Eu-Doped Citrate-Coated Carbonated Apatite Luminescent Nanoprobes for Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation and Characterization of Eu:cit-cAp Nanocarriers
2.3. Doxo Adsorption and Release Using Eu:cit-cAp Nanocarriers
2.4. Luminescence Spectroscopy
2.5. Cytotoxicity Tests
3. Results and Discussion
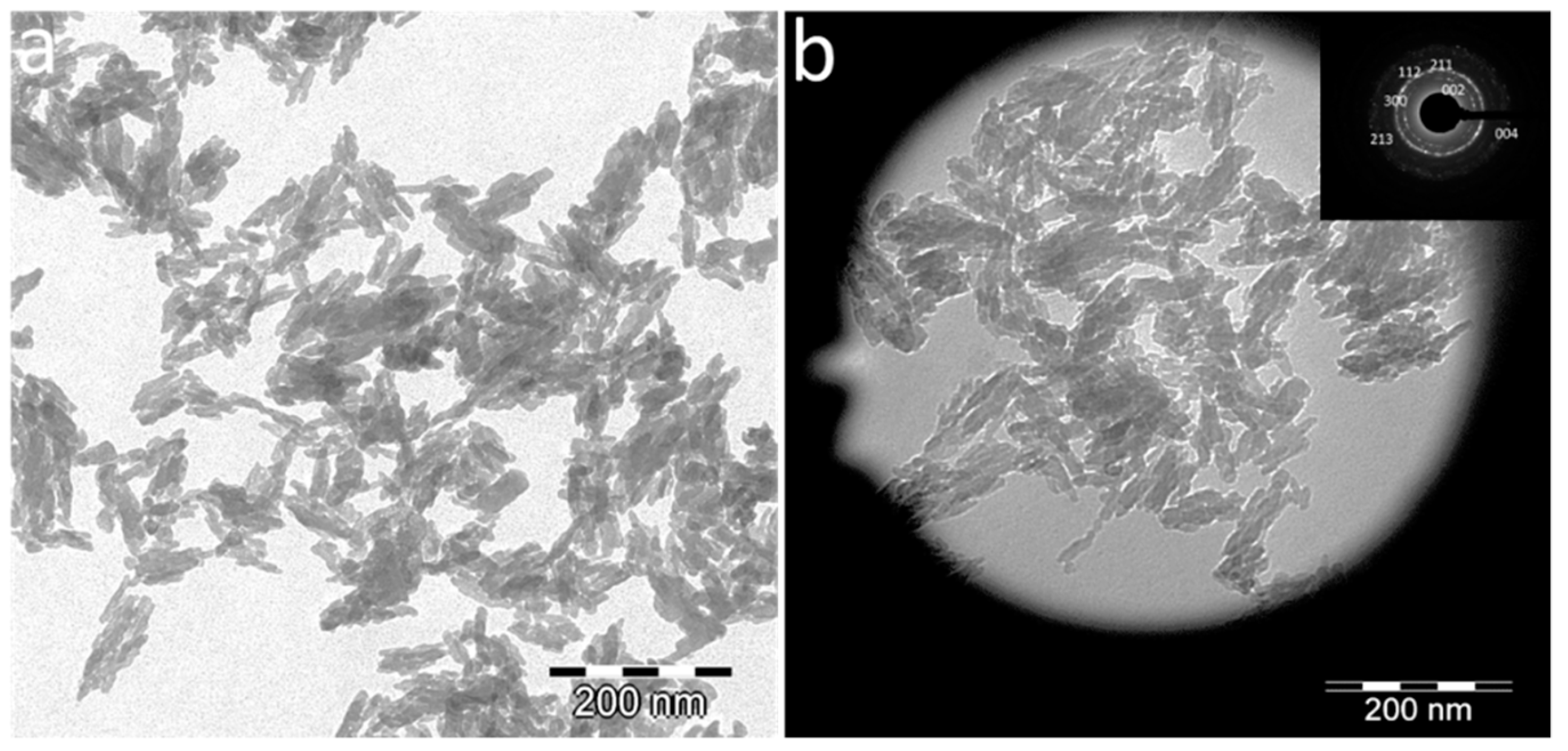
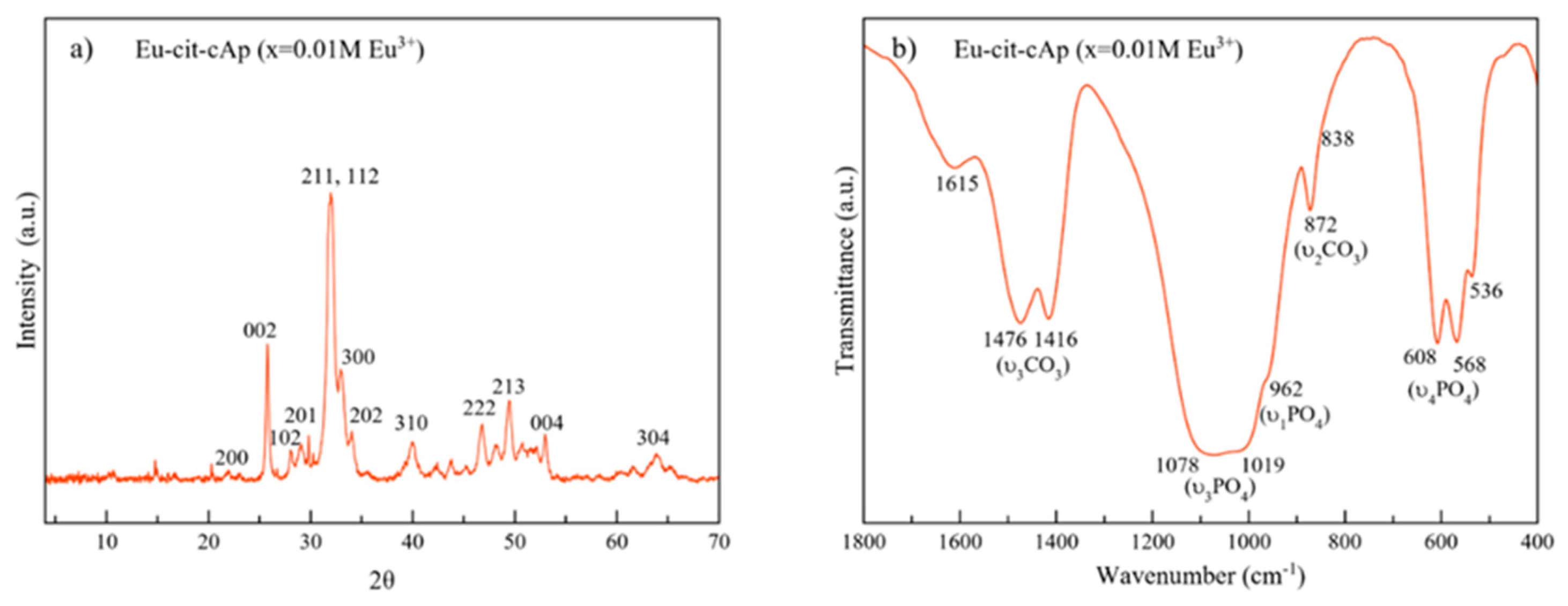
3.1. Physicochemical and Morphological Characteristics of Eu:cit-cAp Nanocarriers
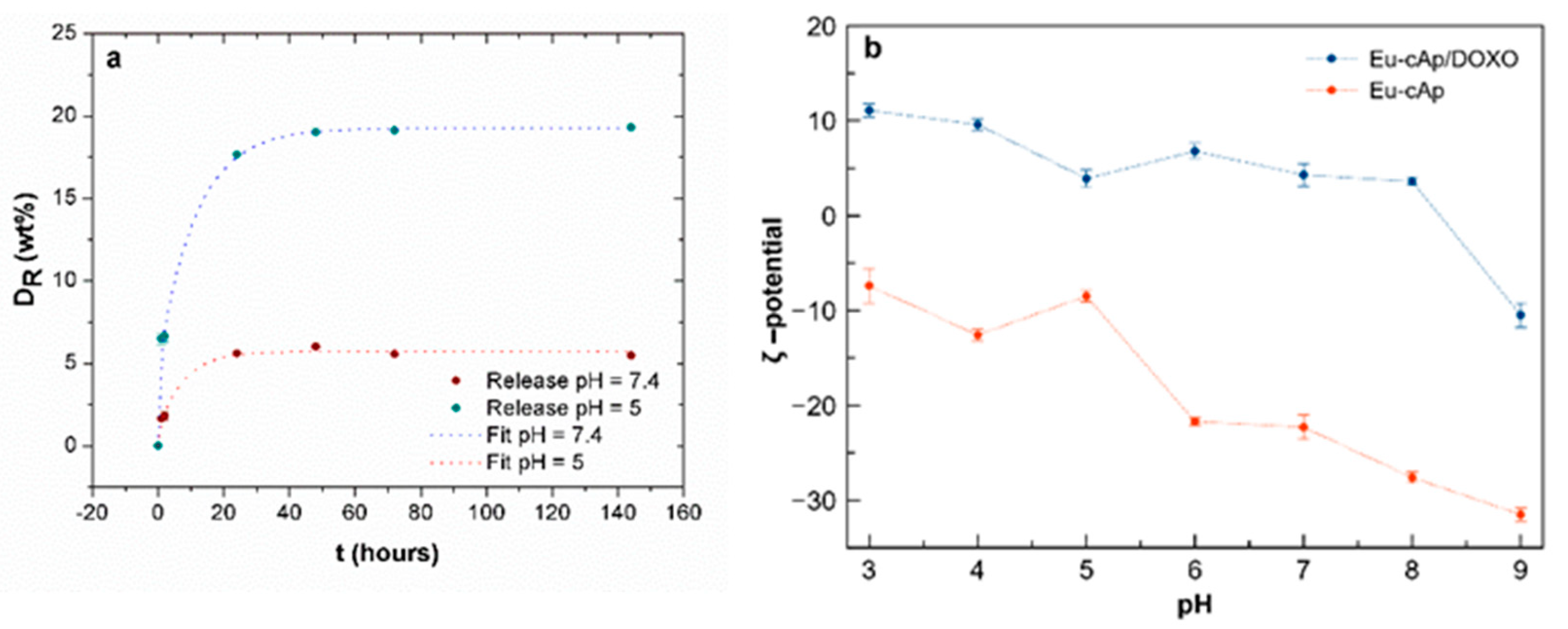
3.2. Doxo Adsorption and Release
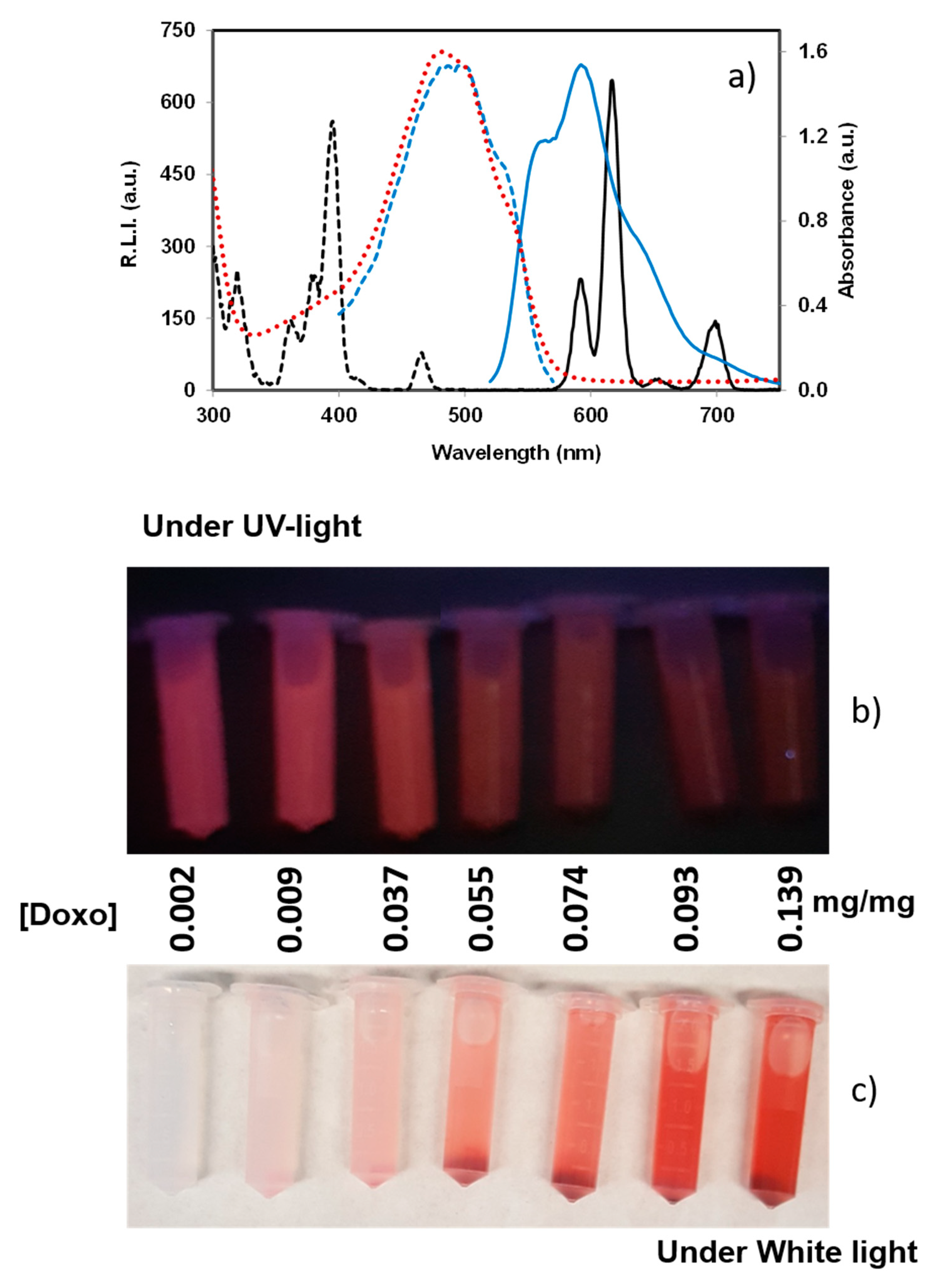
3.3. Luminescence Properties
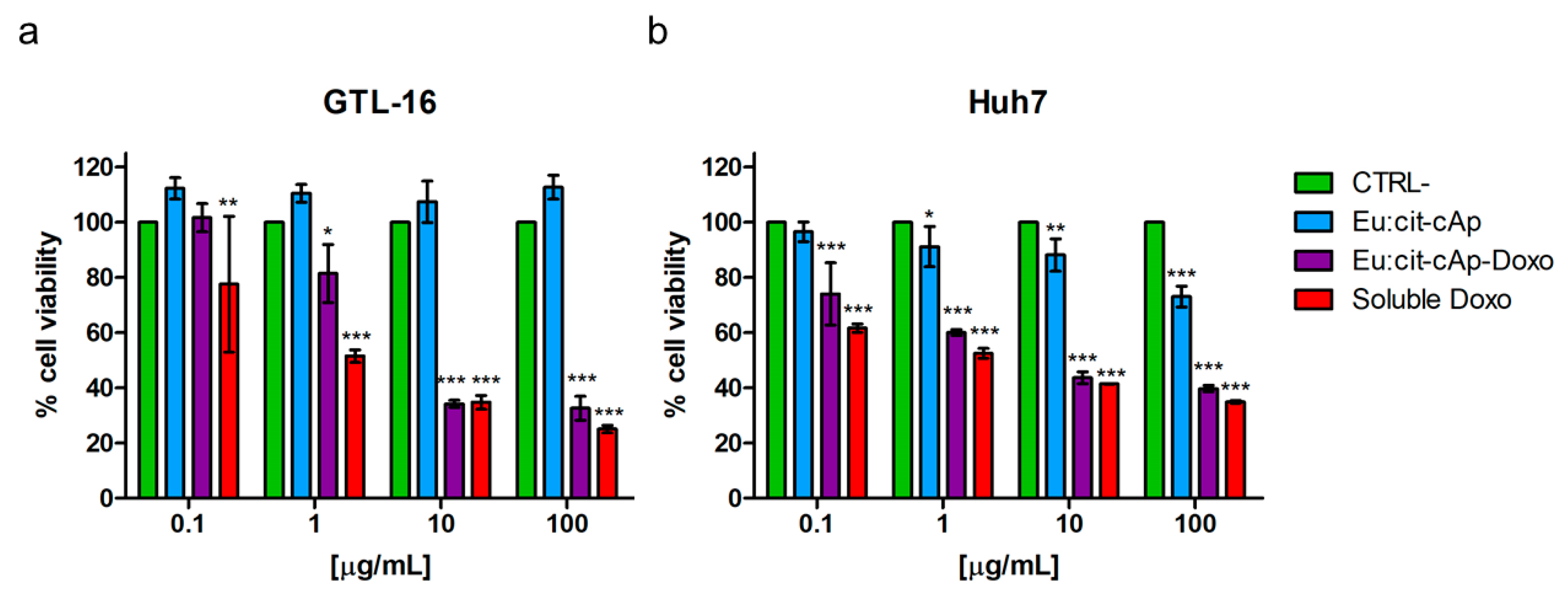
3.4. Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Srinivasan, M.; Rajabi, M.; Mousa, S.A. Multifunctional nanomaterials and their applications in drug delivery and cancer therapy. Nanomaterials 2015, 5, 1690–1703. [Google Scholar] [CrossRef]
- Yu, M.K.; Park, J.; Jon, S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Mattos, B.D.; Tardy, B.L.; Moody, V.M.; Xiao, G.; Ejima, H.; Cui, J.; Liang, K.; Richardson, J.J. Porous Inorganic and Hybrid Systems for Drug Delivery: Future Promise in Combatting Drug Resistance and Translation to Botanical Applications. Curr. Med. Chem. 2019, 26, 6107–6131. [Google Scholar] [CrossRef]
- Sreenivasan, V.K.A.; Zvyagin, A.V.; Goldys, E.M. Luminescent nanoparticles and their applications in the life sciences. J. Phys. Condens. Matter 2013, 25, 194101. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.-J. Quantum dots for fluorescent biosensing and bioimaging applications. Analyst 2013, 138, 2506–2515. [Google Scholar] [CrossRef]
- Fu, C.-C.; Lee, H.-Y.; Chen, K.; Lim, T.-S.; Wu, H.-Y.; Lin, P.-K.; Wei, P.K.; Tsao, P.H.; Chang, H.-C.; Fann, W. Characterization and application of single fluorescent nanodiamonds as cellular biomarkers. Proc. Natl. Acad. Sci. USA 2007, 104, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Wang, F.; Tan, W.B.; Zhang, Y.; Fan, X.P.; Wang, M.Q. Luminescent nanomaterials for biological labelling. Nanotechnology 2006, 17, R1–R13. [Google Scholar] [CrossRef]
