Systemically Administered, Target-Specific, Multi-Functional Therapeutic Recombinant Proteins in Regenerative Medicine
Abstract
1. Drug Delivery in Regenerative Medicine
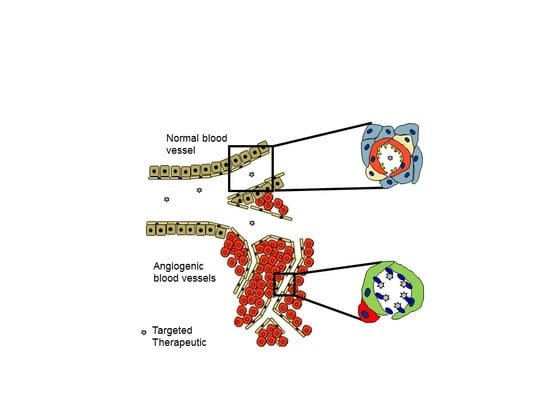
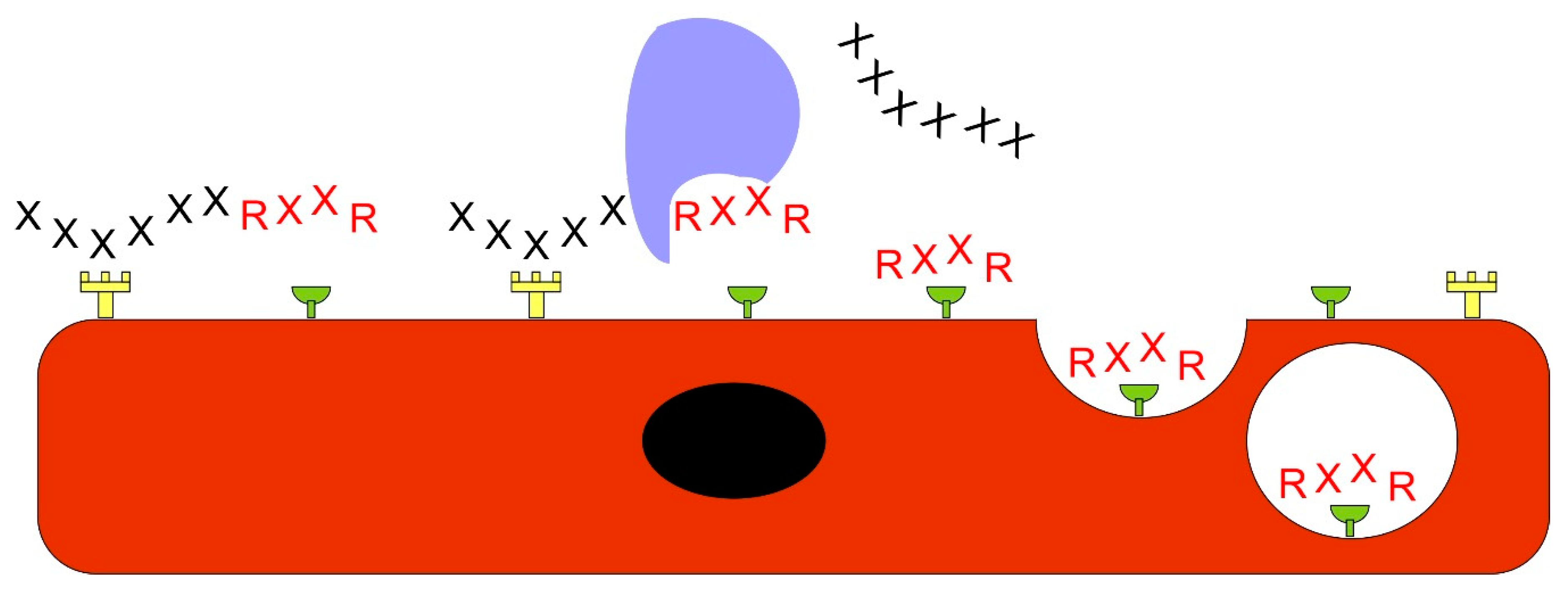
Vascular Heterogeneity-Zip Code-System in Vasculature Enables Tissue-Specific Drug Delivery
Ligands Targeting Injured Tissues
Systemically Administered Anti-Fibrotic Molecule, Car-Decorin
Bystander Effect–A Novel Approach to Drug Delivery
Targeted Nanoparticles And Stem Cells for Regenerative Medicine
2. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAA | Abdominal aorta aneurysm |
| BMP-4 | Bone morphogenetic protein-4 |
| BGN | Biglycan |
| CDR | Complementary determining regions |
| CendR | C-end rule |
| CTGF/CCN2 | Connective tissue growth factor |
| DCN | Decorin |
| ECM | Extracellular matrix |
| EGFR | Epidermal growth factor receptor |
| ERBB | Epidermal growth factor family receptor |
| FGF | Fibroblast growth factor |
| GAG | Glycosaminoglycan |
| HIF-1α | Hypoxia inducible factor-1α |
| HSPG | Heparan sulphate proteoglycan |
| IFNγ | Interferon-γ |
| IGF2 | Insulin-like growth factor 2 |
| KO | Knockout |
| NRP-1 | Neuropilin-1 |
| PAH | Pulmonary arterial hypertension |
| PDGF | Platelet derived growth factor |
| PLGF | Placental growth factor |
| SLRP | Small leucine-rich proteoglycan |
| TGF-β | Transforming growth factor-β |
| TNFα | Tumor necrosis factor α |
| VEGF | Vascular endothelial growth factor |
References
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.M.; Briquez, P.S.; Maruyama, K.; Hubbell, J.A. Extracellular matrix-inspired growth factor delivery systems for bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Geer, D.J.; Swartz, D.D.; Andreadis, S.T. Biomimetic delivery of keratinocyte growth factor upon cellular demand for accelerated wound healing in vitro and in vivo. Am. J. Pathol. 2005, 167, 1575–1586. [Google Scholar] [CrossRef]
- Zhao, Y.; Shimizu, T.; Nishihira, J.; Koyama, Y.; Kushibiki, T.; Honda, A.; Watanabe, H.; Abe, R.; Tabata, Y.; Shimizu, H. Tissue regeneration using macrophage migration inhibitory factor-impregnated gelatin microbeads in cutaneous wounds. Am. J. Pathol. 2005, 167, 1519–1529. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Briquez, P.S.; Hubbell, J.A.; Cochran, J.R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016, 30, 1–12. [Google Scholar] [CrossRef]
- Martino, M.M.; Briquez, P.S.; Guc, E.; Tortelli, F.; Kilarski, W.W.; Metzger, S.; Rice, J.J.; Kuhn, G.A.; Muller, R.; Swartz, M.A.; et al. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science 2014, 343, 885–888. [Google Scholar] [CrossRef]
- Arap, W.; Pasqualini, R.; Ruoslahti, E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998, 279, 377–380. [Google Scholar] [CrossRef]
