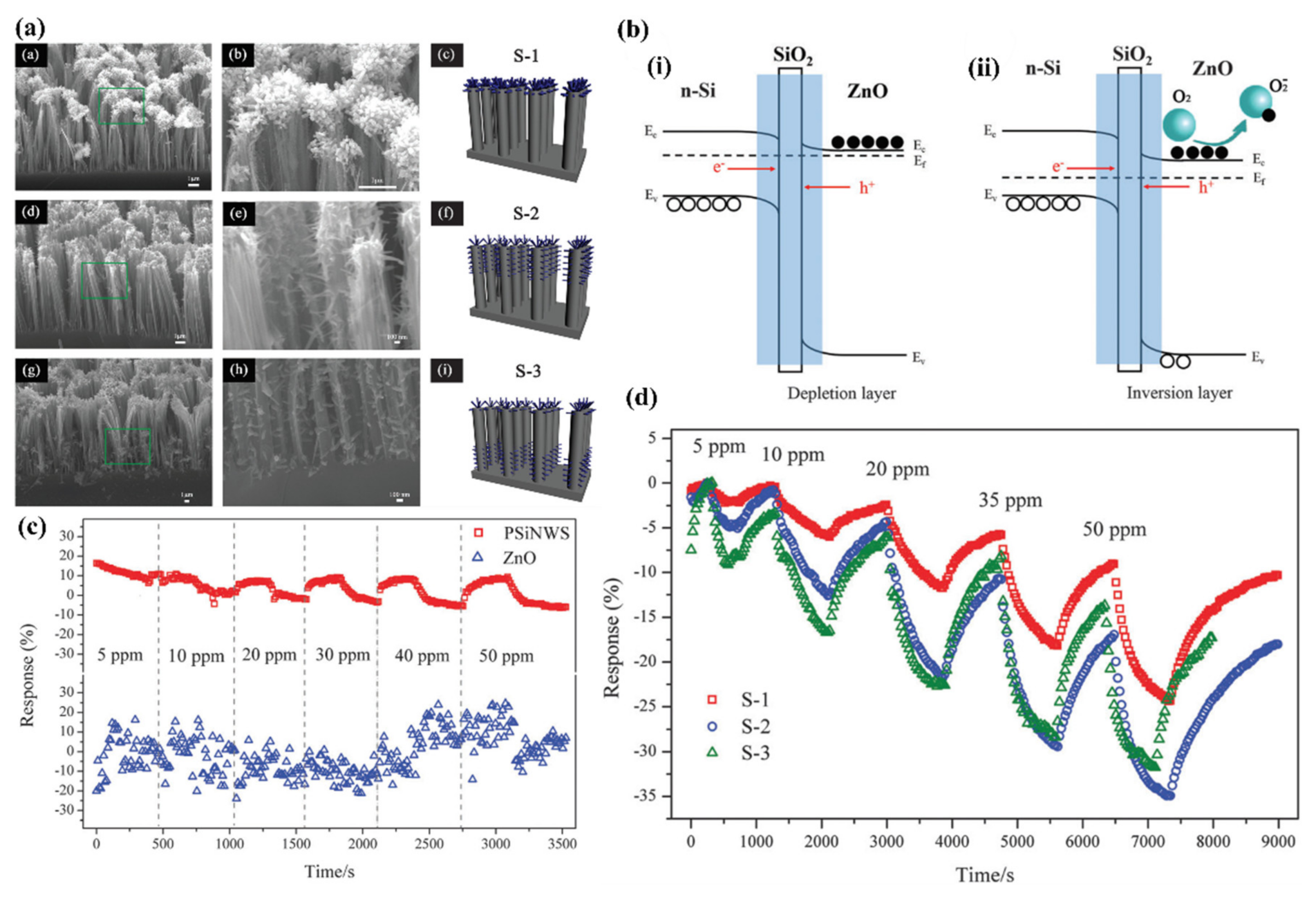
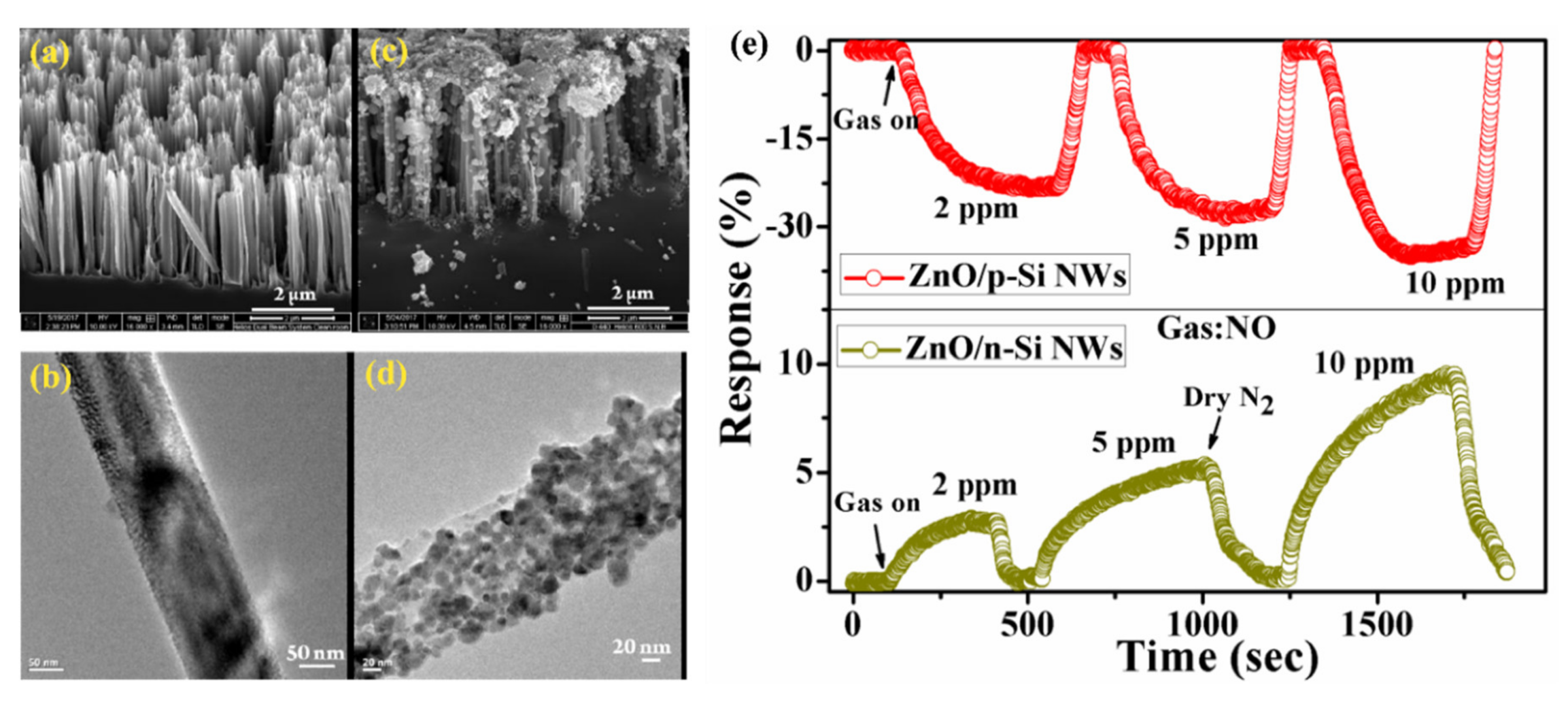
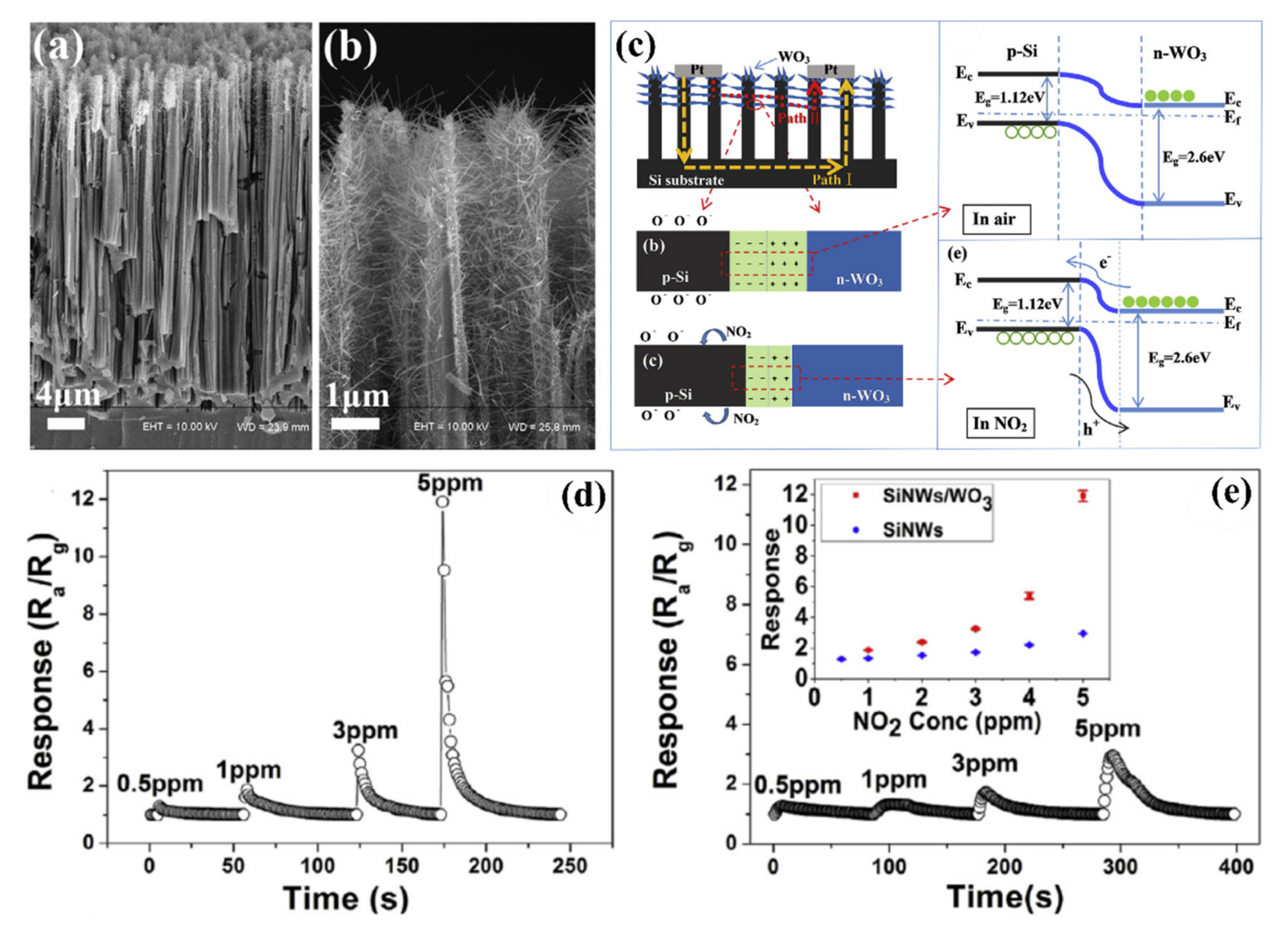
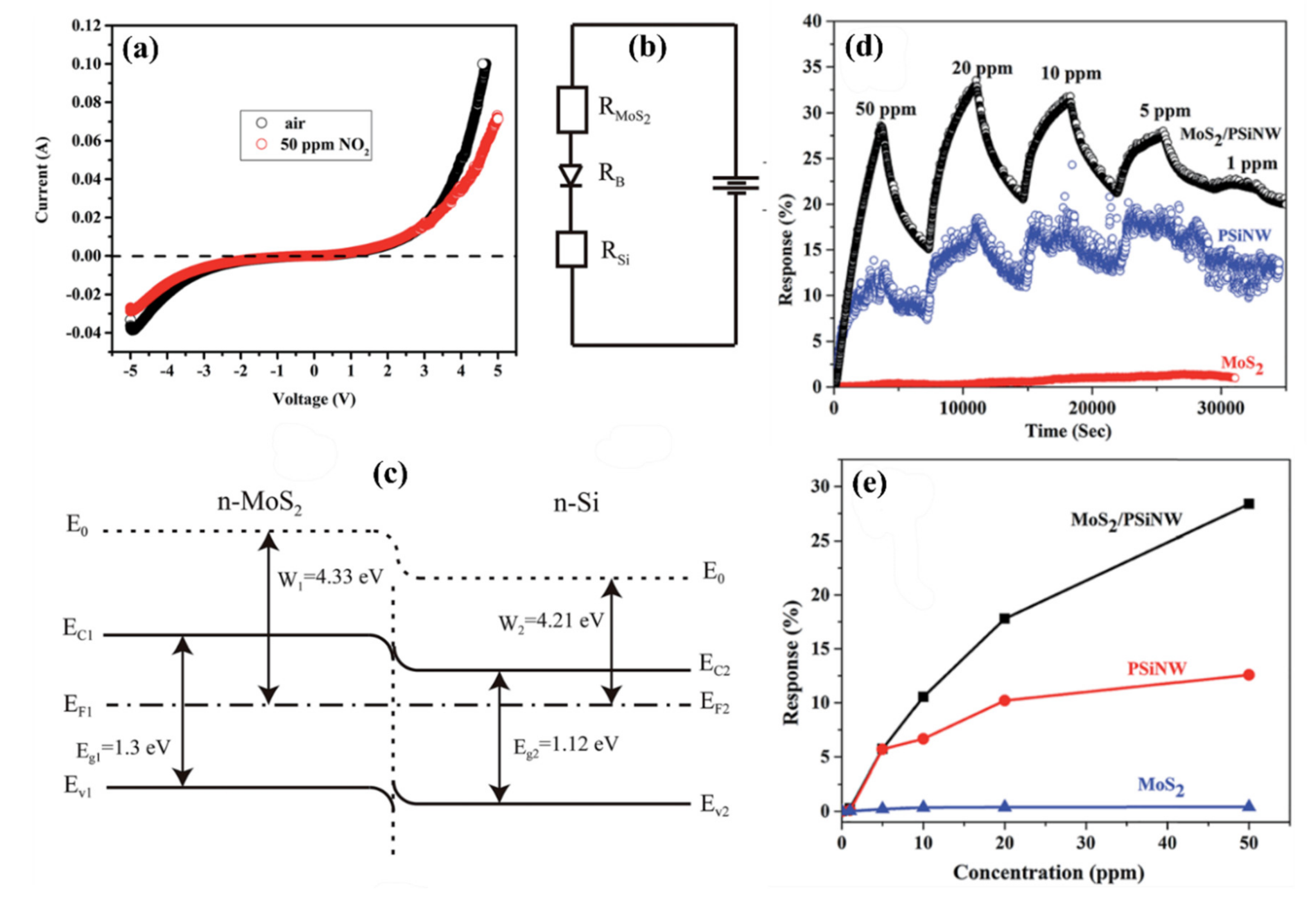
5.3.2. Heterojunctions with Inorganic Semiconductors
5.3.3. Heterojunctions with Organic Semiconductors
5.4. Carbon Materials
5.5. Chemical Surface Modification
5.6. Integration of Multiple Functionalization Methods
6. Conclusions
7. Future Prospective
Author Contributions
Funding
Conflicts of Interest
References
- Tyagi, D.; Wang, H.; Huang, W.; Hu, L.; Tang, Y.; Guo, Z.; Ouyang, Z.; Zhang, H. Recent advances in two-dimensional-material-based sensing technology toward health and environmental monitoring applications. Nanoscale 2020, 12, 3535–3559. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.; Pimentel, A.; Gonçalves, A.; Pereira, S.; Branquinho, R.; Barquinha, P.; Fortunato, E.M.R. Metal oxide nanostructures for sensor applications IOPscience. Semicond. Sci. Technol. 2019, 34, 043001. [Google Scholar] [CrossRef] [Green Version]
- Jian, Y.; Hu, W.; Zhao, Z.; Cheng, P.; Haick, H.; Yao, M.; Wu, W. Gas Sensors Based on Chemi-Resistive Hybrid Functional Nanomaterials Chemi-resistive Hybrids Data process Sensors Hybrids with regulated charge transport. Nano-Micro Lett. 2020, 12, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Hanh, N.H.; Van Duy, L.; Hung, C.M.; Van Duy, N.; Heo, Y.W.; Van Hieu, N.; Hoa, N.D. VOC gas sensor based on hollow cubic assembled nanocrystal Zn2SnO4 for breath analysis. Sens. Actuators A Phys. 2020, 302. [Google Scholar] [CrossRef]
- Mirzaei, A.; Lee, J.H.; Majhi, S.M.; Weber, M.; Bechelany, M.; Kim, H.W.; Kim, S.S. Resistive gas sensors based on metal-oxide nanowires. J. Appl. Phys. 2019, 126, 241102. [Google Scholar] [CrossRef] [Green Version]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- Dai, J.; Ogbeide, O.; Macadam, N.; Sun, Q.; Yu, W.; Li, Y.; Su, B.L.; Hasan, T.; Huang, X.; Huang, W. Printed gas sensors. Chem. Soc. Rev. 2020, 49, 1756–1789. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced sensing performance of ZnO nanostructures-based gas sensors: A review. Energy Rep. 2020, 6, 46–62. [Google Scholar] [CrossRef]
- Sui, L.; Yu, T.; Zhao, D.; Cheng, X.; Zhang, X.; Wang, P.; Xu, Y.; Gao, S.; Zhao, H.; Gao, Y.; et al. In situ deposited hierarchical CuO/NiO nanowall arrays film sensor with enhanced gas sensing performance to H2S. J. Hazard. Mater. 2020, 385, 121570. [Google Scholar] [CrossRef]
- Phuoc, P.H.; Hung, C.M.; Van Toan, N.; Van Duy, N.; Hoa, N.D.; Van Hieu, N. One-step fabrication of SnO2 porous nanofiber gas sensors for sub-ppm H2S detection. Sens. Actuators A Phys. 2020, 303, 111722. [Google Scholar] [CrossRef]
- Gas Sensors Market Size, Share, System and Industry Analysis and Market Forecast to 2024 | MarketsandMarketsTM. Available online: https://www.marketsandmarkets.com/Market-Reports/gas-sensor-market-245141093.html?gclid=CjwKCAiA-f78BRBbEiwATKRRBPxF9TvO4zHQf86ymMDNY3E8JARMkv4zQ6ZfD_VYUgjmH9S8rEOV5xoCo78QAvD_BwE (accessed on 2 November 2020).