- Oltolina, F.; Gregoletto, L.; Colangelo, D.; Gómez-Morales, J.; Delgado-López, J.M.; Prat, M. Monoclonal Antibody-Targeted Fluorescein-5-isothiocyanate-Labeled Biomimetic Nanoapatites: A Promising Fluorescent Probe for Imaging Applications. Langmuir 2015, 31, 1766–1775. [Google Scholar] [CrossRef]
- Zhao, Y.; Chang, C.; Gai, P.; Han, L.; Li, F.; Li, B. One-step synthesis of fluorescent organic nanoparticles: The applications to label –free ratiometric fluorescent pH sensor. Sens. Actuator B Chem. 2018, 273, 1479–1486. [Google Scholar] [CrossRef]
- Jaque, D.; Richard, C.; Viana, B.; Soga, K.; Liu, X.; Solé, J.G. Inorganic nanoparticles for optical bioimaging. Adv. Opt. Photonics 2016, 8, 1–103. [Google Scholar] [CrossRef]
- Svechkarev, D.; Mohs, A.M. Organic fluorescent dye-based nanomaterials: Advances in the rational design for imaging and sensing applications. Curr. Med. Chem. 2019, 26, 4042–4064. [Google Scholar] [CrossRef] [PubMed]
- Ranjbarvaziri, S.; Kiani, S.; Akhlaghi, A.; Vosough, A.; Baharvand, H.; Aghdami, N. Quantum dot labeling using positive charged peptides in human hematopoetic and mesenchymal stem cells. Biomaterials 2011, 32, 5195–5205. [Google Scholar] [CrossRef] [PubMed]
- Perera, T.S.H.; Han, Y.; Lu, X.; Wang, X.; Dai, H.; Li, S. Rare earth doped apatite nanomaterials for biological application. J. Nanomater. 2015, 2015, 705390. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez Burbano, D.C.; Sharma, S.K.; Dorenbos, P.; Viana, B.; Capobianco, J.A. Persistent and Photostimulated Red Emission in CaS:Eu2+,Dy3+ Nanophosphors. Adv. Opt. Mater. 2015, 3, 551–557. [Google Scholar] [CrossRef]
- Rosticher, C.; Viana, B.; Maldiney, T.; Richard, C.; Chanéac, C. Persistent luminescence of Eu, Mn, Dy doped calcium phosphates for in-vivo optical imaging. J. Lumin. 2016, 170, 460–466. [Google Scholar] [CrossRef]