- Hussain, S.; Rodriguez-Fernandez, M.; Braun, G.B.; Doyle, F.J.; Ruoslahti, E. Quantity and accessibility for specific targeting of receptors in tumours. Sci. Rep. 2014, 4, 5232. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Rajotte, D. An address system in the vasculature of normal tissues and tumors. Annu. Rev. Immunol. 2000, 18, 813–827. [Google Scholar] [CrossRef] [PubMed]
- AlDeghaither, D.; Smaglo, B.G.; Weiner, L.M. Beyond peptides and mAbs--current status and future perspectives for biotherapeutics with novel constructs. J. Clin. Pharmacol. 2015, 55 (Suppl. 3), S4–S20. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 2010, 188, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Teesalu, T.; Sugahara, K.N.; Ruoslahti, E. Mapping of vascular ZIP codes by phage display. Methods Enzymol. 2012, 503, 35–56. [Google Scholar] [PubMed]
- Huang, R.; Ma, H.; Guo, Y.; Liu, S.; Kuang, Y.; Shao, K.; Li, J.; Liu, Y.; Han, L.; Huang, S.; et al. Angiopep-conjugated nanoparticles for targeted long-term gene therapy of Parkinson’s disease. Pharm. Res. 2013, 30, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.E.; Wasserman, M.A.; Kibbe, M.R. Targeted Nanotherapies for the Treatment of Surgical Diseases. Ann. Surg. 2016, 263, 900–907. [Google Scholar] [CrossRef]
- Dixon, J.E.; Osman, G.; Morris, G.E.; Markides, H.; Rotherham, M.; Bayoussef, Z.; El Haj, A.J.; Denning, C.; Shakesheff, K.M. Highly efficient delivery of functional cargoes by the synergistic effect of GAG binding motifs and cell-penetrating peptides. Proc. Natl. Acad. Sci. USA 2016, 113, E291–E299. [Google Scholar] [CrossRef]
- Järvinen, T.A.H.; Rashid, J.; May, U.; Valmari, T.; Ahsan, F. Systemic targeted delivery of multi-functional recombinant proteins and nanoparticles in regenerative medicine. ACS Biomater. Sci. Eng. 2017, 3, 1273–1282. [Google Scholar] [CrossRef]
- Ruoslahti, E. Tumor penetrating peptides for improved drug delivery. Adv. Drug Deliv. Rev. 2017, 110–111, 3–12. [Google Scholar] [CrossRef]
- Ruoslahti, E. Vascular zip codes in angiogenesis and metastasis. Biochem. Soc. Trans. 2004, 32, 397–402. [Google Scholar] [CrossRef]
- Pasqualini, R.; Ruoslahti, E. Organ targeting in vivo using phage display peptide libraries. Nature 1996, 380, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.; Aaltonen, M.; Pan, P.; Vähätupa, M.; Kaipiainen, P.; May, U.; Prince, S.; Uusitalo-Järvinen, H.; Waheed, A.; Pastorekova, S.; et al. Role of carbonic anhydrases in skin wound healing. Exp. Mol. Med. 2017, 49, e334. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.A.; Järvinen, T.L.; Kääriäinen, M.; Kalimo, H.; Järvinen, M. Muscle injuries: Biology and treatment. Am. J. Sports Med. 2005, 33, 745–764. [Google Scholar] [CrossRef]
- Lee, J.W.; Ko, J.; Ju, C.; Eltzschig, H.K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 2019, 51, 68. [Google Scholar] [CrossRef] [PubMed]