- Gas Sensors Market Segment, Size, Share, Global Trends, 2025 | MRFR. Available online: https://www.marketresearchfuture.com/reports/gas-sensors-market-5459 (accessed on 2 November 2020).
- Hunter, G.W.; Akbar, S.; Bhansali, S.; Daniele, M.; Erb, P.D.; Johnson, K.; Liu, C.-C.; Miller, D.; Oralkan, O.; Hesketh, P.J.; et al. Editors’ Choice—Critical Review—A Critical Review of Solid State Gas Sensors. J. Electrochem. Soc. 2020, 167, 037570. [Google Scholar] [CrossRef]
- Lou, C.; Hou, K.; Zhu, W.; Wang, X.; Yang, X.; Dong, R.; Chen, H.; Guo, L.; Liu, X. Human Respiratory Monitoring Based on Schottky Resistance Humidity Sensors. Materials 2020, 13, 430. [Google Scholar] [CrossRef] [Green Version]
- Feng, P.; Shao, F.; Shi, Y.; Wan, Q. Gas Sensors Based on Semiconducting Nanowire Field-Effect Transistors. Sensors 2014, 14, 17406–17429. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, R.; Giri, P.K. Silicon nanowire heterostructures for advanced energy and environmental applications: A review IOPscience. Nanotechnology 2016, 28, 012001. [Google Scholar] [CrossRef]
- Weber, W.M.; Mikolajick, T. Silicon and germanium nanowire electronics: Physics of conventional and unconventional transistors. Rep. Prog. Phys. 2017, 80, 066502. [Google Scholar] [CrossRef]
- Oosthuizen, D.N.; Motaung, D.E.; Swart, H.C. Gas sensors based on CeO2 nanoparticles prepared by chemical precipitation method and their temperature-dependent selectivity towards H2S and NO2 gases. Appl. Surf. Sci. 2020, 505, 144356. [Google Scholar] [CrossRef]
- Lee, J.H.; Mirzaei, A.; Kim, J.Y.; Kim, J.H.; Kim, H.W.; Kim, S.S. Optimization of the surface coverage of metal nanoparticles on nanowires gas sensors to achieve the optimal sensing performance. Sens. Actuators B Chem. 2020, 302, 127196. [Google Scholar] [CrossRef]
- Sacco, L.; Forel, S.; Florea, I.; Cojocaru, C.S. Ultra-sensitive NO2 gas sensors based on single-wall carbon nanotube field effect transistors: Monitoring from ppm to ppb level. Carbon 2020, 157, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Park, K.R.; Cho, H.B.; Lee, J.; Song, Y.; Kim, W.B.; Choa, Y.H. Design of highly porous SnO2-CuO nanotubes for enhancing H2S gas sensor performance. Sens. Actuators B Chem. 2020, 302, 127179. [Google Scholar] [CrossRef]
- Tonezzer, M. Selective gas sensor based on one single SnO2 nanowire. Sens. Actuators B Chem. 2019, 288, 53–59. [Google Scholar] [CrossRef]
- Hossein-Babaei, F.; Akbari-Saatlu, M. Growth of ZnO nanorods on the surface and edges of a multilayer graphene sheet. Scr. Mater. 2017, 139, 77–82. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, L.X.; Li, M.W.; Yin, Y.Y.; Yin, J.; Zhu, M.Y.; Chen, J.J.; Wang, Y.; Bie, L.J. Ultrathin SnO2 nanosheets with dominant high-energy {001} facets for low temperature formaldehyde gas sensor. Sens. Actuators B Chem. 2019, 289, 186–194. [Google Scholar] [CrossRef]
- Nakate, U.T.; Ahmad, R.; Patil, P.; Yu, Y.T.; Hahn, Y.B. Ultra thin NiO nanosheets for high performance hydrogen gas sensor device. Appl. Surf. Sci. 2020, 506, 144971. [Google Scholar] [CrossRef]
- Zaïbi, F.; Slama, I.; Okolie, C.; Deshmukh, J.; Hawco, L.; Mastouri, M.; Bennett, C.; Mkandawire, M.; Chtourou, R. Electro-performance of functionalized silicon nanowires by conductive polymer-coated with gold nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124450. [Google Scholar] [CrossRef]
- Shehada, N.; Cancilla, J.C.; Torrecilla, J.S.; Pariente, E.S.; Brönstrup, G.; Christiansen, S.; Johnson, D.W.; Leja, M.; Davies, M.P.A.; Liran, O.; et al. Silicon Nanowire Sensors Enable Diagnosis of Patients via Exhaled Breath. ACS Nano 2016, 10, 7047–7057. [Google Scholar] [CrossRef] [Green Version]
- Baraban, L.; Ibarlucea, B.; Baek, E.; Cuniberti, G. Hybrid Silicon Nanowire Devices and Their Functional Diversity. Adv. Sci. 2019, 6, 1900522. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef] [Green Version]
- Hossein-Babaei, F.; Akbari-Saatlu, M. Growing continuous zinc oxide layers with reproducible nanostructures on the seeded alumina substrates using spray pyrolysis. Ceram. Int. 2020, 46, 8567–8574. [Google Scholar] [CrossRef]
- Masoumi, S.; Shokrani, M.; Aghili, S.; Hossein-Babaei, F. Zinc oxide-based direct thermoelectric gas sensor for the detection of volatile organic compounds in air. Sens. Actuators B Chem. 2019, 294, 245–252. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Rao, M.V.; Li, Q. Recent advances in electrochemical sensors for detecting toxic gases: NO2, SO2 and H2S. Sensors 2019, 19, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, A.; Kim, S.S.; Kim, H.W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances. J. Hazard. Mater. 2018, 357, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Babaei, F.; Amini, A. Recognition of complex odors with a single generic tin oxide gas sensor. Sens. Actuators B Chem. 2014, 194, 156–163. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Zhou, P.; Hao, F.; Xu, X.; Gao, S.; Wei, D.; Ao, Y.; Shen, Y. Enhanced NO2 sensing performance of ZnO nanowires functionalized with ultra-fine In2O3 nanoparticles. Sens. Actuators B Chem. 2020, 308, 127729. [Google Scholar] [CrossRef]
- Hossein-Babaei, F.; Ghalamboran, M.; Yousefiazari, E. Electrophoretic deposition of ZnO on highly oriented pyrolytic graphite substrates. Mater. Lett. 2017, 209, 404–407. [Google Scholar] [CrossRef]
- Hieu, N.M.; Van Lam, D.; Hien, T.T.; Chinh, N.D.; Quang, N.D.; Hung, N.M.; Van Phuoc, C.; Lee, S.M.; Jeong, J.R.; Kim, C.; et al. ZnTe-coated ZnO nanorods: Hydrogen sulfide nano-sensor purely controlled by pn junction. Mater. Des. 2020, 191, 108628. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, J.; Liu, Z.; Li, X.; Geng, Y.; Tian, X.; Du, Y.; Qian, Z. Enhanced acetone-sensing properties to ppb detection level using Au/Pd-doped ZnO nanorod. Sens. Actuators B Chem. 2020, 310, 127129. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 80 2001, 293, 1289–1292. [Google Scholar] [CrossRef]
- Cui, Y.; Lieber, C.M. Functional nanoscale electronic devices assembled using silicon nanowire building blocks. Science 80 2001, 291, 851–853. [Google Scholar] [CrossRef] [Green Version]
- Cao, A.; Sudhölter, E.; de Smet, L. Silicon Nanowire-Based Devices for Gas-Phase Sensing. Sensors 2014, 14, 245–271. [Google Scholar] [CrossRef]
- Chen, X.; Wong, C.K.Y.; Yuan, C.A.; Zhang, G. Nanowire-based gas sensors. Sens. Actuators B Chem. 2013, 177, 178–195. [Google Scholar] [CrossRef]
- Hobbs, R.G.; Petkov, N.; Holmes, J.D. Semiconductor nanowire fabrication by bottom-up and top-down paradigms. Chem. Mater. 2012, 24, 1975–1991. [Google Scholar] [CrossRef] [Green Version]