- Maldiney, T.; Sraiki, G.; Viana, B.; Gourier, D.; Richard, C.; Scherman, D.; Bessodes, M.; Van den Eeckhout, K.; Poelman, D.; Smet, P.F. In vivo optical imaging with rare earth doped Ca2Si5N8 persistent luminescence nanoparticles. Opt. Mater. Express 2012, 2, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Gómez–Morales, J.; Iafisco, M.; Delgado–López, J.M.; Sarda, S.; Druet, C. Progress on the preparation of nanocrystalline apatites and surface characterization: Overview of fundamental and applied aspects. Prog. Cryst. Growth Charact. Mater. 2013, 59, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Iafisco, M.; Delgado-Lopez, J.M.; Varoni, E.M.; Tampieri, A.; Rimondini, L.; Gómez-Morales, J.; Prat, M. Cell surface receptor targeted biomimetic apatite nanocrystals for cancer therapy. Small 2013, 9, 3834–3844. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, I.; Delgado-López, J.M.; Duran-Olivencia, M.A.; Iafisco, M.; Tampieri, A.; Colangelo, D.; Prat, M.; Gomez-Morales, J. pH-responsive delivery of doxorubicin from citrate-apatite nanocrystals with tailored carbonate content. Langmuir 2013, 29, 8213–8221. [Google Scholar] [CrossRef]
- Victor, S.P.; Gayathri Devi, M.G.; Paul, W.; Vijayan, V.M.; Muthu, J.; Sharma, C.P. Europium doped calcium deficient hydroxyapatite as theranostic nanoplatforms: Effect of structure and aspect ratio. ACS Biomater. Sci. Eng. 2017, 3, 3588–3595. [Google Scholar] [CrossRef]
- Kalidoss, M.; Basha, R.Y.; Doble, M.; Sampath Kumar, T.S. Theranostic calcium phosphate nanoparticles with potential for multimodal imaging and drug delivery. Front. Bioeng. Biotechnol. 2019, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Maldiney, T.; Ballet, B.; Bessodes, M.; Schermana, D.; Richard, C. Mesoporous persistent nanophosphors for in vivo optical bioimaging and drug-delivery. Nanoscale 2014, 6, 13970–13976. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Lin, J.; Fu, L.H.; Huang, P. Calcium-based biomaterials for diagnosis, treatment, and theranostics. Chem. Soc. Rev. 2018, 47, 357–403. [Google Scholar] [CrossRef]