- Yeo, E.J. Hypoxia and aging. Exp. Mol. Med. 2019, 51, 67. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.A.; Ruoslahti, E. Molecular changes in the vasculature of injured tissues. Am. J. Pathol. 2007, 171, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Urakami, T.; Järvinen, T.A.; Toba, M.; Sawada, J.; Ambalavanan, N.; Mann, D.; McMurtry, I.; Oka, M.; Ruoslahti, E.; Komatsu, M. Peptide-directed highly selective targeting of pulmonary arterial hypertension. Am. J. Pathol. 2011, 178, 2489–2495. [Google Scholar] [CrossRef]
- Toba, M.; Alzoubi, A.; O’Neill, K.; Abe, K.; Urakami, T.; Komatsu, M.; Alvarez, D.; Järvinen, T.A.; Mann, D.; Ruoslahti, E.; et al. A novel vascular homing peptide strategy to selectively enhance pulmonary drug efficacy in pulmonary arterial hypertension. Am. J. Pathol. 2014, 184, 369–375. [Google Scholar] [CrossRef]
- Kean, T.A.H.J.; Duesler, L.; Young, R.G.; Dadabayev, A.; Olenyik, A.; Penn, M.; Wagner, J.; Fink, D.J.; Caplan, A.I.; Dennis, J.E. Development of a peptide-targeted, myocardial ischemia-homing, mesenchymal stem cell. J. Drug Target. 2012, 20, 23–32. [Google Scholar] [CrossRef]
- Shen, Y.; Russo, V.; Zeglinski, M.R.; Sellers, S.L.; Wu, Z.; Oram, C.; Santacruz, S.; Merkulova, Y.; Turner, C.; Tauh, K.; et al. Recombinant Decorin Fusion Protein Attenuates Murine Abdominal Aortic Aneurysm Formation and Rupture. Sci. Rep. 2017, 7, 15857. [Google Scholar] [CrossRef]
- Schneider, M.; Angele, P.; Järvinen, T.A.H.; Docheva, D. Rescue plan for Achilles: Therapeutics steering the fate and functions of stem cells in tendon wound healing. Adv. Drug Deliv. Rev. 2017, 129, 352–375. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, K.; Ishihara, J.; Mansurov, A.; Ishihara, A.; Raczy, M.M.; Yuba, E.; Hubbell, J.A. Targeting inflammatory sites through collagen affinity enhances the therapeutic efficacy of anti-inflammatory antibodies. Sci. Adv. 2019, 5, eaay1971. [Google Scholar] [CrossRef] [PubMed]
- Pilch, J.; Brown, D.M.; Komatsu, M.; Järvinen, T.A.; Yang, M.; Peters, D.; Hoffman, R.M.; Ruoslahti, E. Peptides selected for binding to clotted plasma accumulate in tumor stroma and wounds. Proc. Natl. Acad. Sci. USA 2006, 103, 2800–2804. [Google Scholar] [CrossRef] [PubMed]
- Yanez, C.O.; Morales, A.R.; Yue, X.; Urakami, T.; Komatsu, M.; Järvinen, T.A.; Belfield, K.D. Deep vascular imaging in wounds by two-photon fluorescence microscopy. PLoS ONE 2013, 8, e67559. [Google Scholar] [CrossRef]
- Morgan, C.E.; Dombrowski, A.W.; Rubert Perez, C.M.; Bahnson, E.S.; Tsihlis, N.D.; Jiang, W.; Jiang, Q.; Vercammen, J.M.; Prakash, V.S.; Pritts, T.A.; et al. Tissue-Factor Targeted Peptide Amphiphile Nanofibers as an Injectable Therapy To Control Hemorrhage. ACS Nano 2016, 10, 899–909. [Google Scholar] [CrossRef]