- Jia, C.; Lin, Z.; Huang, Y.; Duan, X. Nanowire Electronics: From Nanoscale to Macroscale. Chem. Rev. 2019, 119, 9074–9135. [Google Scholar] [CrossRef]
- Boukai, A.I.; Bunimovich, Y.; Tahir-Kheli, J.; Yu, J.K.; Goddard, W.A.; Heath, J.R. Silicon nanowires as efficient thermoelectric materials. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; World Scientific Publishing Co.: Singapore, 2010; pp. 116–119. ISBN 9789814317665. [Google Scholar]
- Stern, E.; Klemic, J.F.; Routenberg, D.A.; Wyrembak, P.N.; Turner-Evans, D.B.; Hamilton, A.D.; LaVan, D.A.; Fahmy, T.M.; Reed, M.A. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature 2007, 445, 519–522. [Google Scholar] [CrossRef]
- Noroozi, M.; Jayakumar, G.; Zahmatkesh, K.; Lu, J.; Hultman, L.; Mensi, M.; Marcinkevicius, S.; Hamawandi, B.; Tafti, M.Y.; Ergül, A.B.; et al. Unprecedented Thermoelectric Power Factor in SiGe Nanowires Field-Effect Transistors. ECS J. Solid State Sci. Technol. 2017, 6, Q114–Q119. [Google Scholar] [CrossRef]
- Radamson, H.; Zhang, Y.; He, X.; Cui, H.; Li, J.; Xiang, J.; Liu, J.; Gu, S.; Wang, G. The Challenges of Advanced CMOS Process from 2D to 3D. Appl. Sci. 2017, 7, 1047. [Google Scholar] [CrossRef]
- Harriott, L.R. Limits of lithography. Proc. IEEE 2001, 89, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Fu, N.; Liu, Y.; Ma, X.; Chen, Z. EUV Lithography: State-of-the-Art Review. J. Microelectron. Manuf. 2019, 2, 19020202. [Google Scholar] [CrossRef]
- Seisyan, R.P. Nanolithography in microelectronics: A review. Tech. Phys. 2011, 56, 1061–1073. [Google Scholar] [CrossRef]
- Trivedi, K.; Yuk, H.; Floresca, H.C.; Kim, M.J.; Hu, W. Quantum confinement induced performance enhancement in sub-5-nm lithographic Si nanowire transistors. Nano Lett. 2011, 11, 1412–1417. [Google Scholar] [CrossRef]
- Hashemi, P.; Gomez, L.; Hoyt, J.L. Gate-all-around n-MOSFETs with uniaxial tensile strain-induced performance enhancement scalable to sub-10-nm nanowire diameter. IEEE Electron Device Lett. 2009, 30, 401–403. [Google Scholar] [CrossRef]
- Ahn, J.H.; Choi, S.J.; Han, J.W.; Park, T.J.; Lee, S.Y.; Choi, Y.K. Double-gate nanowire field effect transistor for a biosensor. Nano Lett. 2010, 10, 2934–2938. [Google Scholar] [CrossRef]
- Hållstedt, J.; Hellström, P.E.; Radamson, H.H. Sidewall transfer lithography for reliable fabrication of nanowires and deca-nanometer MOSFETs. Thin Solid Film. 2008, 517, 117–120. [Google Scholar] [CrossRef]
- Radamson, H.; Thylén, L. Monolithic Nanoscale Photonics-Electronics Integration in Silicon and Other Group IV Elements; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; ISBN 9780124199965. [Google Scholar]
- Altamirano-Sánchez, E.; Paraschiv, V.; Demand, M.; Boullart, W. Dry etching fin process for SOI finFET manufacturing: Transition from 32 to 22 nm node on a 6T-SRAM cell. Microelectron. Eng. 2011, 88, 2871–2878. [Google Scholar] [CrossRef]
- French, R.H.; Tran, H.V. Immersion Lithography: Photomask and Wafer-Level Materials. Annu. Rev. Mater. Res. 2009, 39, 93–126. [Google Scholar] [CrossRef]
- Pirati, A.; Peeters, R.; Smith, D.; Lok, S.; van Noordenburg, M.; van Es, R.; Verhoeven, E.; Meijer, H.; Minnaert, A.; van der Horst, J.-W.; et al. EUV lithography performance for manufacturing: Status and outlook. In Proceedings of the Extreme Ultraviolet (EUV) Lithography VII, San Jose, CA, USA, 21–25 February 2016; Panning, E.M., Goldberg, K.A., Eds.; SPIE: Bellingham, WA, USA, 2016; Volume 9776, p. 97760A. [Google Scholar]
- Pirati, A.; Peeters, R.; Smith, D.; Lok, S.; Minnaert, A.; van Noordenburg, M.; Mallmann, J.; Harned, N.; Stoeldraijer, J.; Wagner, C.; et al. Performance overview and outlook of EUV lithography systems. In Proceedings of the Extreme Ultraviolet (EUV) Lithography VI, San Jose, CA, USA, 23–26 February 2015; Wood, O.R., Panning, E.M., Eds.; SPIE: Bellingham, WA, USA, 2015; Volume 9422, p. 94221P. [Google Scholar]
- Yoda, Y.; Hayakawa, A.; Ishiyama, S.; Ohmura, Y.; Fujimoto, I.; Hirayama, T.; Shiba, Y.; Masaki, K.; Shibazaki, Y. Next-generation immersion scanner optimizing on-product performance for 7 nm node. In Proceedings of the Optical Microlithography XXIX; Erdmann, A., Kye, J., Eds.; SPIE: Bellingham, WA, USA, 2016; Volume 9780, p. 978012. [Google Scholar]
- De Bisschop, P. Stochastic effects in EUV lithography: Random, local CD variability, and printing failures. J. Micro Nanolithography MEMS MOEMS 2017, 16, 1. [Google Scholar] [CrossRef]
- De Bisschop, P.; Van de Kerkhove, J.; Mailfert, J.; Vaglio Pret, A.; Biafore, J. Impact of stochastic effects on EUV printability limits. In Proceedings of the Extreme Ultraviolet (EUV) Lithography, V, San Jose, CA, USA, 24–27 February 2014; Wood, O.R., Panning, E.M., Eds.; SPIE: Bellingham, WA, USA, 2014; Volume 9048, p. 904809. [Google Scholar]
- Kim, S.K. Modeling and simulation of line edge roughness for EUV resists. J. Semicond. Technol. Sci. 2014, 14, 61–69. [Google Scholar] [CrossRef]
- Mojarad, N.; Gobrecht, J.; Ekinci, Y. Beyond EUV lithography: A comparative study of efficient photoresists’ performance. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Popescu, C.; Frommhold, A.; McClelland, A.; Roth, J.; Ekinci, Y.; Robinson, A.P.G. Sensitivity enhancement of the high-resolution xMT multi-trigger resist for EUV lithography. In Proceedings of the Extreme Ultraviolet (EUV) Lithography VIII, San Jose, CA, USA, 26 February–2 March 2017; SPIE: Bellingham, WA, USA, 2017; Volume 10143, p. 101430V. [Google Scholar]
- Radamson, H.H.; He, X.; Zhang, Q.; Liu, J.; Cui, H.; Xiang, J.; Kong, Z.; Xiong, W.; Li, J.; Gao, J.; et al. Miniaturization of CMOS. Micromachines 2019, 10, 293. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Syms, R.R.A. NEMS by sidewall transfer lithography. J. Microelectromech. Syst. 2014, 23, 1366–1373. [Google Scholar] [CrossRef]
- Radamson, H.H.; Zhu, H.; Wu, Z.; He, X.; Lin, H.; Liu, J.; Xiang, J.; Kong, Z.; Xiong, W.; Li, J.; et al. State of the Art and Future Perspectives in Advanced CMOS Technology. Nanomaterials 2020, 10, 1555. [Google Scholar] [CrossRef]
- Zhang, Q.; Tu, H.; Yin, H.; Wei, F.; Zhao, H.; Xue, C.; Wei, Q.; Zhang, Z.; Zhang, X.; Zhang, S.; et al. Si Nanowire Biosensors Using a FinFET Fabrication Process for Real Time Monitoring Cellular Ion Actitivies. In Proceedings of the Technical Digest International Electron Devices Meeting (IEDM, Institute of Electrical and Electronics Engineers Inc.), San Francisco, CA, USA, 1–5 December 2018; pp. 29.6.1–29.6.4. [Google Scholar]
- Kanemitsu, Y.; Ogawa, T.; Shiraishi, K.; Takeda, K. Visible photoluminescence from oxidized Si nanometer-sized spheres: Exciton confinement on a spherical shell. Phys. Rev. B 1993, 48, 4883–4886. [Google Scholar] [CrossRef]