- Feiz, M.S.; Meshkini, A. Targeted delivery of adenosine 5’-triphosphate using chitosan-coated mesoporous hydroxyapatite: A theranostic pH-sensitive nanoplatform with enhanced anti-cancer effect. Int. J. Biol. Macromol. 2019, 129, 1090–1102. [Google Scholar] [CrossRef]
- Victor, S.P.; Paul, W.; Vineeth, V.M.; Komeri, R.; Jayabalan, M.; Sharma, C.P. Neodymium doped hydroxyapatite theranostic nanoplatforms for colon specific drug delivery applications. Colloids Surf. B Biointerfaces 2016, 145, 539–547. [Google Scholar] [CrossRef]
- Gómez-Morales, J.; Verdugo-Escamilla, C.; Fernández-Penas, R.; Parra-Milla, C.M.; Drouet, C.; Maube-Bosc, F.; Oltolina, F.; Prat, M.; Fernández-Sánchez, J.F. Luminescent biomimetic citrate-coated europium-doped carbonated apatite nanoparticles for use in bioimaging: Physico-chemistry and cytocompatibility. RSC Adv. 2018, 8, 2385–2397. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Morales, J.; Verdugo-Escamilla, C.; Fernández-Penas, R.; Parra-Milla, C.M.; Drouet, C.; Iafisco, M.; Oltolina, F.; Prat, M.; Fernández-Sánchez, J.F. Bioinspired crystallization, sensitized luminescence and cytocompatibility of citrate-functionalized Ca-substituted europium phosphate monohydrate nanophosphors. J. Colloid Interface Sci. 2019, 538, 174–186. [Google Scholar] [CrossRef] [Green Version]
- Delgado-López, J.M.; Iafisco, M.; Rodríguez, I.; Prat, M.; Gómez-Morales, J.; Tampieri, A. Crystallization of bioinspired citrate-functionalized nanoapatite with tailored carbonate content. Acta Biomater. 2012, 8, 3491–3499. [Google Scholar] [CrossRef]
- Martínez-Casado, F.J.; Iafisco, M.; Delgado-López, J.M.; Martínez-Benito, C.; Ruiz-Pérez, C.; Colangelo, D.; Oltolina, F.; Prat, M.; Gómez-Morales, J. Bioinspired citrate–apatite nanocrystals doped with divalent transition metal ions. Cryst. Growth Des. 2015, 16, 145–153. [Google Scholar] [CrossRef]