- Sellers, D.L.; Bergen, J.M.; Johnson, R.N.; Back, H.; Ravits, J.M.; Horner, P.J.; Pun, S.H. Targeted axonal import (TAxI) peptide delivers functional proteins into spinal cord motor neurons after peripheral administration. Proc. Natl. Acad. Sci. USA 2016, 113, 2514–2519. [Google Scholar] [CrossRef]
- Costantini, T.W.; Eliceiri, B.P.; Putnam, J.G.; Bansal, V.; Baird, A.; Coimbra, R. Intravenous phage display identifies peptide sequences that target the burn-injured intestine. Peptides 2012, 38, 94–99. [Google Scholar] [CrossRef]
- Costantini, T.W.; Putnam, J.G.; Sawada, R.; Baird, A.; Loomis, W.H.; Eliceiri, B.P.; Bansal, V.; Coimbra, R. Targeting the gut barrier: identification of a homing peptide sequence for delivery into the injured intestinal epithelial cell. Surgery 2009, 146, 206–212. [Google Scholar] [CrossRef]
- Mann, A.P.; Scodeller, P.; Hussain, S.; Joo, J.; Kwon, E.; Braun, G.B.; Molder, T.; She, Z.G.; Kotamraju, V.R.; Ranscht, B.; et al. A peptide for targeted, systemic delivery of imaging and therapeutic compounds into acute brain injuries. Nat. Commun. 2016, 7, 11980. [Google Scholar] [CrossRef]
- Joo, J.; Kwon, E.J.; Kang, J.; Skalak, M.; Anglin, E.J.; Mann, A.P.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Porous silicon-graphene oxide core-shell nanoparticles for targeted delivery of siRNA to the injured brain. Nanoscale Horiz. 2016, 1, 407–414. [Google Scholar] [CrossRef]
- Wang, Y.; Newman, M.R.; Ackun-Farmmer, M.; Baranello, M.P.; Sheu, T.A.H.J.; Puzas, J.E.; Benoit, D.S.W. Fracture-Targeted Delivery of beta-Catenin Agonists via Peptide-Functionalized Nanoparticles Augments Fracture Healing. ACS Nano 2017, 11, 9445–9458. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Guo, B.; Wu, H.; Shao, N.; Li, D.; Liu, J.; Dang, L.; Wang, C.; Li, H.; Li, S.; et al. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy. Nat. Med. 2015, 21, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Yu, X.; Carbone, E.J.; Nelson, C.; Kan, H.M.; Lo, K.W. Poly aspartic acid peptide-linked PLGA based nanoscale particles: potential for bone-targeting drug delivery applications. Int. J. Pharm. 2014, 475, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Joo, J.; Kang, J.; Kim, B.; Braun, G.B.; She, Z.G.; Kim, D.; Mann, A.P.; Molder, T.; Teesalu, T.; et al. Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy. Nat. Biomed. Eng. 2018, 2, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Whitney, M.A.; Crisp, J.L.; Nguyen, L.T.; Friedman, B.; Gross, L.A.; Steinbach, P.; Tsien, R.Y.; Nguyen, Q.T. Fluorescent peptides highlight peripheral nerves during surgery in mice. Nat. Biotechnol. 2011, 29, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, H.L.; Whitney, M.A.; Gross, L.A.; Friedman, B.; Adams, S.R.; Crisp, J.L.; Hussain, T.; Frei, A.P.; Novy, K.; Wollscheid, B.; et al. Laminin targeting of a peripheral nerve-highlighting peptide enables degenerated nerve visualization. Proc. Natl. Acad. Sci. USA 2016, 113, 12774–12779. [Google Scholar] [CrossRef]
