Highly Efficient Antimicrobial Activity of CuxFeyOz Nanoparticles against Important Human Pathogens
Abstract
:1. Introduction
2. Materials and Methods
3. Results
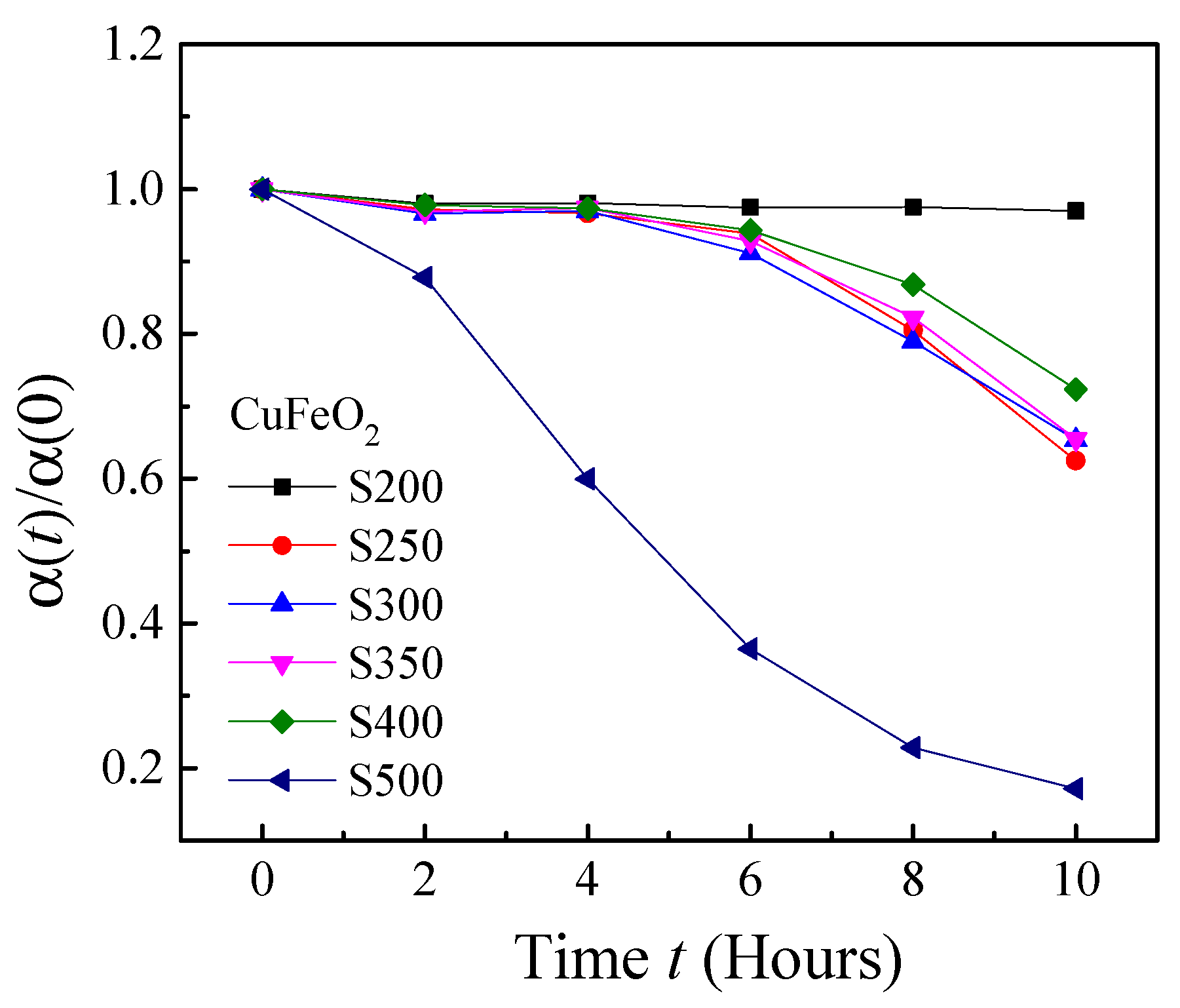
3.1. Synthesis and Characterizations of CuxFeyOz NPs
3.2. Antimicrobal Characterizations of S500
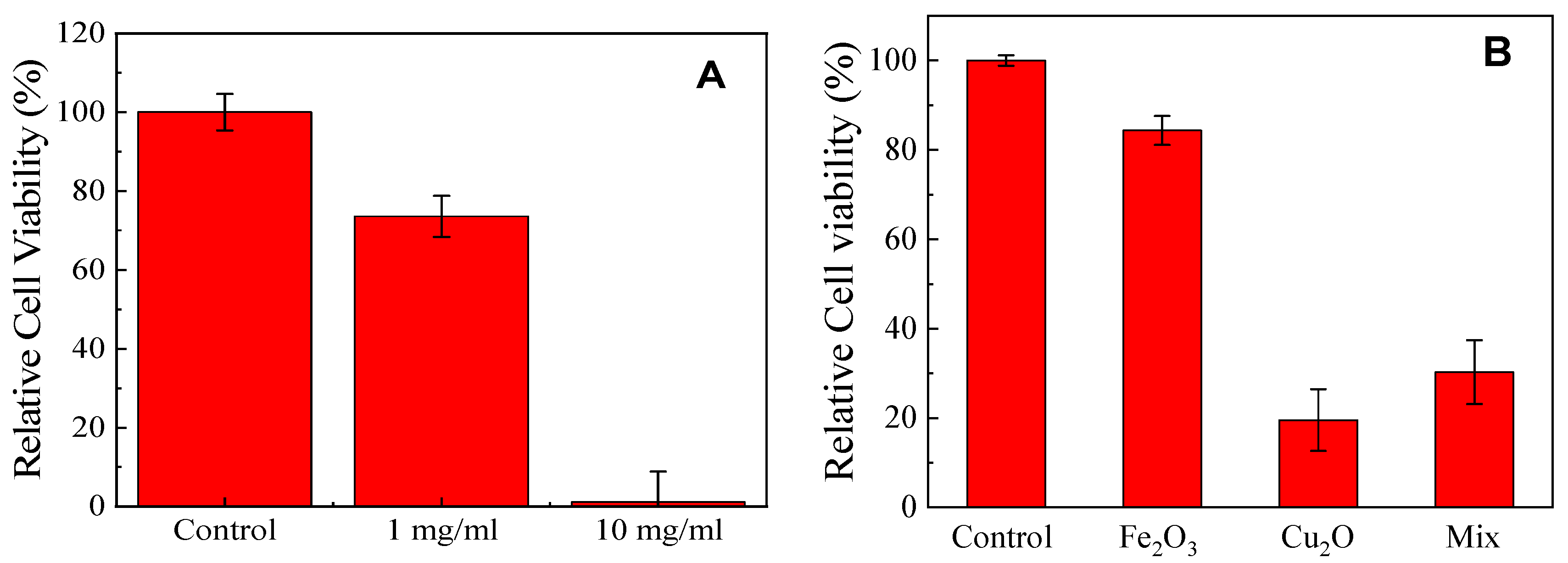
3.3. Cytotoxicity of the CuxFeyOz NP Sample S500
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fauci, A.S. Infectious diseases: Considerations for the 21st century. Clin. Infect. Dis. 2001, 32, 675–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. The World Health Report 2000: Health Systems: Improving Performance; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Pinner, R.W.; Teutsch, S.M.; Simonsen, L.; Klug, L.A.; Graber, J.M.; Clarke, M.J.; Berkelman, R.L. Trends in infectious diseases mortality in the United States. Jama 1996, 275, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [Green Version]
- Dwyer-Lindgren, L.; Bertozzi-Villa, A.; Stubbs, R.W.; Morozoff, C.; Kutz, M.J.; Huynh, C.; Barber, R.M.; Shackelford, K.A.; Mackenbach, J.P.; Van Lenthe, F.J. US county-level trends in mortality rates for major causes of death, 19802–014. Jama 2016, 316, 2385–2401. [Google Scholar] [CrossRef] [Green Version]
- CDC. Outbreak of Listeria Infections Linked to Deli Ham. Available online: https://www.cdc.gov/listeria/outbreaks/countryham-10-18/index.html (accessed on 26 May 2020).
- CDC. Outbreak of Salmonella Infections Linked to Ground Beef. Available online: https://www.cdc.gov/salmonella/newport-10-18/index.html (accessed on 26 May 2020).