- Beretta, G.; Zunino, F. Molecular Mechanisms of Anthracycline Activity. In Anthracycline Chemistry and Biology II; Krohn, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 283, pp. 1–19. [Google Scholar]
- Kremer, L.C.M.; van Dalen, E.C.; Offringa, M.; Voute, P.A. Frequency and risk factors of anthracycline-induced clinical heart failure in children: A systematic review. Ann. Oncol. 2002, 13, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Longhi, A.; Ferrari, S.; Bacci, G.; Specchia, S. Long-term follow-up of patients with doxorubicin-induced cardiac toxicity after chemotherapy for osteosarcoma. Anti-cancer Drug 2007, 18, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Wood, J. Doxorubicin. In The Cytotoxics Handbook, 4th ed.; Allwood, A.S., Wright, P., Eds.; Radcliffe Medical Press, Ltd.: Oxon, UK, 2002; p. 8. [Google Scholar]
- Agrawal, P.; Barthwal, S.K.; Barthwal, R. Studies on self-aggregation of anthracycline drugs by restrained molecular dynamics approach using nuclear magnetic resonance spectroscopy supported by absorption, fluorescence, diffusion ordered spectroscopy and mass spectrometry. Eur. J. Med. Chem. 2009, 44, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- García Rubia, G.; Peigneux, A.; Jabalera, Y.; Puerma, J.; Oltolina, F.; Colangelo, D.; Gómez Morales, J.; Prat, M.; Jimenez-Lopez, C. pH-dependent adsorption-release of doxorubicin on MamC-biomimetic magnetite nanoparticles. Langmuir 2018, 34, 13713–13724. [Google Scholar] [CrossRef] [PubMed]
- Beijnen, J.H.; van der Houwen, O.A.G.J.; Underberg, W.J.M. Aspects of the degradation kinetics of doxorubicin in aqueous solution. Int. J. Pharm. 1986, 32, 123–131. [Google Scholar] [CrossRef]
- Turiel, E.; Perez-Conde, C.; Martin-Esteban, A. Assessment of the cross-reactivity and binding sites characterisation of a propazine-imprinted polymer using the Langmuir-Freundlich isotherm. Analyst 2003, 128, 137–141. [Google Scholar] [CrossRef]