- Lehmann, V.; Gösele, U. Porous silicon formation: A quantum wire effect. Appl. Phys. Lett. 1991, 58, 856–858. [Google Scholar] [CrossRef]
- Megouda, N.; Hadjersi, T.; Szunerits, S.; Boukherroub, R. Electroless chemical etching of silicon in aqueous NH 4 F/ AgNO 3 /HNO 3 solution. Appl. Surf. Sci. 2013, 284, 894–899. [Google Scholar] [CrossRef]
- Liu, K.; Qu, S.; Tan, F.; Bi, Y.; Lu, S.; Wang, Z. Ordered silicon nanowires prepared by template-assisted morphological design and metal-assisted chemical etching. Mater. Lett. 2013, 101, 96–98. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Vorobjova, A.I.; Vinnik, D.A. Template assisted ni nanowires fabrication. Mater. Sci. Forum 2019, 946, 235–241. [Google Scholar] [CrossRef]
- Jansen, H.; De Boer, M.; Legtenberg, R.; Elwenspoek, M. The black silicon method: A universal method for determining the parameter setting of a fluorine-based reactive ion etcher in deep silicon trench etching with profile control. J. Micromech. Microeng. 1995, 5, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Hållstedt, J.; Blomqvist, M.; Persson, P.O.Å.; Hultman, L.; Radamson, H.H. The effect of carbon and germanium on phase transformation of nickel on epitaxial layers articles you may be interested in. J. Appl. Phys. 2004, 95, 2397. [Google Scholar] [CrossRef]
- Zhou, Y.; Ogawa, M.; Han, X.; Wang, K.L. Alleviation of Fermi-level pinning effect on metal/germanium interface by insertion of an ultrathin aluminum oxide. Appl. Phys. Lett. 2008, 93, 202105. [Google Scholar] [CrossRef]
- Sze, S.M.; Ng, K.K. Physics of Semiconductor Devices; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; ISBN 9780470068328. [Google Scholar]
- Chin, Y.K.; Pey, K.L.; Singh, N.; Lo, G.Q.; Tan, L.H.; Zhu, G.; Zhou, X.; Wang, X.C.; Zheng, H.Y. Excimer laser-annealed dopant segregated Schottky (ELA-DSS) Si nanowire gate-all-around (GAA) pFET with near zero effective Schottky barrier height (SBH). In Proceedings of the Technical Digest International Electron Devices Meeting (IEDM), Baltimore, MD, USA, 7–9 December 2009. [Google Scholar]
- Nishi, Y.; Doering, R. Handbook of Semiconductor Manufacturing Technology; Doering, R., Nishi, Y., Eds.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781315213934. [Google Scholar]
- Nava, F.; Tu, K.N.; Thomas, O.; Senateur, J.P.; Madar, R.; Borghesi, A.; Guizzetti, G.; Gottlieb, U.; Laborde, O.; Bisi, O. Electrical and optical properties of silicide single crystals and thin films. Mater. Sci. Rep. 1993, 9, 141–200. [Google Scholar] [CrossRef]
- Wu, Y.; Xiang, J.; Yang, C.; Lu, W.; Lieber, C.M. Single-crystal metallic nanowires and metal/semiconductor nanowire heterostructures. Nature 2004, 430, 61–65. [Google Scholar] [CrossRef]
- Zhang, Z.; Pagette, F.; D’Emic, C.; Yang, B.; Lavoie, C.; Zhu, Y.; Hopstaken, M.; Maurer, S.; Murray, C.; Guillorn, M.; et al. Sharp reduction of contact resistivities by effective schottky barrier lowering with silicides as diffusion sources. IEEE Electron Device Lett. 2010, 31, 731–733. [Google Scholar] [CrossRef]
- Chen, R.; Dayeh, S.A. Metal-Semiconductor Compound Contacts to Nanowire Transistors; Springer: Singapore, 2019; pp. 111–158. [Google Scholar]
- Lin, Y.C.; Chen, Y.; Shailos, A.; Huang, Y. Detection of spin polarized carrier in silicon nanowire with single crystal MnSi as magnetic contacts. Nano Lett. 2010, 10, 2281–2287. [Google Scholar] [CrossRef]
- Chou, Y.C.; Wu, W.W.; Cheng, S.L.; Yoo, B.Y.; Myung, N.; Chen, L.J.; Tut, K.N. In-situ TEM Observation of repeating events of nucleation in epitaxial growth of nano CoSi 2 in nanowires of Si. Nano Lett. 2008, 8, 2194–2199. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C.; Lu, K.C.; Wu, W.W.; Bai, J.; Chen, L.J.; Tu, K.N.; Huang, Y. Single crystalline PtSi nanowires, PtSi/Si/PtSi nanowire heterostructures, and nanodevices. Nano Lett. 2008, 8, 913–918. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Y.C.; Zhong, X.; Cheng, H.C.; Duan, X.; Huang, Y. Kinetic manipulation of silicide phase formation in Si nanowire templates. Nano Lett. 2013, 13, 3703–3708. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Y.C.; Huang, C.W.; Wang, C.W.; Chen, L.J.; Wu, W.W.; Huang, Y. Kinetic competition model and size-dependent phase selection in 1-D nanostructures. Nano Lett. 2012, 12, 3115–3120. [Google Scholar] [CrossRef]
- Reza, S.; Bosman, G.; Islam, M.S.; Kamins, T.I.; Sharma, S.; Williams, R.S. Noise in silicon nanowires. IEEE Trans. Nanotechnol. 2006, 5, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Kamins, T.I.; Islam, M.S.; Williams, R.S.; Marshall, A.F. Structural characteristics and connection mechanism of gold-catalyzed bridging silicon nanowires. J. Cryst. Growth 2005, 280, 562–568. [Google Scholar] [CrossRef]
- Reeves, G.K.; Harrison, H.B. Obtaining the Specific Contact Resistance from Transmission Line Model Measurements. IEEE Electron Device Lett. 1982, 3, 111–113. [Google Scholar] [CrossRef]
- Smith, J.T.; Zhao, Y.; Yang, C.; Appenzeller, J. Effects of nanoscale contacts to silicon nanowires on contact resistance: Characterization and modeling. In Proceedings of the Device Research Conference (DRC)—Conference Digest, South Bend, IN, USA, 21–23 June 2010; pp. 139–140. [Google Scholar]
- Chaudhry, A.; Ramamurthi, V.; Fong, E.; Islam, M.S. Ultra-low contact resistance of epitaxially interfaced bridged silicon nanowires. Nano Lett. 2007, 7, 1536–1541. [Google Scholar] [CrossRef]
- Singh, D.; Fisher, T.S.; Murthy, J.Y. Thermal contact resistance of a silicon nanowire on a substrate. In Proceedings of the 2007 ASME InterPack Conference (IPACK 2007); American Society of Mechanical Engineers Digital Collection: New York, NY, USA, 2007; Volume 2, pp. 1007–1017. [Google Scholar]
- Hu, C.; Xu, P.; Fu, C.; Zhu, Z.; Gao, X.; Jamshidi, A.; Noroozi, M.; Radamson, H.; Wu, D.; Zhang, S.L. Characterization of Ni(Si,Ge) films on epitaxial SiGe(100) formed by microwave annealing. Appl. Phys. Lett. 2012, 101, 092101. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lin, C.Y.; Huang, C.C.; Chien, C.H. The effect of pulsed laser annealing on the nickel silicide formation. Microelectron. Eng. 2010, 87, 2540–2543. [Google Scholar] [CrossRef]
- Lu, W.; Lieber, C.M. Semiconductor nanowires. J. Phys. D. Appl. Phys. 2006, 39, R387. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Huang, Y.; Wang, S.; Zhang, S. Critical review: Growth mechanisms of the self-assembling of silicon wires. J. Vac. Sci. Technol. A 2020, 38, 010802. [Google Scholar] [CrossRef]