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [Green Version]
- Furno, F.; Morley, K.S.; Wong, B.; Sharp, B.L.; Arnold, P.L.; Howdle, S.M.; Bayston, R.; Brown, P.D.; Winship, P.D.; Reid, H.J. Silver nanoparticles and polymeric medical devices: A new approach to prevention of infection? J. Antimicrob. Chemother. 2004, 54, 1019–1024. [Google Scholar] [CrossRef] [Green Version]
- Marambio-Jones, C.; Hoek, E.M. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Hassan, I.A.; Parkin, I.P.; Nair, S.P.; Carmalt, C.J. Antimicrobial activity of copper and copper (I) oxide thin films deposited via aerosol-assisted CVD. J. Mater. Chem. B 2014, 2, 2855–2860. [Google Scholar] [CrossRef]
- Zhu, L.; Basnet, P.; Larson, S.R.; Jones, L.P.; Howe, J.Y.; Tripp, R.A.; Zhao, Y. Visible Light-Induced Photoeletrochemical and Antimicrobial Properties of Hierarchical CuBi2O4 by Facile Hydrothermal Synthesis. ChemistrySelect 2016, 1, 1518–1524. [Google Scholar] [CrossRef]
- Meziani, M.J.; Dong, X.; Zhu, L.; Jones, L.P.; LeCroy, G.E.; Yang, F.; Wang, S.; Wang, P.; Zhao, Y.; Yang, L. Visible-light-activated bactericidal functions of carbon “Quantum” dots. ACS Appl. Mater. Interfaces 2016, 8, 10761–10766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abo-Zeid, Y.; Williams, G.R. The potential anti-infective applications of metal oxide nanoparticles: A systematic review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1592. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria-"A Battle of the Titans". Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Huang, G.; Yu, J.C.; Wong, P.K. Advances in photocatalytic disinfection of bacteria: Development of photocatalysts and mechanisms. J. Environ. Sci. 2015, 34, 232–247. [Google Scholar] [CrossRef]
- Nararom, M.; Thepa, S.; Kongkiattikajorn, J.; Songprakorp, R. Disinfection of water containing Escherichia coli by use of a compound parabolic concentrator: Effect of global solar radiation and reactor surface treatment. Res. Chem. Intermed. 2015, 41, 6543–6558. [Google Scholar] [CrossRef]
- Marugan, J.; van Grieken, R.; Pablos, C.; Lucila Satuf, M.; Cassano, A.E.; Alfano, O.M. Photocatalytic inactivation of Escherichia coli aqueous suspensions in a fixed-bed reactor. Catal. Today 2015, 252, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Markowska-Szczupak, A.; Ulfig, K.; Morawski, A.W. The application of titanium dioxide for deactivation of bioparticulates: An overview. Catal. Today 2011, 169, 249–257. [Google Scholar] [CrossRef]
- Liou, J.-W.; Chang, H.-H. Bactericidal Effects and Mechanisms of Visible Light-Responsive Titanium Dioxide Photocatalysts on Pathogenic Bacteria. Arch. Immunol. Et Ther. Exp. 2012, 60, 267–275. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef]
- Janpetch, N.; Vanichvattanadecha, C.; Rujiravanit, R. Photocatalytic disinfection of water by bacterial cellulose/N-F co-doped TiO2 under fluorescent light. Cellulose 2015, 22, 3321–3335. [Google Scholar] [CrossRef]
- Bogdan, J.; Zarzynska, J.; Plawinska-Czarnak, J. Comparison of Infectious Agents Susceptibility to Photocatalytic Effects of Nanosized Titanium and Zinc Oxides: A Practical Approach. Nanoscale Res. Lett. 2015, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, M.T.; Alazba, A.A. A review of nanomaterials based membranes for removal of contaminants from polluted waters. Membr. Water Treat. 2014, 5, 123–146. [Google Scholar] [CrossRef]