- Langmuir, I. The adsoprtion of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Giordano, S.; Ponzetto, C.; Di Renzo, M.F.; Cooper, C.S.; Comoglio, P.M. Tyrosine kinase receptor indistinguishable from the c-met protein. Nature 1989, 339, 155–156. [Google Scholar] [CrossRef]
- Nakabayashi, H.; Taketa, K.; Miyano, K.; Yamane, T.; Sato, J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982, 42, 3858–3863. [Google Scholar]
- Vandecandelaere, N.; Rey, C.; Drouet, C. Biomimetic apatite-based biomaterials: On the critical impact of synthesis and post-synthesis parameters. J. Mater. Sci. Mater. Med. 2012, 23, 2593–2606. [Google Scholar] [CrossRef] [Green Version]
- Socrates, G. Infrared and Raman characteristic groups frequencies. In Tables and Charts, 3rd ed.; John Wiley and Sons, Ltd.: Chichester, UK, 2001. [Google Scholar]
- Sips, R. On the structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Rill, C.; Kolar, Z.I.; Kickelbick, G.; Wolterbeek, H.T.; Peters, J.A. Kinetics and thermodynamics of adsorption on hydroxyapatite of the [160 Tb]Terbium complexes of the bone-targeting ligands DOTP and BPPED. Langmuir 2009, 25, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Andrade, J.D. Cooperative adsorption of proteins onto hydroxyapatite. J. Colloid Interface Sci. 1998, 200, 104–113. [Google Scholar] [CrossRef]
- Iafisco, M.; Drouet, C.; Adamiano, A.; Pascaud, P.; Montesi, M.; Panseri, S.; Sarda, S.; Tampieri, A. Superparamagnetic iron-doped nanocrystalline apatite as a delivery system for doxorubicin. J. Mater. Chem. B 2016, 4, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Geisow, M.J.; Evans, W.H. pH in the endosome. Measurements during pinocytosis and receptor-mediated endocytosis. Exp. Cell Res. 1984, 150, 36–46. [Google Scholar] [CrossRef]
- Hemmilä, I.; Dakubu, S.; Mukkala, V.-M.; Siitaria, H.; Lövgrena, T. Europium as a label in time-resolved immunofluorometric assays. Anal. Biochem. 1984, 137, 335–343. [Google Scholar] [CrossRef]
- Richardson, F.S. Terbium(III) and europium(III) ions as luminescent probes and stains for biomolecular systems. Chem. Rev. 1982, 82, 541–552. [Google Scholar] [CrossRef]