- Kayes, B.M.; Filler, M.A.; Putnam, M.C.; Kelzenberg, M.D.; Lewis, N.S.; Atwater, H.A. Growth of vertically aligned Si wire arrays over large areas (>1 cm2) with Au and Cu catalysts. Appl. Phys. Lett. 2007, 91, 103110. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, V.; Senz, S.; Gösele, U. Diameter-dependent growth direction of epitaxial silicon nanowires. Nano Lett. 2005, 5, 931–935. [Google Scholar] [CrossRef]
- Schubert, L.; Werner, P.; Zakharov, N.D.; Gerth, G.; Kolb, F.M.; Long, L.; Gösele, U.; Tan, T.Y. Silicon nanowhiskers grown on <111> Si substrates by molecular-beam epitaxy. Appl. Phys. Lett. 2004, 84, 4968–4970. [Google Scholar] [CrossRef]
- Morales, A.M.; Lieber, C.M. A laser ablation method for the synthesis of crystalline semiconductor nanowires. Science 80 1998, 279, 208–211. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y.F.; Tang, Y.H.; Lee, C.S.; Lee, S.T. SiO2-enhanced synthesis of Si nanowires by laser ablation. Appl. Phys. Lett. 1998, 73, 3902–3904. [Google Scholar] [CrossRef]
- Schmidt, V.; Wittemann, J.V.; Senz, S.; Gósele, U. Silicon nanowires: A review on aspects of their growth and their electrical properties. Adv. Mater. 2009, 21, 2681–2702. [Google Scholar] [CrossRef]
- Pan, H.; Lim, S.; Poh, C.; Sun, H.; Wu, X.; Feng, Y.; Lin, J. Growth of Si nanowires by thermal evaporation. Nanotechnology 2005, 16, 417–421. [Google Scholar] [CrossRef]
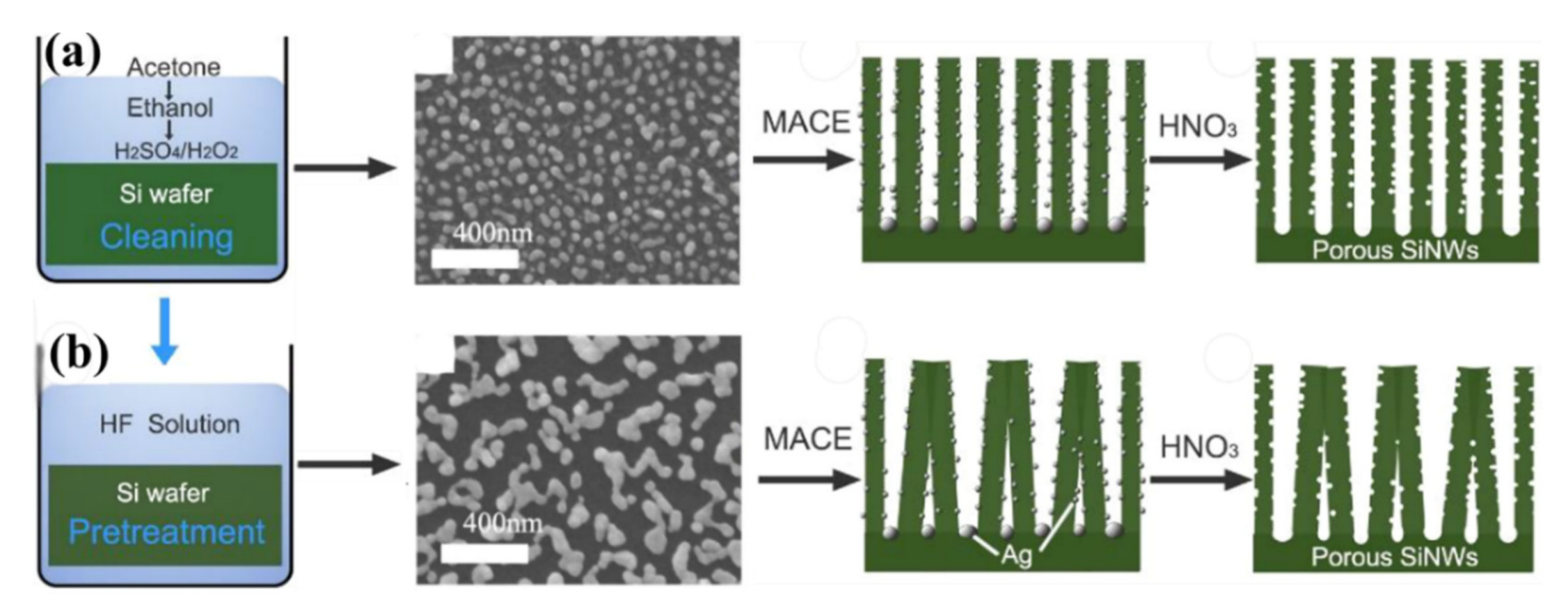
- Kwon, Y.J.; Mirzaei, A.; Na, H.G.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Oum, W.; Kim, S.S.; Kim, H.W. Porous Si nanowires for highly selective room-temperature NO2 gas sensing. Nanotechnology 2018, 29, 294001. [Google Scholar] [CrossRef]
- Qin, Y.; Jiang, Y.; Zhao, L. Modulation of Agglomeration of Vertical Porous Silicon Nanowires and the Effect on Gas-Sensing Response. Adv. Eng. Mater. 2018, 20, 1700893. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kang, S.Y.; Choi, S.W.; Kwon, Y.J.; Choi, M.S.; Bang, J.H.; Kim, S.S.; Kim, H.W. Fabrication and gas sensing properties of vertically aligned Si nanowires. Appl. Surf. Sci. 2018, 427, 215–226. [Google Scholar] [CrossRef]
- Chang, Y.; Qu, H.; Duan, X.; Mu, L.; Reed, M.A. VOC detection using multimode E-nose composed of bulk acoustic wave resonator and silicon nanowire field effect transistor array. In Proceedings of the IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016. [Google Scholar]
- Demami, F.; Ni, L.; Rogel, R.; Salaun, A.C.; Pichon, L. Silicon nanowires based resistors as gas sensors. Sens. Actuators B Chem. 2012, 170, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Luo, L.; Xi, Y.; Song, J.; Wang, Y.; Yang, L.; Wang, A.; Chen, Y.; Han, N.; Wang, F. Reduced Graphene Oxide-Coated Si Nanowires for Highly Sensitive and Selective Detection of Indoor Formaldehyde. Nanoscale Res. Lett. 2019, 14, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Jiang, Y.; Zhao, L. Enhanced humidity resistance of porous SiNWs via OTS functionalization for rarefied NO2 detection. Sens. Actuators B Chem. 2019, 283, 61–68. [Google Scholar] [CrossRef]
- Qin, Y.; Cui, Z.; Wen, Z.; Bai, Y. Highly sensitive NO2 sensors based on core-shell array of silicon nanowires/polypyrrole and new insight into gas sensing mechanism of organic/inorganic hetero-contact. Polym. Compos. 2019, 40, 3275–3284. [Google Scholar] [CrossRef]
- Rajan, N.K.; Routenberg, D.A.; Reed, M.A. Optimal signal-to-noise ratio for silicon nanowire biochemical sensors. Appl. Phys. Lett. 2011, 98, 264107. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.P.A.; Zheng, G.; Lieber, C.M. Subthreshold regime has the optimal sensitivity for nanowire FET biosensors. Nano Lett. 2010, 10, 547–552. [Google Scholar] [CrossRef] [Green Version]
- Hakim, M.; Tanzeem, S.; Sun, K.; Ashburn, P. Low Cost Mass Manufacturable Silicon Nano-Sensors for Detection of Molecules in Gas Phase. SF J. Nanochem. Nanotechnol. 2018, 1, 1006. [Google Scholar]
- Patolsky, F.; Lieber, C.M. Nanowire nanosensors. Mater. Today 2005, 8, 20–28. [Google Scholar] [CrossRef]
- Shalev, G.; Landman, G.; Amit, I.; Rosenwaks, Y.; Levy, I. Specific and label-free femtomolar biomarker detection with an electrostatically formed nanowire biosensor. NPG Asia Mater. 2013, 41. [Google Scholar] [CrossRef] [Green Version]
- Shalev, G. The Electrostatically Formed Nanowire: A Novel Platform for Gas-Sensing Applications. Sensors 2017, 17, 471. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Dong, X.; Wang, Z.; Wang, Y.; Hou, Z. The Humidity-Induced Sensitivity Amplification Effect in an Ionization Gas Sensor with Silicon Nanostructures. IEEE Electron Device Lett. 2020, 41, 908–911. [Google Scholar] [CrossRef]
- Liu, H.; Wang, B.; Han, Y.; Yang, Z.; Hou, Z.; Huang, Y. A Unique Ionization Gas Sensor with Extraordinary Susceptibility of Sub-1-Volt. IEEE Sens. J. 2020, 20, 3423–3428. [Google Scholar] [CrossRef]
- Mahapatra, N.; Ben-Cohen, A.; Vaknin, Y.; Henning, A.; Hayon, J.; Shimanovich, K.; Greenspan, H.; Rosenwaks, Y. Electrostatic Selectivity of Volatile Organic Compounds Using Electrostatically Formed Nanowire Sensor. ACS Sens. 2018, 3, 709–715. [Google Scholar] [CrossRef]
- Swaminathan, N.; Henning, A.; Jurca, T.; Hayon, J.; Shalev, G.; Rosenwaks, Y. Effect of varying chain length of n-alcohols and n-alkanes detected with electrostatically-formed nanowire sensor. Sens. Actuators B Chem. 2017, 248, 240–246. [Google Scholar] [CrossRef]
- Swaminathan, N.; Henning, A.; Vaknin, Y.; Shimanovich, K.; Godkin, A.; Shalev, G.; Rosenwaks, Y. Dynamic Range Enhancement Using the Electrostatically Formed Nanowire Sensor. ACS Sens. 2016, 1, 688–695. [Google Scholar] [CrossRef]
- Henning, A.; Swaminathan, N.; Godkin, A.; Shalev, G.; Amit, I.; Rosenwaks, Y. Tunable diameter electrostatically formed nanowire for high sensitivity gas sensing. Nano Res. 2015, 8, 2206–2215. [Google Scholar] [CrossRef]
- Henning, A.; Molotskii, M.; Swaminathan, N.; Vaknin, Y.; Godkin, A.; Shalev, G.; Rosenwaks, Y. Electrostatic Limit of Detection of Nanowire-Based Sensors. Small 2015, 11, 4931–4937. [Google Scholar] [CrossRef]
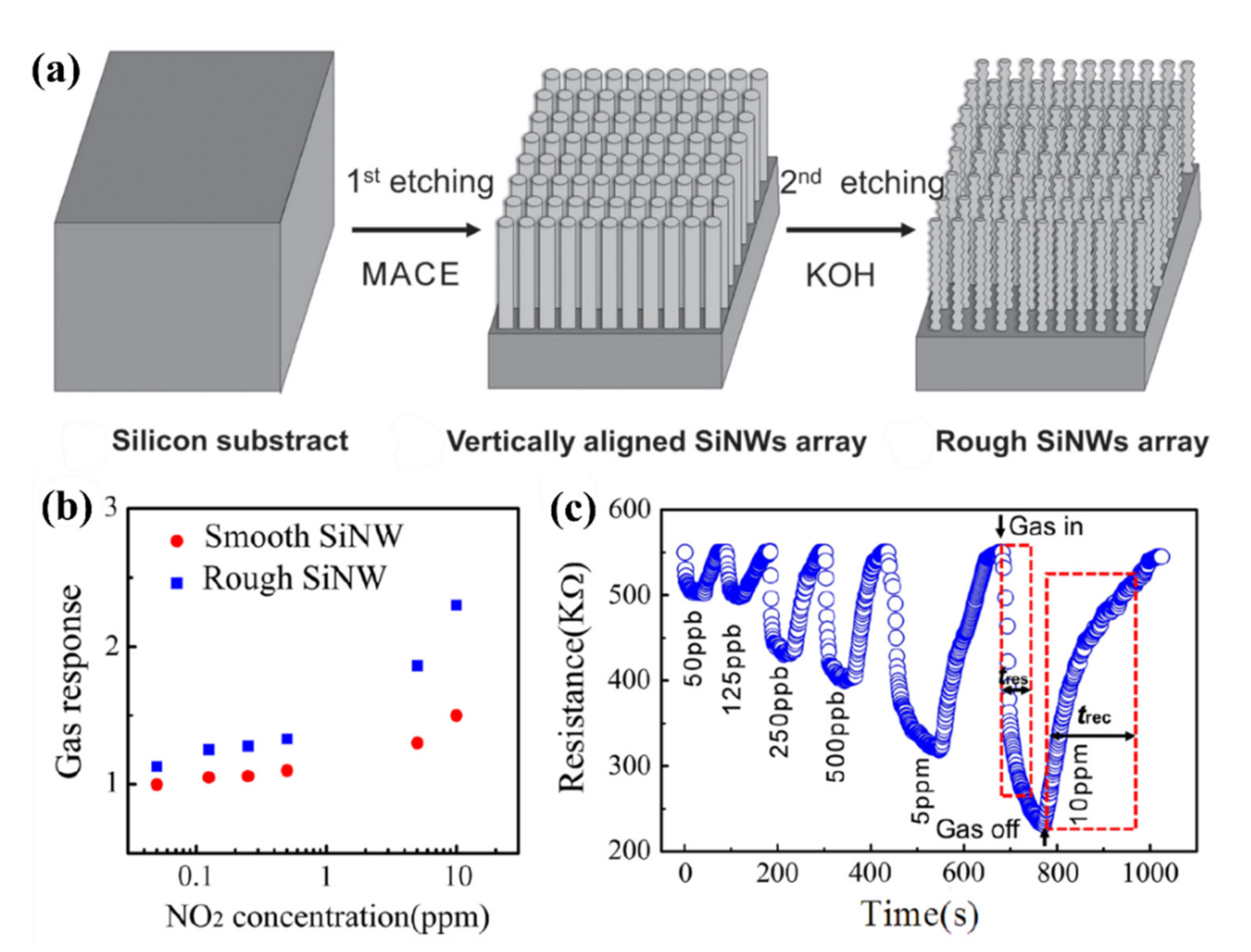
- Qin, Y.; Liu, Y.; Wang, Y. Aligned Array of Porous Silicon Nanowires for Gas-Sensing Application. ECS J. Solid State Sci. Technol. 2016, 5, P380–P383. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, Y.; Liu, Y.; Nanotechnology, X.Z.U. KOH post-etching-induced rough silicon nanowire array for H2 gas sensing application. Nanotechnology 2016, 27, 465502. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, Y.; Liu, Y. Vertically aligned silicon nanowires with rough surface and its NO2 sensing properties. J. Mater. Sci. Mater. Electron. 2016, 27, 11319–11324. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, M.; Wang, Z.; Liu, X.; Yuan, L. A systematic study of the impact of etching time to the sensitivity of SiNW sensor fabricated by MACEtch process. Mater. Sci. Semicond. Process. 2016, 56, 307–312. [Google Scholar] [CrossRef]