- Hossain, F.; Perales-Perez, O.J.; Hwang, S.; Roman, F. Antimicrobial nanomaterials as water disinfectant: Applications, limitations and future perspectives. Sci. Total Environ. 2014, 466, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Larsen, G.K.; Jadeja, R.P.; Hung, Y.-C.; Zhao, Y. α-Fe2O3 Nanocolumns and Nanorods Fabricated by Electron Beam Evaporation for Visible Light Photocatalytic and Antimicrobial Applications. ACS Appl. Mater. Interfaces 2013, 5, 2085–2095. [Google Scholar] [CrossRef]
- Ocsoy, I.; Paret, M.L.; Ocsoy, M.A.; Kunwar, S.; Chen, T.; You, M.; Tan, W. Nanotechnology in Plant Disease Management: DNA-Directed Silver Nanoparticles on Graphene Oxide as an Antibacterial against Xanthomonas perforans. ACS Nano 2013, 7, 8972–8980. [Google Scholar] [CrossRef] [Green Version]
- Ocsoy, I.; Temiz, M.; Celik, C.; Altinsoy, B.; Yilmaz, V.; Duman, F. A green approach for formation of silver nanoparticles on magnetic graphene oxide and highly effective antimicrobial activity and reusability. J. Mol. Liq. 2017, 227, 147–152. [Google Scholar] [CrossRef]
- Baldemir, A.; Köse, N.B.; Ildız, N.; İlgün, S.; Yusufbeyoğlu, S.; Yilmaz, V.; Ocsoy, I. Synthesis and characterization of green tea (Camellia sinensis (L.) Kuntze) extract and its major components-based nanoflowers: A new strategy to enhance antimicrobial activity. RSC Adv. 2017, 7, 44303–44308. [Google Scholar] [CrossRef] [Green Version]
- Celik, C.; Ildiz, N.; Ocsoy, I. Building block and rapid synthesis of catecholamines-inorganic nanoflowers with their peroxidase-mimicking and antimicrobial activities. Sci. Rep. 2020, 10, 2903. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Wang, W.; Sun, S.; Zhang, L.; Wang, L.; Chang, J. Crystallography facet-dependent antibacterial activity: The case of Cu2O. Ind. Eng. Chem. Res. 2011, 50, 10366–10369. [Google Scholar] [CrossRef]
- Applerot, G.; Lellouche, J.; Lipovsky, A.; Nitzan, Y.; Lubart, R.; Gedanken, A.; Banin, E. Understanding the antibacterial mechanism of CuO nanoparticles: Revealing the route of induced oxidative stress. Small 2012, 8, 3326–3337. [Google Scholar] [CrossRef] [PubMed]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.S.; Autrup, H. Toxicity of silver nanoparticles—Nanoparticle or silver ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Yu, X.; Xu, C.; Li, X.; Li, Z.; Wei, D.; Liu, Y. New Toxicity Mechanism of Silver Nanoparticles: Promoting Apoptosis and Inhibiting Proliferation. PLoS ONE 2015, 10, e0122535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munro, C.J.; Nguyen, M.A.; Falgons, C.; Chaudhry, S.; Olagunju, M.O.; Bode, A.; Bobé, C.; Portela, M.E.; Knecht, M.R.; Collins, K.M. Identification of toxicity effects of Cu2O materials on C. elegans as a function of environmental ionic composition. Environ. Sci. Nano 2020, 7, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Siddiqui, M.A.; Akhtar, M.J.; Ahmad, I.; Pant, A.B.; Alhadlaq, H.A. Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells. Biochem. Biophys. Res. Commun. 2010, 396, 578–583. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; De Rijk, E.; Bonetto, A.; Wohlleben, W.; Stone, V.; Brunelli, A.; Badetti, E.; Marcomini, A.; Gosens, I.; Cassee, F.R. Toxicity of copper oxide and basic copper carbonate nanoparticles after short-term oral exposure in rats. Nanotoxicology 2019, 13, 50–72. [Google Scholar] [CrossRef]
- Pegalajar-Jurado, A.; Wold, K.A.; Joslin, J.M.; Neufeld, B.H.; Arabea, K.A.; Suazo, L.A.; McDaniel, S.L.; Bowen, R.A.; Reynolds, M.M. Reprint of: Nitric oxide-releasing polysaccharide derivative exhibits 8-log reduction against Escherichia coli, Acinetobacter baumannii and Staphylococcus aureus. J. Control. Release 2015, 220, 617–623. [Google Scholar] [CrossRef]