- Zollfrank, C.; Scheel, H.; Brungs, S.; Greil, P. Europium(III) Orthophosphates: Synthesis, characterization, and optical Properties. Cryst. Growth Des. 2008, 8, 766–770. [Google Scholar] [CrossRef]
- Gauthler, T.D.; Shane, E.D.; Guerin, W.F.; Seltz, W.R.; Grant, C.L. Fluorescence quenching method for determining equilibrium constants for polycyclic aromatic hydrocarbons binding to dissolved humic materials. Environ. Sci. Technol. 1986, 20, 1162–1166. [Google Scholar] [CrossRef]
- Parker, C.A. Photoluminescence of Solutions; Elsevier Publishing, Co.: Amsterdam, The Netherlands; London, UK; New York, NY, USA, 1968. [Google Scholar]
- Lakowicz, J. Principles of Fluorescence Spectroscopy; Plenum: New York, NY, USA, 1983. [Google Scholar]
- Zhai, W.; Wang, C.; Yu, P.; Wang, Y.; Mao, L. Single-Layer MnO2 nanosheets suppressed fluorescence of 7-hydroxycoumarin: Mechanistic study and application for sensitive sensing of ascorbic acid in vivo. Anal. Chem. 2014, 86, 12206–12213. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, T.; Nie, Z.; Liu, Y.; Yao, S. Non-redox modulated fluorescence strategy for sensitive and selective ascorbic acid detection with highly photoluminescent nitrogen-doped carbon nanoparticles via solid-state synthesis. Anal. Chem. 2015, 87, 8524–8530. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Fang, A.; Wen, Y.; Li, H.; Zhang, Y.; Yao, S. Rapid and highly-sensitive uric acid sensing based on enzymatic catalysis-induced upconversion inner filter effect. Biosens. Bioelectron. 2016, 86, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liu, Y.; Kong, R.; Chen, G.; Wang, H.; Wang, X.; Xia, L.; Qu, F. Turn-on fluorescence detection of β-glucuronidase using RhB@MOF-5 as an ultrasensitive nanoprobe. Sens. Actuator B Chem. 2019, 295, 1–6. [Google Scholar] [CrossRef]
- ISO 10993-5:2009. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Available online: https://www.iso.org/standard/36406.html (accessed on 5 November 2018).