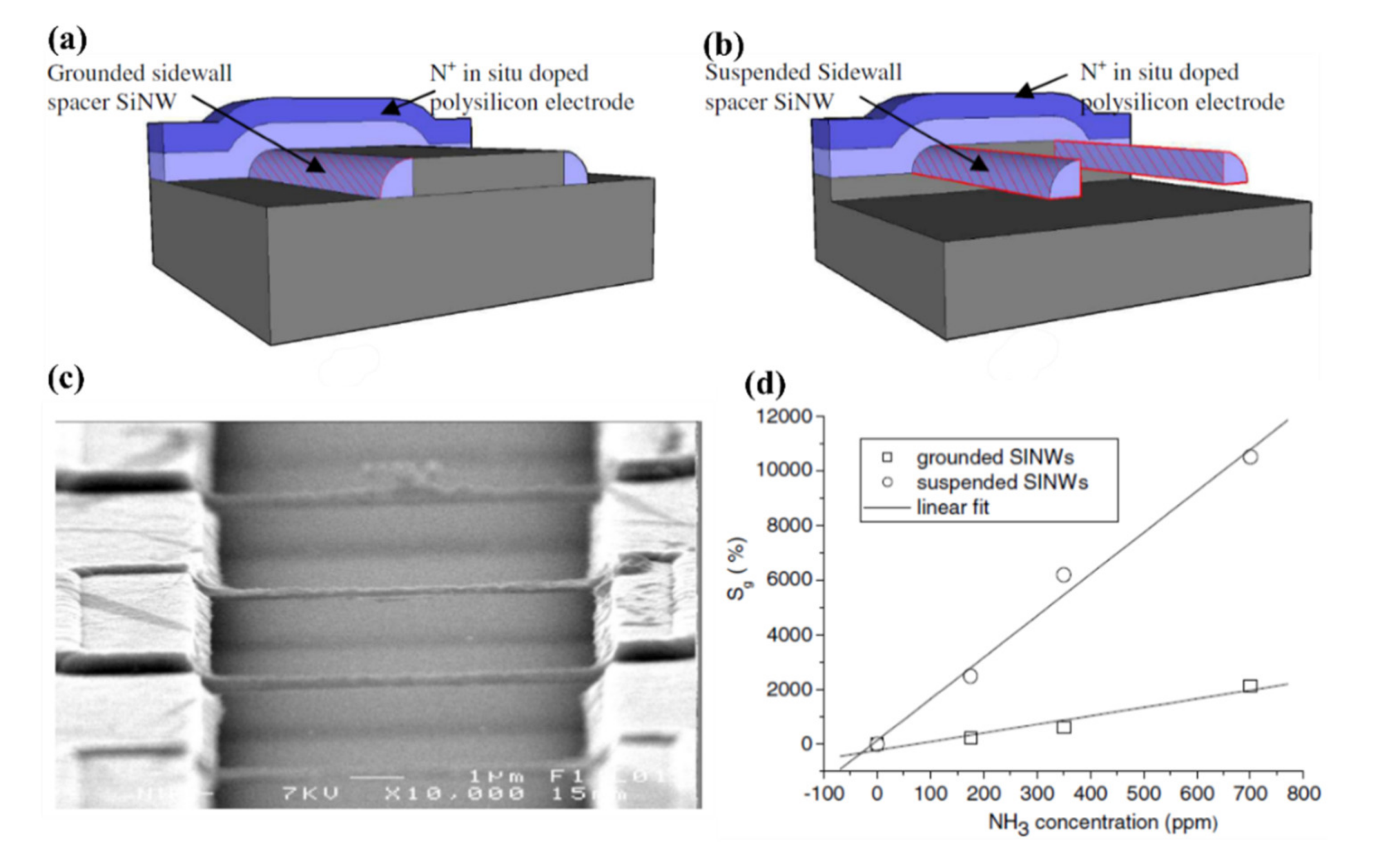
- Pichon, L.; Salaün, A.C.; Wenga, G.; Rogel, R.; Jacques, E. Ammonia sensors based on suspended silicon nanowires. Procedia Eng. 2014, 87, 1003–1006. [Google Scholar] [CrossRef]
- Pichon, L.; Rogel, R.; Jacques, E.; Salaun, A.C. N-type in-situ doping effect on vapour-liquid-solid silicon nanowire properties for gas sensing applications. Phys. Status Solidi 2014, 11, 344–348. [Google Scholar] [CrossRef]
- Lin, L.; Liu, D.; Chen, Q.; Zhou, H.; Wu, J. A vertical tip-tip contact silicon nanowire array for gas sensing. Nanoscale 2016, 8, 17757–17764. [Google Scholar] [CrossRef]
- Yang, X.; Gao, A.; Wang, Y.; Li, T. Wafer-level and highly controllable fabricated silicon nanowire transistor arrays on (111) silicon-on-insulator (SOI) wafers for highly sensitive detection in liquid and gaseous environments. Nano Res. 2018, 11, 1520–1529. [Google Scholar] [CrossRef]
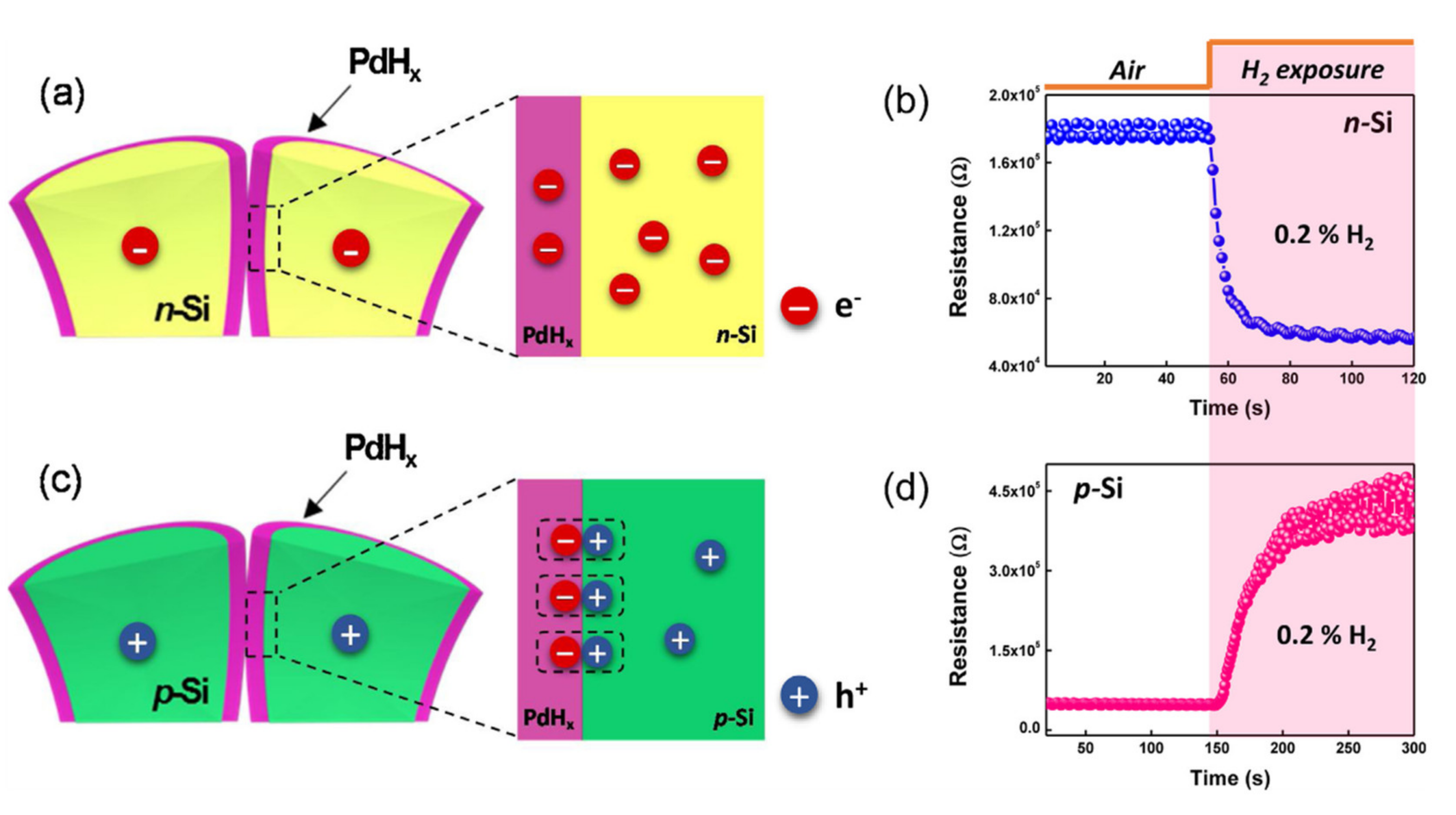
- Baek, J.; Jang, B.; Kim, M.H.; Kim, W.; Kim, J.; Rim, H.J.; Shin, S.; Lee, T.; Cho, S.; Lee, W. High-performance hydrogen sensing properties and sensing mechanism in Pd-coated p-type Si nanowire arrays. Sens. Actuators B Chem. 2018, 256, 465–471. [Google Scholar] [CrossRef]
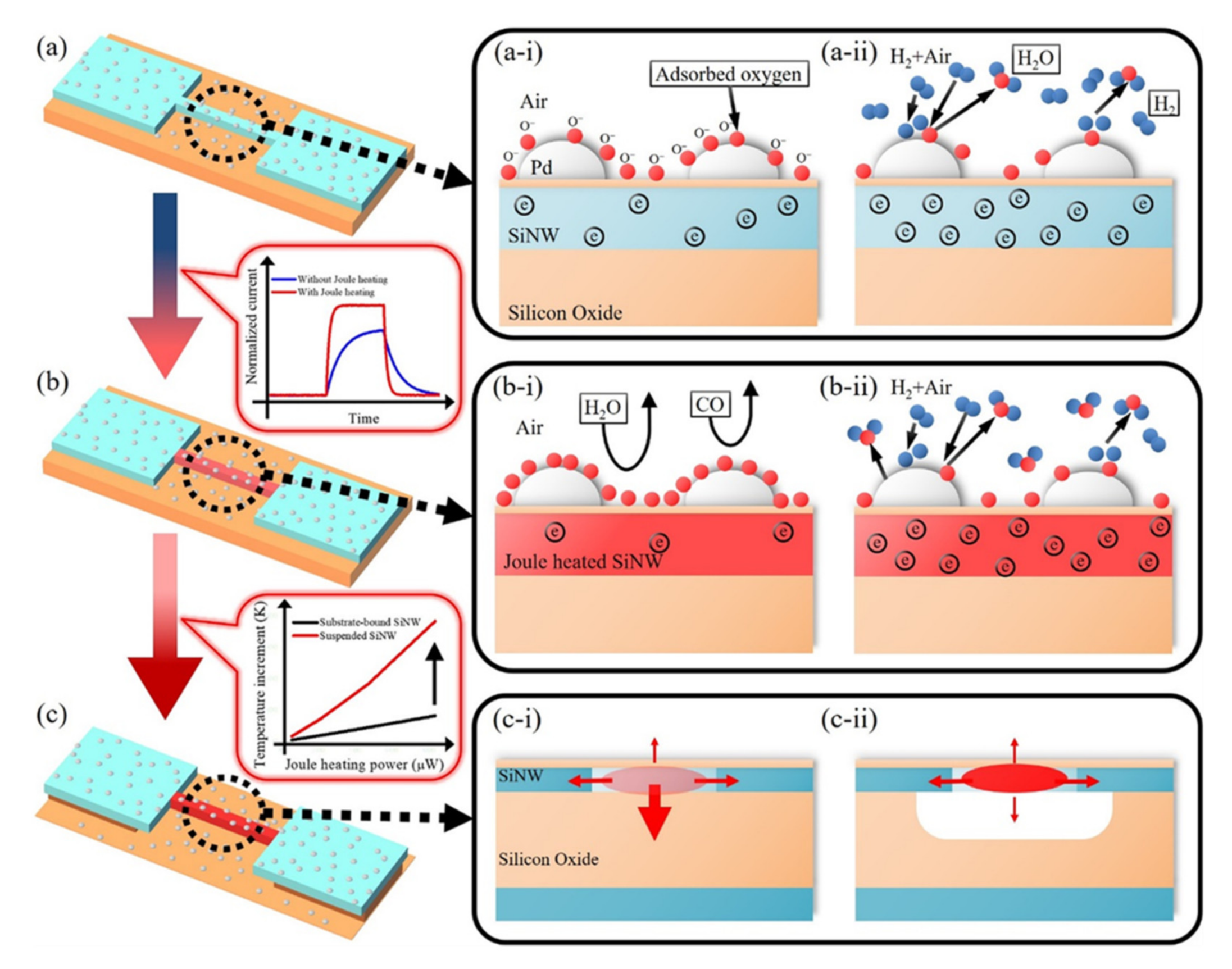
- Ahn, J.H.; Yun, J.; Il Moon, D.; Choi, Y.K.; Park, I. Self-heated silicon nanowires for high performance hydrogen gas detection. Nanotechnology 2015, 26, 095501. [Google Scholar] [CrossRef]
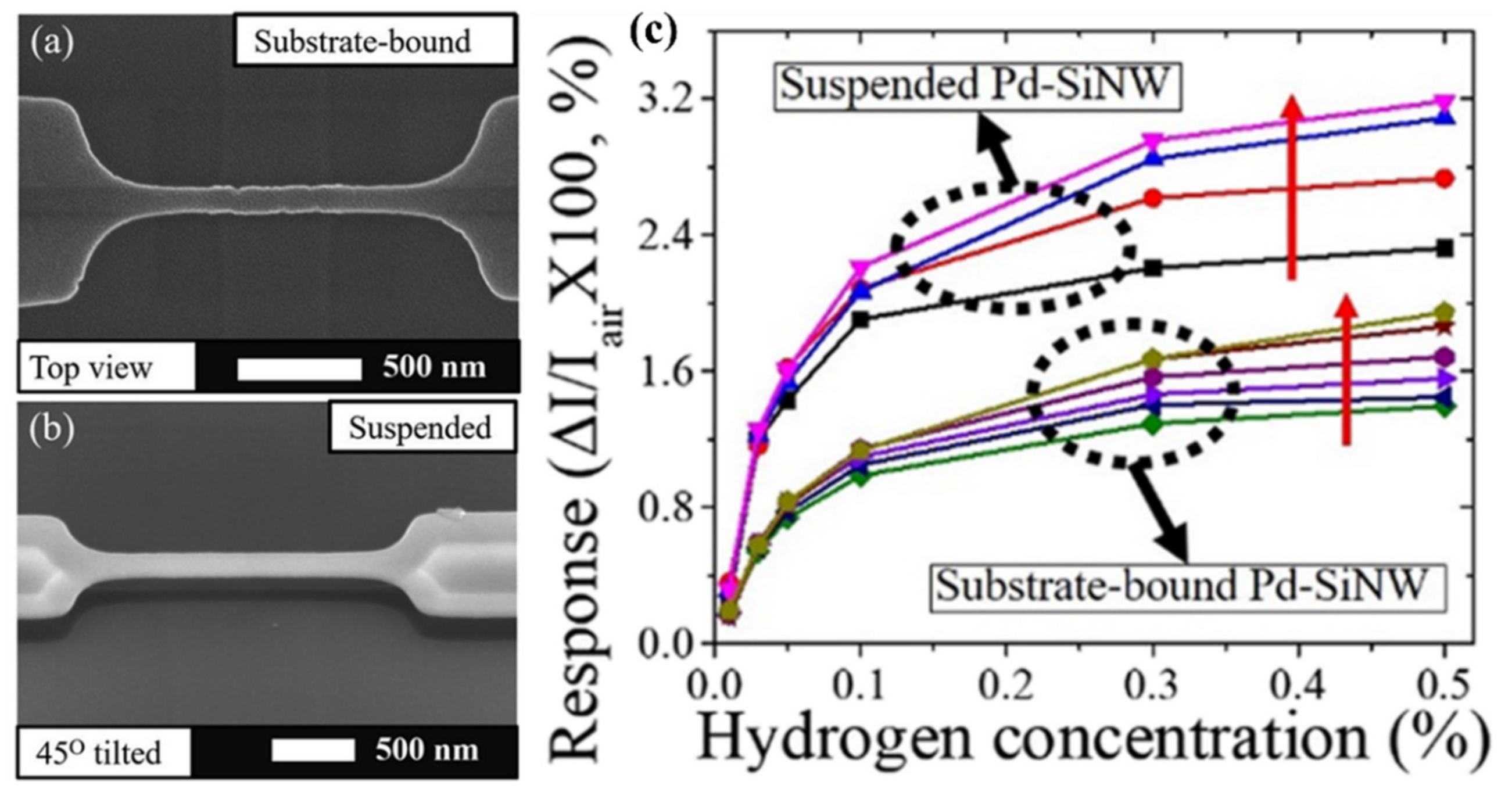
- Yun, J.; Ahn, J.H.; Il Moon, D.; Choi, Y.K.; Park, I. Joule-Heated and Suspended Silicon Nanowire Based Sensor for Low-Power and Stable Hydrogen Detection. ACS Appl. Mater. Interfaces 2019, 11, 42349–42357. [Google Scholar] [CrossRef]
- Choi, B.; Ahn, J.H.; Lee, J.J.; Yoon, J.; Lee, J.J.; Jeon, M.; Kim, D.M.D.H.; Kim, D.M.D.H.; Park, I.; Choi, S.J. A bottom-gate silicon nanowire field-effect transistor with functionalized palladium nanoparticles for hydrogen gas sensors. Solid. State. Electron. 2015, 114, 76–79. [Google Scholar] [CrossRef]
- Yun, J.; Ahn, J.H.; Choi, Y.K.; Park, I. Ultra-low power hydrogen sensor by suspended and palladium coated silicon nanowire. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017; pp. 1079–1082. [Google Scholar]
- Ahn, J.H.; Yun, J.; Choi, Y.K.; Park, I. Palladium nanoparticle decorated silicon nanowire field-effect transistor with side-gates for hydrogen gas detection. Appl. Phys. Lett. 2014, 104, 013508. [Google Scholar] [CrossRef]
- Zhu, L.S.; Zhang, J.; Xu, X.W.; Yu, Y.Z.; Wu, X.; Yang, T.; Wang, X.H. Room temperature H2 detection based on Pd/SiNWs/p-Si Schottky diode structure. Sens. Actuators B Chem. 2016, 227, 515–523. [Google Scholar] [CrossRef]
- Baba Ahmed, L.; Naama, S.; Keffous, A.; Hassein-Bey, A.; Hadjersi, T. H2 sensing properties of modified silicon nanowires. Prog. Nat. Sci. Mater. Int. 2015, 25, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Hassan, K.; Uddin, A.S.M.I.; Chung, G.S. Hydrogen sensing properties of Pt/Pd bimetal decorated on highly hydrophobic Si nanowires. Int. J. Hydrog. Energy 2016, 41, 10991–11001. [Google Scholar] [CrossRef]
- Kim, D.; Park, C.; Choi, W.; Shin, S.H.; Jin, B.; Baek, R.H.; Lee, J.S. Improved Long-Term Responses of Au-Decorated Si Nanowire FET Sensor for NH3 Detection. IEEE Sens. J. 2020, 20, 2270–2277. [Google Scholar] [CrossRef]
- Naama, S.; Hadjersi, T.; Keffous, A.; Nezzal, G. CO2 gas sensor based on silicon nanowires modified with metal nanoparticles. Mater. Sci. Semicond. Process. 2015, 38, 367–372. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, D.; Zhang, T.; Cui, Z. Ultrasensitive Silicon Nanowire Sensor Developed by a Special Ag Modification Process for Rapid NH3 Detection. ACS Appl. Mater. Interfaces 2017, 9, 28766–28773. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, D.; Wang, Z.; Jiang, Y. Ag nanoparticles-functionalized rough silicon nanowires array and its unique response characteristics to ultrararefied NO2. Sens. Actuators B Chem. 2018, 258, 730–738. [Google Scholar] [CrossRef]
- Hsu, H.F.; Chen, C.A.; Liu, S.W.; Tang, C.K. Fabrication and Gas-Sensing Properties of Ni-Silicide/Si Nanowires. Nanoscale Res. Lett. 2017, 12, 182. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.J.; Heo, K.; Lee, H.; Jin, J.H.; Chang, H.; Park, M.; Lee, H.B.R.; Kim, H.; Lee, B.Y. Real-time detection of chlorine gas using Ni/Si shell/core nanowires. Nanoscale Res. Lett. 2015, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zeng, P.; Zhang, P.; Hu, M.; Ma, S.Y.; Yan, W.-J. Synthesis and room-temperature NO2 gas sensing properties of a WO3 nanowires/porous silicon hybrid structure IOPscience. Chin. Phys. B 2014, 23, 058103. [Google Scholar] [CrossRef]
- Bang, J.H.; Choi, M.S.; Mirzaei, A.; Oum, W.; Han, S.; Kim, S.S.; Kim, H.W. Porous Si/SnO2 nanowires heterostructures for H2S gas sensing. Ceram. Int. 2020, 46, 604–611. [Google Scholar] [CrossRef]
- Liu, D.; Lin, L.; Chen, Q.; Zhou, H.; Wu, J. Low Power Consumption Gas Sensor Created from Silicon Nanowires/TiO2 Core-Shell Heterojunctions. ACS Sens. 2017, 2, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Li, Z.; Wang, G.; Chen, C.; Lv, S.; Li, M. ZnO nanorod/porous silicon nanowire hybrid structures as highly-sensitive NO2 gas sensors at room temperature. Phys. Chem. Chem. Phys. 2016, 18, 4835–4841. [Google Scholar] [CrossRef]
- Samanta, C.; Ghatak, A.; Raychaudhuri, A.K.; Ghosh, B. ZnO/Si nanowires heterojunction array-based nitric oxide (NO) gas sensor with noise-limited detectivity approaching 10 ppb. Nanotechnology 2019, 30, 305501. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, Z.; Liu, D.; Wang, K. Dendritic composite array of silicon nanowires/WO3 nanowires for sensitive detection of NO2 at room temperature. Mater. Lett. 2017, 207, 29–32. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, M.; Liu, X.; Wei, Y.; Li, N.; Qin, Y. Synthesis of the cactus-like silicon nanowires/tungsten oxide nanowires composite for room-temperature NO2 gas sensor. J. Alloy. Compd. 2016, 679, 391–399. [Google Scholar] [CrossRef]
- Han, J.W.; Rim, T.; Baek, C.K.; Meyyappan, M. Chemical Gated Field Effect Transistor by Hybrid Integration of One-Dimensional Silicon Nanowire and Two-Dimensional Tin Oxide Thin Film for Low Power Gas Sensor. ACS Appl. Mater. Interfaces 2015, 7, 21263–21269. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wu, D.; Bu, K.; Xu, T.; Shi, Z.; Xu, J.; Tian, Y.; Li, X. Dual-mode high-sensitivity humidity sensor based on MoS2/Si nanowires array heterojunction. J. Alloy. Compd. 2017, 726, 632–637. [Google Scholar] [CrossRef]