- Jin, T.; Sun, D.; Su, J.; Zhang, H.; Sue, H.J. Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella enteritidis, and Escherichia coli O157: H7. J. Food Sci. 2009, 74, M46–M52. [Google Scholar] [CrossRef]
- Esteban-Cubillo, A.; Pecharromán, C.; Aguilar, E.; Santarén, J.; Moya, J.S. Antibacterial activity of copper monodispersed nanoparticles into sepiolite. J. Mater. Sci. 2006, 41, 5208–5212. [Google Scholar] [CrossRef]
- Baranwal, A.; Srivastava, A.; Kumar, P.; Bajpai, V.K.; Maurya, P.K.; Chandra, P. Prospects of Nanostructure Materials and Their Composites as Antimicrobial Agents. Front. Microbiol. 2018, 9, 422. [Google Scholar] [CrossRef] [Green Version]
- Tran, C.D.; Makuvaza, J.; Munson, E.; Bennett, B. Biocompatible Copper Oxide Nanoparticle Composites from Cellulose and Chitosan: Facile Synthesis, Unique Structure, and Antimicrobial Activity. ACS Appl. Mater. Interfaces 2017, 9, 42503–42515. [Google Scholar] [CrossRef] [Green Version]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Liu, M.; Sunada, K.; Miyauchi, M.; Hashimoto, K. A facile one-step hydrothermal synthesis of rhombohedral CuFeO2 crystals with antivirus property. Chem. Commun. 2012, 48, 7365–7367. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Kumaran, S.; Wu, H.-F. One-pot synthesis of CuFeO2 nanoparticles capped with glycerol and proteomic analysis of their nanocytotoxicity against fungi. RSC Adv. 2016, 6, 97629–97635. [Google Scholar] [CrossRef]
- Köferstein, R. Synthesis, phase evolution and properties of phase-pure nanocrystalline BiFeO3 prepared by a starch-based combustion method. J. Alloys Compd. 2014, 590, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Mahieux, P.Y.; Aubert, J.E.; Cyr, M.; Coutand, M.; Husson, B. Quantitative mineralogical composition of complex mineral wastes--contribution of the Rietveld method. Waste Manag. 2010, 30, 378–388. [Google Scholar] [CrossRef]
- Leon-Reina, L.; Garcia-Mate, M.; Alvarez-Pinazo, G.; Santacruz, I.; Vallcorba, O.; De la Torre, A.G.; Aranda, M.A. Accuracy in Rietveld quantitative phase analysis: A comparative study of strictly monochromatic Mo and Cu radiations. J. Appl. Crystallogr. 2016, 49, 722–735. [Google Scholar] [CrossRef]
- Shanmugam, C.; Sivasubramanian, G.; Parthasarathi, B.; Baskaran, K.; Balachander, R.; Parameswaran, V.R. Antimicrobial, free radical scavenging activities and catalytic oxidation of benzyl alcohol by nano-silver synthesized from the leaf extract of Aristolochia indica L.: A promenade towards sustainability. Appl. Nanosci. 2016, 6, 711–723. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.M.; Roy, A.S.; Mondal, P.; Mubarak, M.; Mondal, S.; Hossain, D.; Banerjee, S.; Santra, S.C. Synthesis, catalytic oxidation and antimicrobial activity of copper(II) Schiff base complex. J. Mol. Catal. A Chem. 2011, 336, 106–114. [Google Scholar] [CrossRef]
- Edison, T.J.I.; Sethuraman, M. Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Process Biochem. 2012, 47, 1351–1357. [Google Scholar] [CrossRef]
- Brown, G.T.; Darwent, J.R. Methyl orange as a probe for photooxidation reactions of colloidal titanium dioxide. J. Phys. Chem. 1984, 88, 4955–4959. [Google Scholar] [CrossRef]
- Giannousi, K.; Lafazanis, K.; Arvanitidis, J.; Pantazaki, A.; Dendrinou-Samara, C. Hydrothermal synthesis of copper based nanoparticles: Antimicrobial screening and interaction with DNA. J. Inorg. Biochem. 2014, 133, 24–32. [Google Scholar] [CrossRef]
- Meghana, S.; Kabra, P.; Chakraborty, S.; Padmavathy, N. Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv. 2015, 5, 12293–12299. [Google Scholar] [CrossRef]