- Shi, M.; Ho, K.; Keating, A.; Shoichet, M.S. Doxorubicin-conjugated immuno-nanoparticles for intracellular anticancer drug delivery. Adv. Funct. Mater. 2009, 19, 1689–1696. [Google Scholar] [CrossRef]
- Padhye, S.S.; Guin, S.; Yao, H.-P.; Zhou, Y.-Q.; Zhang, R.; Wang, M.-H. Sustained expression of the RON receptor tyrosine kinase by pancreatic cancer stem cells as a potential targeting moiety for antibody-directed chemotherapeutics. Mol. Pharm. 2011, 8, 2310–2319. [Google Scholar] [CrossRef]
- Peigneux, A.; Oltolina, F.; Colangelo, D.; Iglesias, G.R.; Delgado, A.V.; Prat, M.; Jiménez-López, C. Functionalized biomimetic magnetic nanoparticles as effective nanocarriers for targeted chemotherapy. Part. Part. Syst. Charact. 2019, 36, 1900057. [Google Scholar] [CrossRef]
- Oltolina, F.; Colangelo, D.; Miletto, I.; Clemente, N.; Miola, M.; Verné, E.; Prat, M.; Follenzi, A. Tumor targeting by monoclonal antibody functionalized magnetic nanoparticles. Nanomaterials 2019, 9, 1575. [Google Scholar] [CrossRef] [Green Version]
- Manzoor, A.A.; Lindner, L.H.; Landon, C.D.; Park, J.-Y.; Simnick, A.J.; Dreher, M.R.; Das, S.; Hanna, G.; Park, W.; Chilkoti, A.; et al. Overcoming limitations in nanoparticle drug delivery: Triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012, 72, 5566–5575. [Google Scholar] [CrossRef] [Green Version]




| Parameter | Doxo/Eu:cit-cAp | R2 |
|---|---|---|
| KLF [mL mg−1] | 44 ± 2 | |
| Qmax [mg Doxo mg Eu:cit-cAp−1] | 0.28 ± 0.02 | 0.94597 |
| r | 6 ± 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabalera, Y.; Oltolina, F.; Prat, M.; Jimenez-Lopez, C.; Fernández-Sánchez, J.F.; Choquesillo-Lazarte, D.; Gómez-Morales, J. Eu-Doped Citrate-Coated Carbonated Apatite Luminescent Nanoprobes for Drug Delivery. Nanomaterials 2020, 10, 199. https://doi.org/10.3390/nano10020199
Jabalera Y, Oltolina F, Prat M, Jimenez-Lopez C, Fernández-Sánchez JF, Choquesillo-Lazarte D, Gómez-Morales J. Eu-Doped Citrate-Coated Carbonated Apatite Luminescent Nanoprobes for Drug Delivery. Nanomaterials. 2020; 10(2):199. https://doi.org/10.3390/nano10020199
Chicago/Turabian StyleJabalera, Ylenia, Francesca Oltolina, Maria Prat, Concepcion Jimenez-Lopez, Jorge F. Fernández-Sánchez, Duane Choquesillo-Lazarte, and Jaime Gómez-Morales. 2020. "Eu-Doped Citrate-Coated Carbonated Apatite Luminescent Nanoprobes for Drug Delivery" Nanomaterials 10, no. 2: 199. https://doi.org/10.3390/nano10020199