- Wu, D.; Lou, Z.; Wang, Y.; Xu, T.; Shi, Z.; Xu, J.; Tian, Y.; Li, X. Construction of MoS2/Si nanowire array heterojunction for ultrahigh-sensitivity gas sensor IOPscience. Nanotechnology 2017, 28, 435503. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Z.; Wang, G.; Liao, J.; Lv, S.; Zhu, Z. Highly enhanced response of MoS2/porous silicon nanowire heterojunctions to NO2 at room temperature. RSC Adv. 2018, 8, 11070–11077. [Google Scholar] [CrossRef] [Green Version]
- Wong, Y.C.; Ang, B.C.; Haseeb, A.S.M.A.; Baharuddin, A.A.; Wong, Y.H. Review—Conducting Polymers as Chemiresistive Gas Sensing Materials: A Review. J. Electrochem. Soc. 2020, 167, 037503. [Google Scholar] [CrossRef]
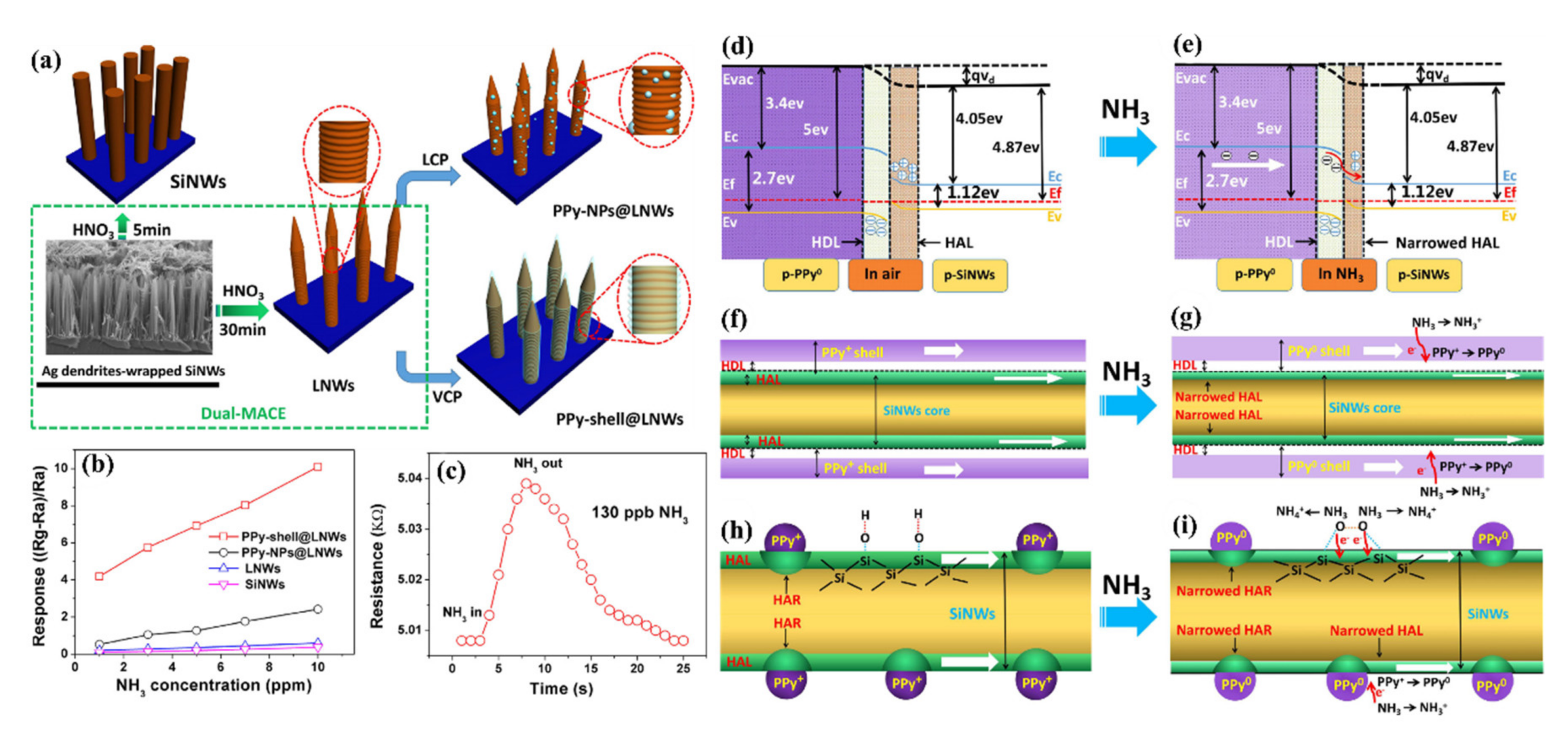
- Qin, Y.; Cui, Z.; Zhang, T.; Liu, D. Polypyrrole shell (nanoparticles)-functionalized silicon nanowires array with enhanced NH3-sensing response. Sens. Actuators B Chem. 2018, 258, 246–254. [Google Scholar] [CrossRef]
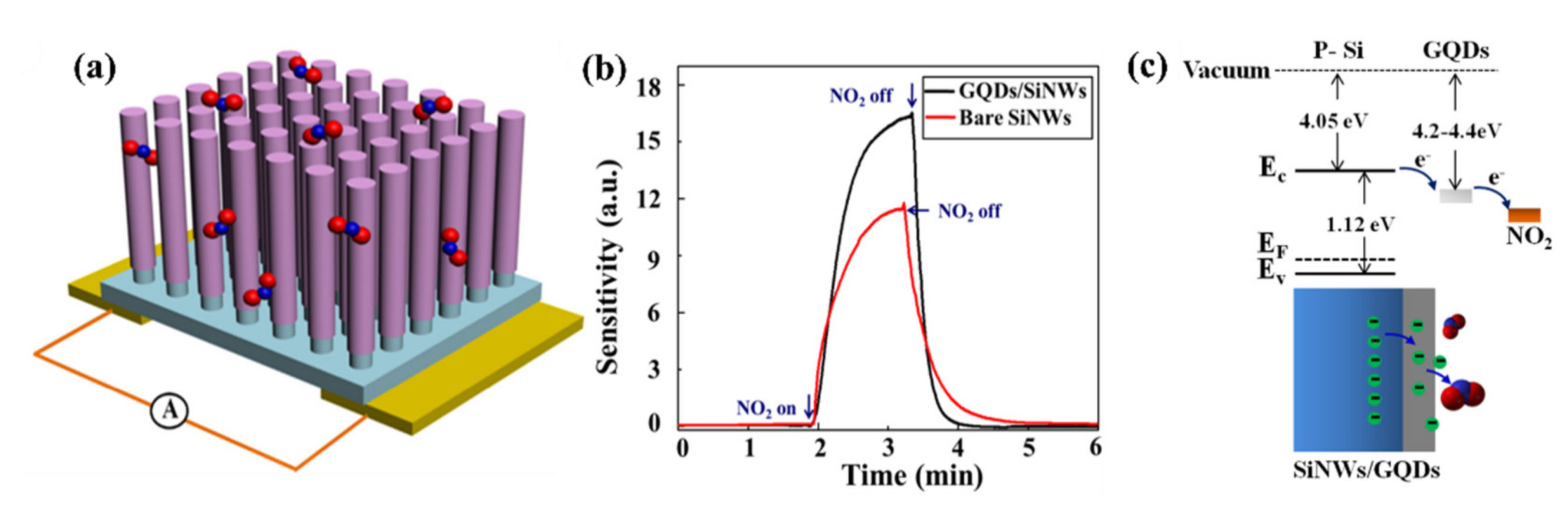
- Li, T.Y.; Duan, C.Y.; Zhu, Y.X.; Chen, Y.F.; Wang, Y. Graphene quantum dots modified silicon nanowire array for ultrasensitive detection in the gas phase. J. Phys. D Appl. Phys. 2017, 50. [Google Scholar] [CrossRef]
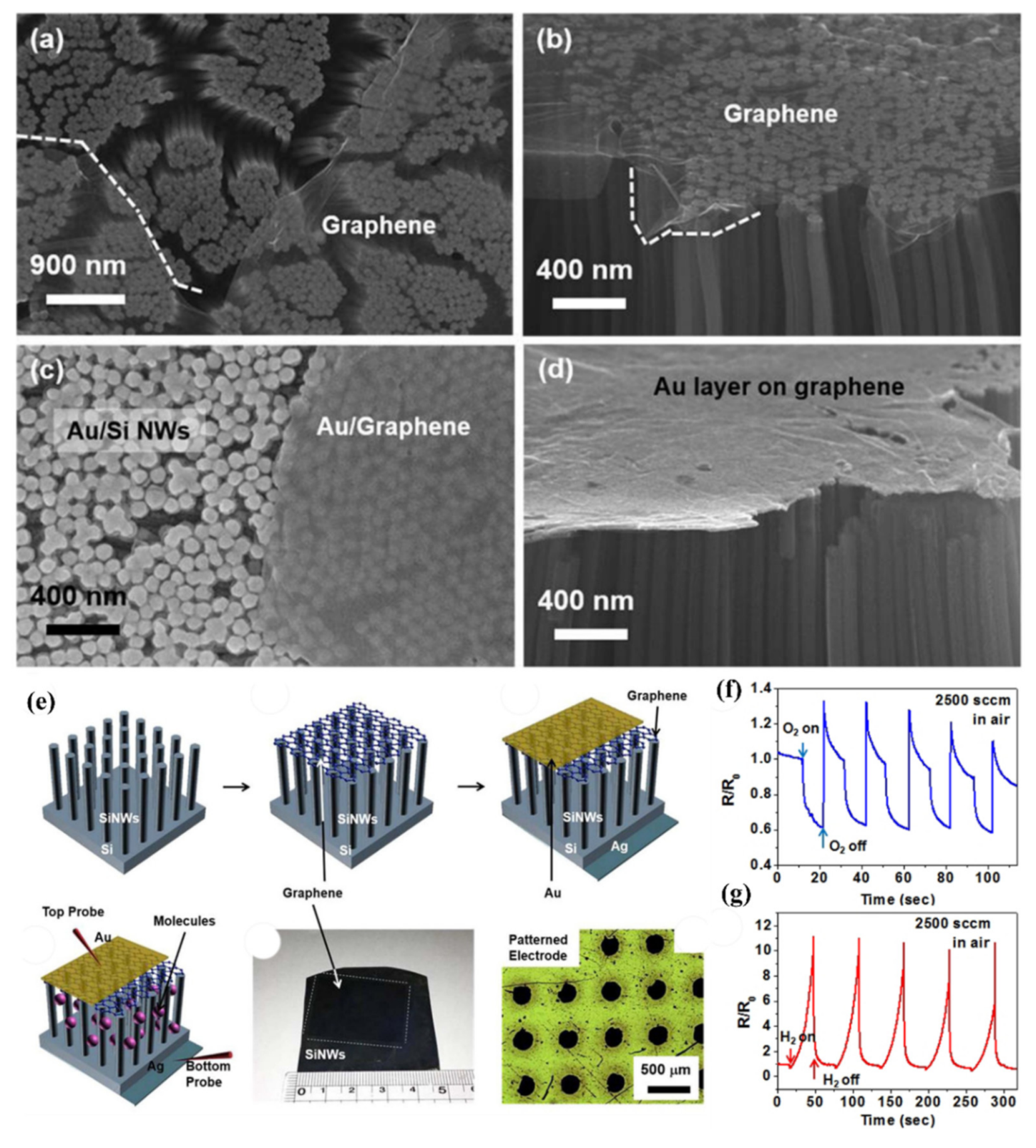
- Kim, J.; Oh, S.D.; Kim, J.H.; Shin, D.H.; Kim, S.; Choi, S.H. Graphene/Si-nanowire heterostructure molecular sensors. Sci. Rep. 2014, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Cancilla, J.C.; Torrecilla, J.S.; Haick, H. Artificial sensing intelligence with silicon nanowires for ultraselective detection in the gas phase. Nano Lett. 2014, 14, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Halpern, J.M.; Wang, B.; Haick, H. Controlling the sensing properties of silicon nanowires via the bonds nearest to the silicon nanowire surface. ACS Appl. Mater. Interfaces 2015, 7, 11315–11321. [Google Scholar] [CrossRef]
- Shehada, N.; Brönstrup, G.; Funka, K.; Christiansen, S.; Leja, M.; Haick, H. Ultrasensitive silicon nanowire for real-world gas sensing: Noninvasive diagnosis of cancer from breath volatolome. Nano Lett. 2015, 15, 1288–1295. [Google Scholar] [CrossRef]
- Gao, A.; Wang, Y.Y.; Yang, X.; Wang, Y.Y.; Li, T. Ultrasensitive bioelectronic nose based on CMOS-compatible silicon nanowire array. In Proceedings of the 2017 IEEE Sensors, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar]
- Liu, X.; Chen, S.; Wang, H.; Gao, A.; Wang, Y.; Li, T.; Zhang, H.; Huang, Z.; Cheng, Z. Amino Monolayer Modified Nanowire Array for Trinitrotoluene Detection. Sens. Mater. 2018, 30, 2669–2677. [Google Scholar] [CrossRef]
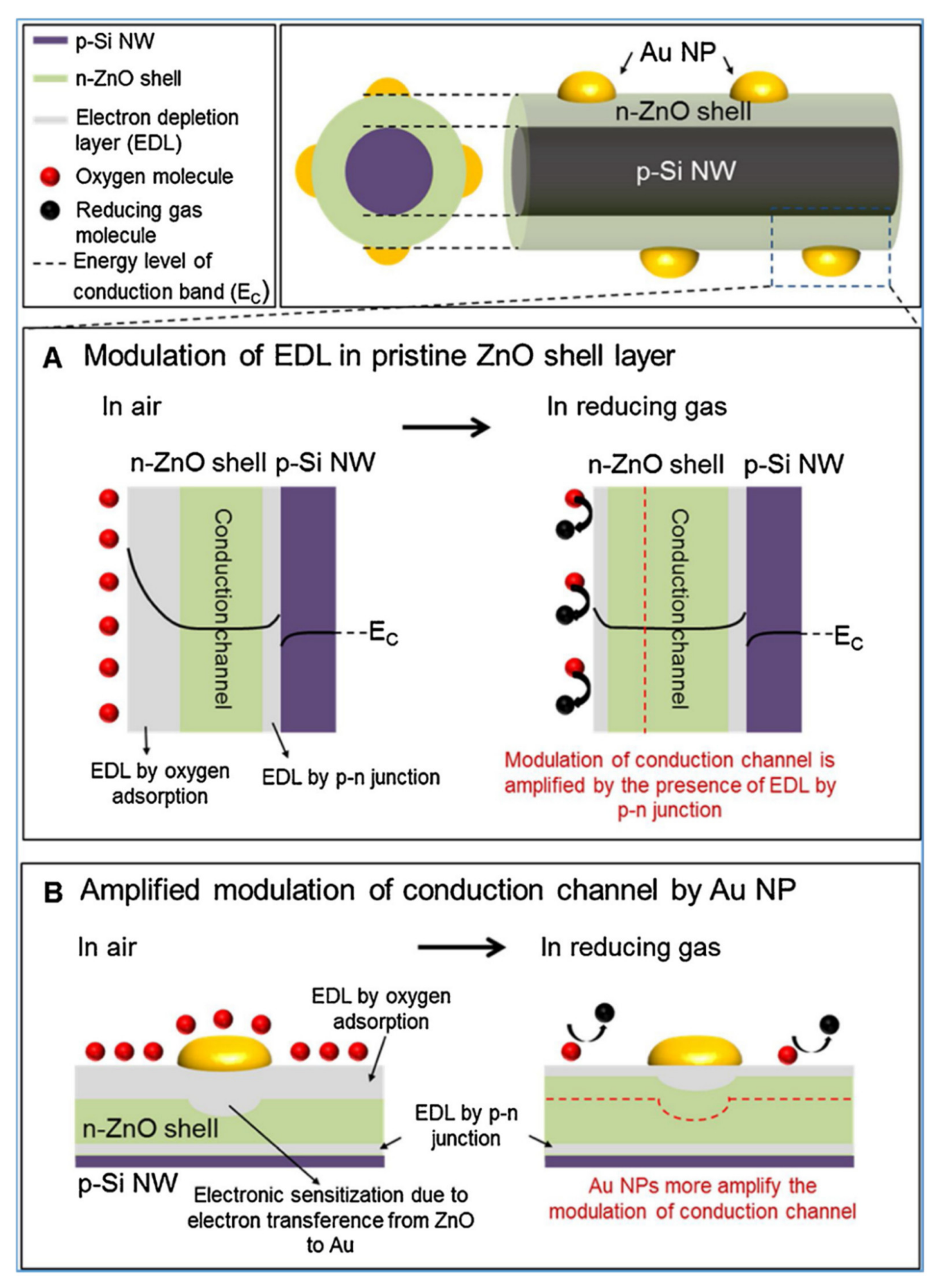
- Choi, M.S.; Mirzaei, A.; Bang, J.H.; Oum, W.; Jung Kwon, Y.; Kim, J.H.; Choi, S.W.; Kim, S.S.; Kim, H.W. Selective H2S-sensing performance of Si nanowires through the formation of ZnO shells with Au functionalization. Sens. Actuators B Chem. 2019, 289, 1–14. [Google Scholar] [CrossRef]
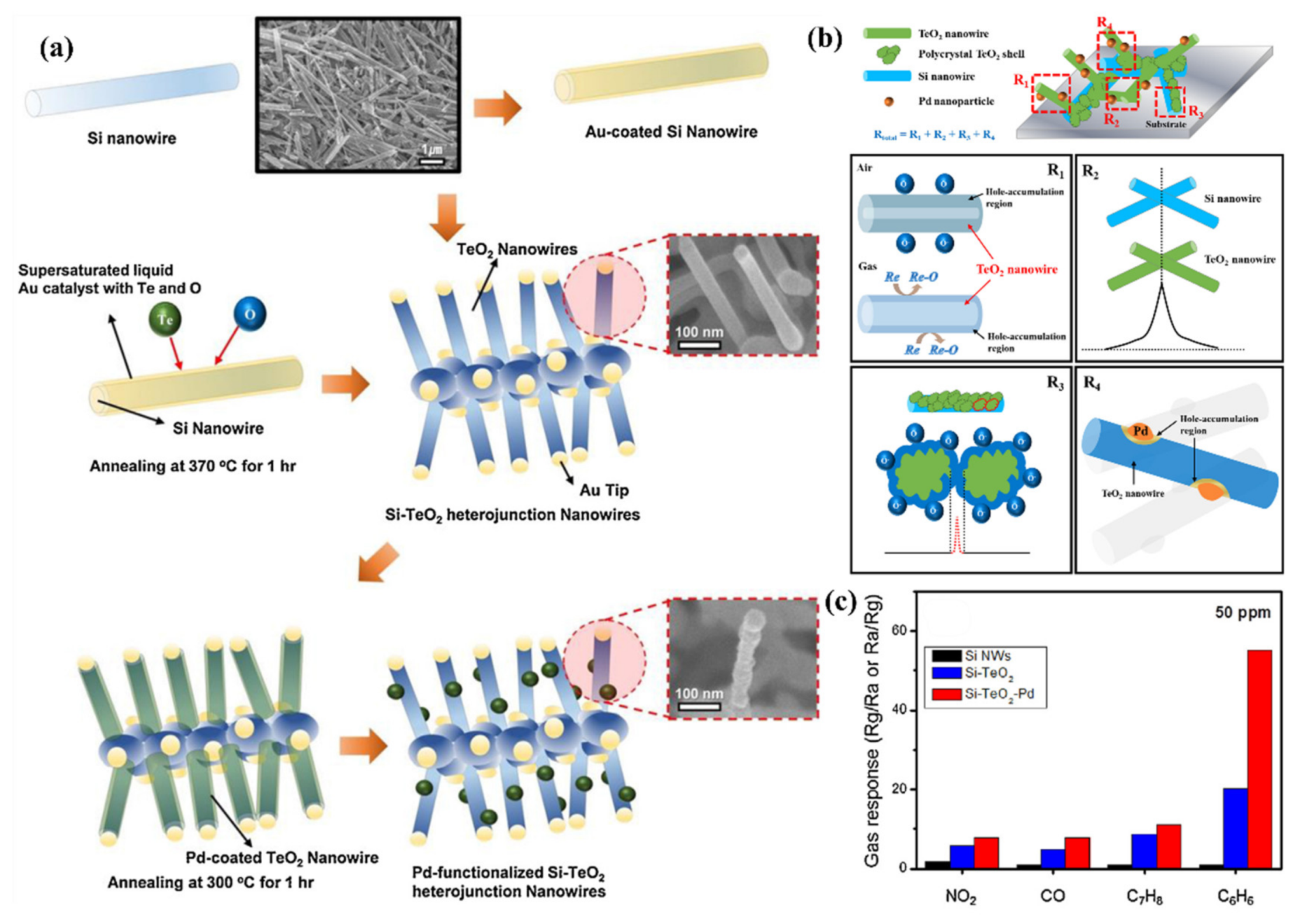
- Kwon, Y.J.; Choi, S.W.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Kim, S.S.; Kim, H.W. Enhancement of the benzene-sensing performance of Si nanowires through the incorporation of TeO2 heterointerfaces and Pd-sensitization. Sens. Actuators B Chem. 2017, 244, 1085–1097. [Google Scholar] [CrossRef]
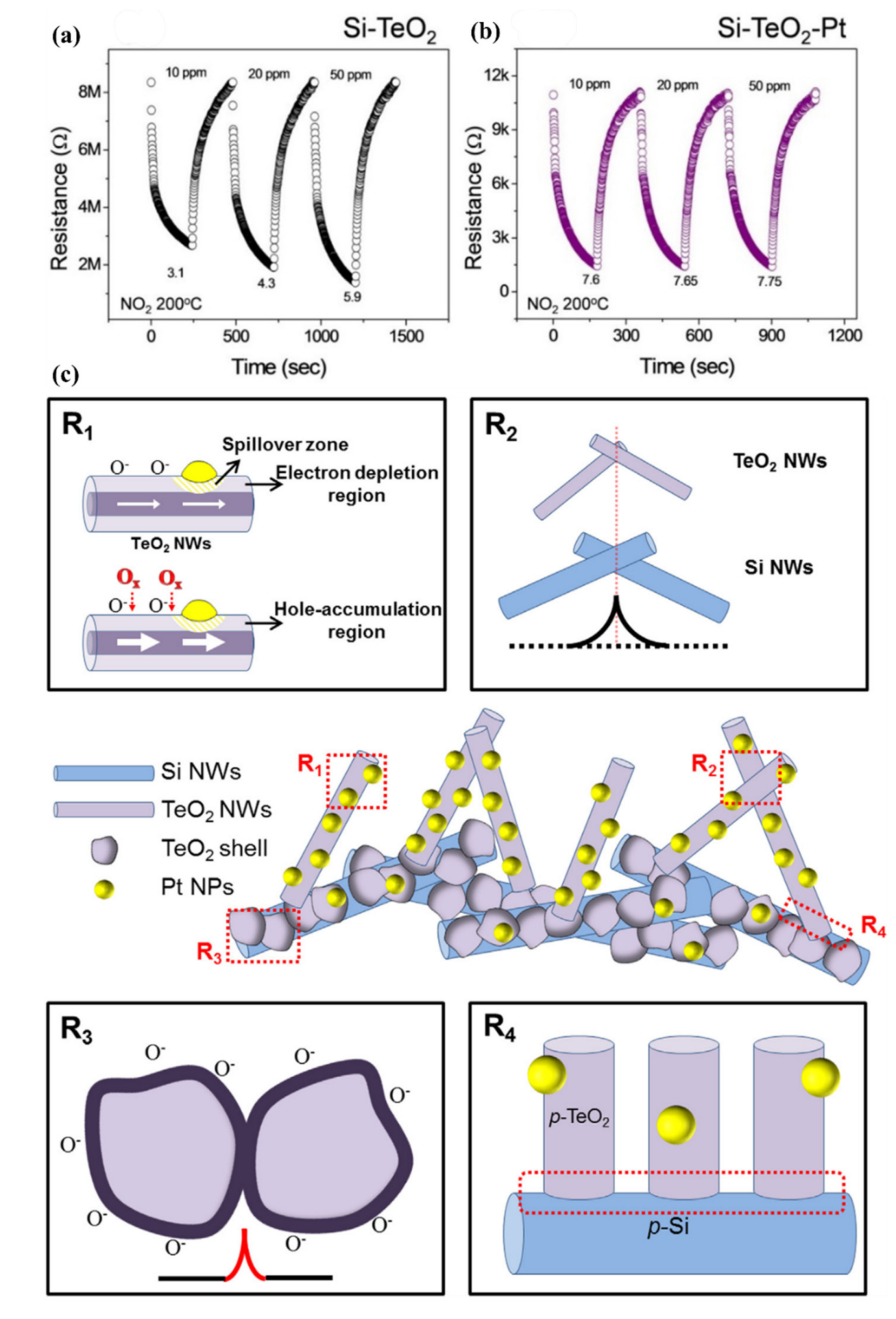
- Bang, J.H.; Choi, M.S.; Mirzaei, A.; Han, S.; Lee, H.Y.; Choi, S.W.; Kim, S.S.; Kim, H.W. Hybridization of silicon nanowires with TeO2 branch structures and Pt nanoparticles for highly sensitive and selective toluene sensing. Appl. Surf. Sci. 2020, 525, 146620. [Google Scholar] [CrossRef]
- Qin, Y.; Zang, J.; Wen, Z. Synergistic functionalization of aligned silicon nanowires by Ag nanoparticles&PPy wrapping for improving gas-sensing response at high humidity level. Phys. E Low-Dimens. Syst. Nanostructures 2020, 118, 113957. [Google Scholar] [CrossRef]
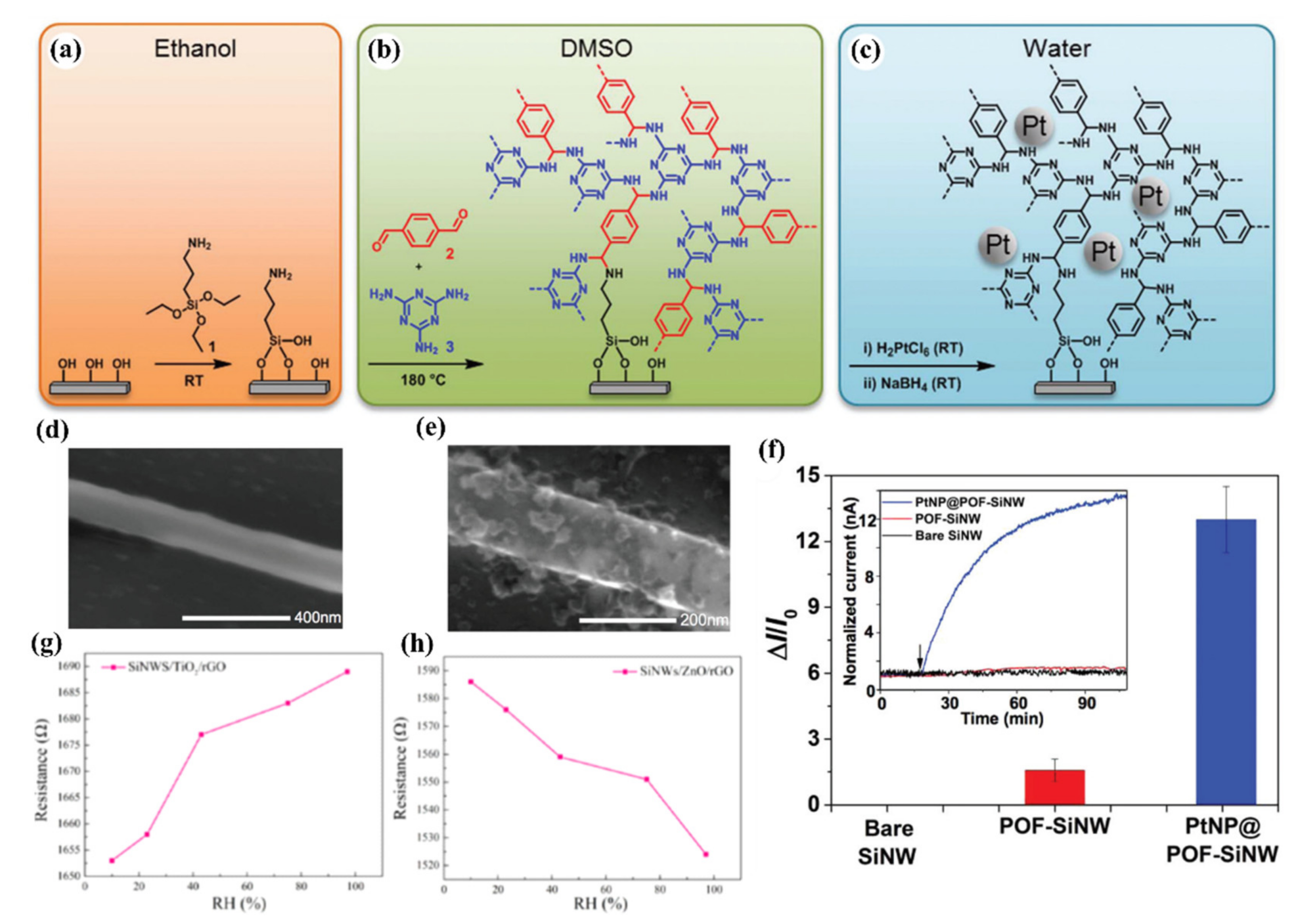
- Cao, A.; Shan, M.; Paltrinieri, L.; Evers, W.H.; Chu, L.; Poltorak, L.; Klootwijk, J.H.; Seoane, B.; Gascon, J.; Sudhölter, E.J.R.; et al. Enhanced vapour sensing using silicon nanowire devices coated with Pt nanoparticle functionalized porous organic frameworks. Nanoscale 2018, 10, 6884–6891. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Jia, C.; Shi, F.; Zeng, L.; Lin, P.; Dong, L.; Shi, Z.; Tian, Y.; Li, X.; Jie, J. Mixed-dimensional PdSe 2 /SiNWA heterostructure based photovoltaic detectors for self-driven, broadband photodetection, infrared imaging and humidity sensing. J. Mater. Chem. A 2020, 8, 3632–3642. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, Z.; Xiao, L.; Lv, R. Carbon Nanotube-Silicon Nanowire Heterojunction Solar Cells with Gas-Dependent Photovoltaic Performances and Their Application in Self-Powered NO2 Detecting. Nanoscale Res. Lett. 2016, 11, 299. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Hu, M.; Zeng, P.; Li, M.; Yan, W.; Qin, Y. Synthesis and low-temperature gas sensing properties of tungsten oxide nanowires/porous silicon composite. Sens. Actuators B Chem. 2014, 192, 341–349. [Google Scholar] [CrossRef]
- Lo, Y.-R.; Chen, H.-M.P.; Yang, Y.-S.; Lu, M.-P. Gas Sensing Ability on Polycrystalline-Silicon Nanowire. ECS J. Solid State Sci. Technol. 2018, 7, Q3104–Q3107. [Google Scholar] [CrossRef] [Green Version]








| Phase | Reaction Temperature (°C) | Crystal Structure | Shottky Barrier Height (eV) | Interfacial Plane Structure | Ref. |
|---|---|---|---|---|---|
| MnSi | 650 | Cubic | 0.65 | MnSi (−2 −1 4)‖Si (3 4 5) | [88] |
| MnSi [1 −2 0]‖Si [3 −1 −1] | |||||
| CoSi2 | 800 | Cubic | 0.64 | CoSi2 (−1 1 1)‖Si (−1 1 1) | [89] |
| CoSi2 [1 1 0]‖Si [1 1 0] | |||||
| PtSi | 520 | Orthorhombic | 0.88 | PtSi (1 0 1)‖Si (1 1 1) | [90] |
| PtSi [0 1 0]‖Si [1 −1 0] | |||||
| NiSi | 450 | Orthorhombic | 0.65 | NiSi (−1 1 0)‖Si (1 −1 1) | [91] |
| NiSi [0 0 1]‖Si [1 1 0] | |||||
| NiSi2 | 300~650 | Cubic | 0.66 | NiSi2 (1 1 1)‖Si (1 1 1) | [92] |
| NiSi2 [−1 1 0]‖Si [−1 1 0] |
| Year | Approach | SiNW Size | Functionalization | WT | Target(s) | Detection Limit | Ref. |
|---|---|---|---|---|---|---|---|
| 2018 | TD | D: 29–56 nm | Bare | RT | VOC | No Data | [126] |
| 2017 | TD | D: 20 nm | Bare | RT | Ethanol | 26 ppm | [123] |
| 2017 | TD | No Data | Bare | 50–60 °C | VOC | 50 ppm | [127] |
| 2016 | TD | D: 29 nm | Bare | RT | Ethanol, Acetone | ∼26 ppm ethanol,∼40 ppm acetone | [128] |
| 2015 | TD | D: 16–46 nm | Bare | RT | Ethanol | 100 ppm | [129] |
| 2015 | TD | D: 22–115 nm | Bare | RT | Ethanol | No Data | [130] |
| Year | Approach | SiNW Size | Functionalization | WT | Target(s) | Detection Limit | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Resistor | 2018 | TD | D: 30 nm | Bare | 100 °C | H2 | 10 ppm | [112] |