- Antonoglou, O.; Lafazanis, K.; Mourdikoudis, S.; Vourlias, G.; Lialiaris, T.; Pantazaki, A.; Dendrinou-Samara, C. Biological relevance of CuFeO2 nanoparticles: Antibacterial and anti-inflammatory activity, genotoxicity, DNA and protein interactions. Mater. Sci. Eng. C 2019, 99, 264–274. [Google Scholar] [CrossRef]
- Bai, H.; Liu, Z.; Sun, D.D. Hierarchical ZnO/Cu “corn-like” materials with high photodegradation and antibacterial capability under visible light. Phys. Chem. Chem. Phys. 2011, 13, 6205–6210. [Google Scholar] [CrossRef]
- Bondarenko, O.; Ivask, A.; Käkinen, A.; Kahru, A. Sub-toxic effects of CuO nanoparticles on bacteria: Kinetics, role of Cu ions and possible mechanisms of action. Environ. Pollut. 2012, 169, 81–89. [Google Scholar] [CrossRef]
- Fan, W.; Wang, X.; Cui, M.; Zhang, D.; Zhang, Y.; Yu, T.; Guo, L. Differential oxidative stress of octahedral and cubic Cu2O micro/nanocrystals to Daphnia magna. Environ. Sci. Technol. 2012, 46, 10255–10262. [Google Scholar] [CrossRef]
- Von Moos, N.; Slaveykova, V.I. Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae–state of the art and knowledge gaps. Nanotoxicology 2014, 8, 605–630. [Google Scholar] [CrossRef]
- Wu, P.; Imlay, J.A.; Shang, J.K. Mechanism of Escherichia coli inactivation on palladium-modified nitrogen-doped titanium dioxide. Biomaterials 2010, 31, 7526–7533. [Google Scholar] [CrossRef] [Green Version]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Midander, K.; Cronholm, P.; Karlsson, H.L.; Elihn, K.; Möller, L.; Leygraf, C.; Wallinder, I.O. Surface characteristics, copper release, and toxicity of nano-and micrometer-sized copper and copper (II) oxide particles: A cross-disciplinary study. Small 2009, 5, 389–399. [Google Scholar] [CrossRef]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [Green Version]








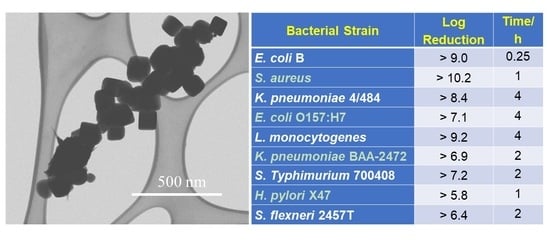
| Material | Bacterial Strain | Reduction | Test Time | Reference |
|---|---|---|---|---|
| Silver (Ag) | E. coli/S. aureus | 5 log | 0.5 h/1.5 h | [33] |
| Copper oxide (CuO) | E. coli | 3 log | 3 h | [35] |
| Cuprous oxide (Cu2O) | E. coli | 7 log | 5 min | [34] |
| Nitric oxide (NO) | E. coli/A. baumannii/S. aureus | 8 log | 24 h | [41] |
| Zinc oxide (ZnO) | E. coli | 6 log | 48 h | [42] |
| Copper (Cu) | E. coli/S. aureus | 4 log | 24 h | [43] |
| Bacteria Strain | Log Reduction | Time/h |
|---|---|---|
| E. coli B (ECB) (−) | >9.0 | 0.25 |
| S. aureus (SA) (+) | >10.2 | 1 |
| K. pneumoniae 4/484 (KP4/484) (−) | >8.4 | 4 |
| E. coli O157:H7 (EHEC) (−) | >7.1 | 4 |
| Listeria (LT) (+) | >9.2 | 4 |
| K. pneumoniae BAA-2472 (KP2472) (−) | >6.9 | 2 |
| S. Typhimurium 700408 (SET) (−) | >7.2 | 2 |
| H. pylori X47 (HP) (−) | >5.8 | 1 |
| S. flexneri 2457T (SF) (−) | >6.4 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Pearson, D.W.; Benoit, S.L.; Xie, J.; Pant, J.; Yang, Y.; Mondal, A.; Handa, H.; Howe, J.Y.; Hung, Y.-C.; et al. Highly Efficient Antimicrobial Activity of CuxFeyOz Nanoparticles against Important Human Pathogens. Nanomaterials 2020, 10, 2294. https://doi.org/10.3390/nano10112294
Zhu L, Pearson DW, Benoit SL, Xie J, Pant J, Yang Y, Mondal A, Handa H, Howe JY, Hung Y-C, et al. Highly Efficient Antimicrobial Activity of CuxFeyOz Nanoparticles against Important Human Pathogens. Nanomaterials. 2020; 10(11):2294. https://doi.org/10.3390/nano10112294
Chicago/Turabian StyleZhu, Lu, David W. Pearson, Stéphane L. Benoit, Jing Xie, Jitendra Pant, Yanjun Yang, Arnab Mondal, Hitesh Handa, Jane Y. Howe, Yen-Con Hung, and et al. 2020. "Highly Efficient Antimicrobial Activity of CuxFeyOz Nanoparticles against Important Human Pathogens" Nanomaterials 10, no. 11: 2294. https://doi.org/10.3390/nano10112294