| 2018 | TD | D: 50 nm L: 10 μm | Bare | RT | NO2 | 10 ppm | [110] | |
| 2018 | TD | D: 50–125 nm L: 31 μm | Bare | RT | NO2 | 0.25 ppm | [111] | |
| 2016 | TD | D: 50–200 nm L: 25–30 μm | Bare | RT | NO2 | 18 ppb | [137] | |
| 2016 | TD | D: 90 nm L: 42 μm | Bare | RT | H2 | 50 ppm | [132] | |
| 2016 | TD | D: 100 nm L: 11–25 μm | Porous SiNWs | RT | NO2 | 50 ppb | [131] | |
| 2016 | TD | D: 550 nm | Bare | RT | NO2 | 1 ppm | [134] | |
| 2016 | TD | D: 90 nm L: 36 μm | Bare | RT | NO2 | 50 ppb | [133] | |
| 2014 | TD | W: 100 nm, L: 7.26 μm | Polycrystalline SiNWs | RT | NH3 | 2 ppm | [135] | |
| 2014 | BU | D: 150 nm L: ~20 μm | phosphorous in-situ doped | RT | NH3 | 2 ppm | [136] | |
| FET | 2018 | TD | W: 100 nm | Bare | RT | NO2 | 1 ppm | [138] |
| 2017 | TD | No Data | Bare | RT | Ethanol | No Data | [113] |
| Year | Approach | SiNW Size | Functionalization | WT | Target(s) | Detection Limit | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Resistor | 2019 | TD | W: 160 nm, L: 500 nm | Pd | RT | H2 | 0.01% | [141] |
| 2018 | TD | D: 200 nm L: 30 μm | Pd-coated | RT | H2 | 2 ppm | [139] | |
| 2018 | TD | L: 20 μm | Ag | RT | NO2 | 10 ppb | [151] | |
| 2017 | TD | W: 215 nm | Ni-Silicide | 250 °C | O2 | No Data | [152] | |
| 2017 | TD | L: 30 μm | Ag | RT | NH3 | 330 ppb | [150] | |
| 2017 | TD | W: 160 nm | Pd | 40 °C, 60 °C | H2 | No Data | [143] | |
| 2016 | TD | D: 100–200 nm L: 8–12 μm | Pt/Pd | 75 °C | H2 | 1 ppm | [147] | |
| 2016 | TD | D: 40–80 nm L: 22 μm | Pd | RT | H2 | 300 ppm | [145] | |
| 2015 | TD | D: 20–100 nm L: 13 μm | Pt, Pd, Ag, Au | RT | H2 | 15 ppm | [146] | |
| 2015 | TD | L: 1.35 μm | Pt, Au | RT | CO2 | 0.5 mbar | [149] | |
| 2015 | TD | L: 1 μm, W: 110 nm, H: 40 nm | Pd | RT | H2 | 0.1% | [140] | |
| 2015 | BU | D: 60 nm L: 1–4 μm | Ni | RT | Chlorine | 5 ppm | [153] | |
| FET | 2020 | TD | W: 70 nm L: 10 µm | Au | RT | NH3 | 1 ppm | [148] |
| 2015 | TD | W: 70 nm, L:10 μm, H: 80 nm | Pd | RT | H2 | 0.01% | [142] | |
| 2014 | TD | W: 100 nm, L:1 μm, H: 50 nm | Pd | RT | H2 | 0.1% | [144] |
| Year | Approach | SiNW Size | Functionalization | WT | Target(s) | Detection Limit | Gases Checked for Selectivity | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Resistor | 2020 | TD | L: 1–3 μm, | TiO2 + Pt | 200 °C | Toluene | 10 ppm | CO, C6H6, NO2 | [176] |
| 2020 | TD | D: 100–200 nm, L: 8 μm | PdSe2 | RT | Humidity | 11% | - | [179] | |
| 2020 | TD | L: 20 μm | PPy + Ag | RT | NH3 | 200 ppb | Acetone, Methanol, Ethanol, Isopropanol, Cyclohexanol | [177] | |
| 2020 | TD | L: 30 μm | SnO2 | 100 °C | H2S | 10 ppm | CO, Acetone, Ethanol, Benzene, Toluene | [155] | |
| 2020 | TD | L: 10 μm | (TiO2 or ZnO) + rGO | RT | Humidity | 10% | - | [15] | |
| 2019 | TD | W: 100~300 nm, L: 24 μm | rGO | 300 °C | HCHO | 35 ppb | Ethanol, Acetone, Ammonia, Methanol, Xylene, Toluene | [115] | |
| 2019 | TD | W: 80–100 nm, L: 2.5–3 μm | ZnO | RT | NO | 10 ppb | NH3, CH4, H2S, NO2, H2O | [158] | |
| 2019 | TD | W: 280-500 nm | OTS-Porous SiNWs | RT | NO2 | 5 ppb | NO, Ethanol, Acetone, Methanol, CH4, O2, NH3 | [116] | |
| 2019 | TD | W: ~100 nm L: 20 μm | LSiNWs/PPy | RT | NO2 | 50 ppb | Ethanol, Acetone, Methanol, CH4, H2 | [117] | |
| 2019 | TD | W: 100 nm | ZnO + Au | 300 °C | H2S | 5 ppm | Ethanol, Acetone, NO2 | [174] | |
| 2018 | TD | D: 100 nm L: 30 μm | PPy | RT | NH3 | 130 ppb | Ethanol, Acetone, Methanol, CH4, H2 | [166] | |
| 2018 | TD | D: 200 nm | MoS2 | RT | NO2 | 1 ppm | - | [164] | |
| 2017 | TD | W: 70 nm | OBP | RT | Nonanoic acid | 10 ppb | Hexanoic acid | [172] | |
| 2017 | TD | L: 35 μm | WO3 | RT | NO2 | 0.5 ppm | Ethanol, Acetone, CH4, NH3, H2 | [159] | |
| 2017 | TD | D: 400 nm | TiO2 | RT | CH4 | 20 ppm | Ethanol, Acetone | [156] | |
| 2017 | TD | L: 3.4 μm | MoS2 | RT | NO | 10 ppb | NO2, NH3, O2, H2 | [163] | |
| 2017 | TD | L: 7 μm | GQD | RT | NO2 | 10 ppm | SO2, N2, O2, H2 | [167] | |
| 2017 | TD | No Data | Pd + TeO2 | 200 °C | Benzene | 10 ppm | CO, Toluene, NO2, H2S, Ethanol, Acetone | [175] | |
| 2017 | TD | L: 5 μm | MoS2 | RT | Humidity | 11% | - | [162] | |
| 2016 | TD | W: 375 nm L: 7.6 μm | ZnO | RT | NO2 | 5 ppm | Methanol, CH4, NO2 | [157] | |
| 2016 | TD | D: 400–500 nm, L: 2–2.2 μm | WO3 | RT | NO2 | 0.25 ppm | Ethanol, Acetone, Methanol, NH3, Isopropanol | [160] | |
| 2016 | TD | L: 300 nm | Carbon Nanotube | RT | NO2 | 10 ppm | - | [180] | |
| 2014 | TD | L: 8 μm | WO3 | 100 °C | NO2 | 2 ppm | Ethanol, Acetone, NH3 | [181] | |
| 2014 | TD | No Data | WO3 | RT | NO2 | 100 ppb | Ethanol, Methanol, NH3, Isopropy alochol | [154] | |
| 2014 | TD | D: 50 nm | Graphene | RT | O2, H2 | No Data | - | [168] | |
| FET | 2018 | TD | W: 100 nm | Amine group | RT | TNT | 1 ppb | 4-nitrophenol 1,3-dinitrobenzene 2-nitrochlorobenzene | [173] |
| 2018 | TD | W: 150 nm | Pt NPs + POFs | RT | Methanol | No Data | Ethanol, Isopropanol, Acetaldehyde | [178] | |
| 2018 | TD | W: 75 nm L: 2 µm | Poly-SiNWs | RT | NH3, Ethanol | 1–100 ppm | O2,H2, CH4, CO2, H2O | [182] | |
| 2016 | BU | D: 40 ± 8 nm L: 8.5 ± 1.5 μm | Different modification | RT | VOC | Different for every gas | Different VOCs; selectivity depends on learning process and used type of signal | [28] | |
| 2015 | BU | D: 40 ± 8 nm L: 8.5 ± 1.5 μm | Propyl, Propenyl, Propynyl | RT | VOC | Different for every gas | Different VOCs; selectivity depends on learning process and used type of signal | [170] | |
| 2015 | BU | D: 40 ± 8 nm L: 8.5 ± 1.5 μm | Different modification | RT | Cancer (VOC) | Different for every gas | Different VOCs; selectivity depends on learning process and used type of signal | [171] | |
| 2014 | BU | D: 40 ± 8 nm L: 8.5 ± 1.5 μm | Different modification | RT | VOC | Different for every gas | Different VOCs; selectivity depends on learning process and used type of signal | [169] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbari-Saatlu, M.; Procek, M.; Mattsson, C.; Thungström, G.; Nilsson, H.-E.; Xiong, W.; Xu, B.; Li, Y.; Radamson, H.H. Silicon Nanowires for Gas Sensing: A Review. Nanomaterials 2020, 10, 2215. https://doi.org/10.3390/nano10112215
Akbari-Saatlu M, Procek M, Mattsson C, Thungström G, Nilsson H-E, Xiong W, Xu B, Li Y, Radamson HH. Silicon Nanowires for Gas Sensing: A Review. Nanomaterials. 2020; 10(11):2215. https://doi.org/10.3390/nano10112215
Chicago/Turabian StyleAkbari-Saatlu, Mehdi, Marcin Procek, Claes Mattsson, Göran Thungström, Hans-Erik Nilsson, Wenjuan Xiong, Buqing Xu, You Li, and Henry H. Radamson. 2020. "Silicon Nanowires for Gas Sensing: A Review" Nanomaterials 10, no. 11: 2215. https://doi.org/10.3390/nano10112215