- Järvinen, T.A.; Ruoslahti, E. Target-seeking antifibrotic compound enhances wound healing and suppresses scar formation in mice. Proc. Natl. Acad. Sci. USA 2010, 107, 21671–21676. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.A. Design of target-seeking antifibrotic compounds. Methods Enzymol. 2012, 509, 243–261. [Google Scholar]
- Krusius, T.; Ruoslahti, E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc. Natl. Acad. Sci. USA 1986, 83, 7683–7687. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Mann, D.M.; Ruoslahti, E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 1990, 346, 281–284. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Ruoslahti, E. Expression of human proteoglycan in Chinese hamster ovary cells inhibits cell proliferation. Nature 1988, 336, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Border, W.A.; Noble, N.A.; Yamamoto, T.; Harper, J.R.; Yamaguchi, Y.; Pierschbacher, M.D.; Ruoslahti, E. Natural inhibitor of transforming growth factor-β protects against scarring in experimental kidney disease. Nature 1992, 360, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.A.; Prince, S. Decorin: A Growth Factor Antagonist for Tumor Growth Inhibition. Biomed. Res. Int. 2015, 2015, 654765. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.A.H.; Ruoslahti, E. Generation of a multi-functional, target organ-specific, anti-fibrotic molecule by molecular engineering of the extracellular matrix protein, decorin. Br. J. Pharmacol. 2019, 176, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Sainio, A.O.; Järvelainen, H.T. Decorin-mediated oncosuppression - a potential future adjuvant therapy for human epithelial cancers. Br. J. Pharmacol. 2019, 176, 5–15. [Google Scholar] [CrossRef]
- Border, W.A.; Ruoslahti, E. Transforming growth factor-β in disease: the dark side of tissue repair. J. Clin. Invest. 1992, 90, 1–7. [Google Scholar] [CrossRef]
- Hildebrand, A.; Romaris, M.; Rasmussen, L.M.; Heinegard, D.; Twardzik, D.R.; Border, W.A.; Ruoslahti, E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem. J. 1994, 302 (Pt 2), 527–534. [Google Scholar] [CrossRef]
- Andrianifahanana, M.; Wilkes, M.C.; Gupta, S.K.; Rahimi, R.A.; Repellin, C.E.; Edens, M.; Wittenberger, J.; Yin, X.; Maidl, E.; Becker, J.; et al. Profibrotic TGFβ responses require the cooperative action of PDGF and ErbB receptor tyrosine kinases. FASEB J. 2013, 27, 4444–4454. [Google Scholar] [CrossRef]
- Vial, C.; Gutierrez, J.; Santander, C.; Cabrera, D.; Brandan, E. Decorin interacts with connective tissue growth factor (CTGF)/CCN2 by LRR12 inhibiting its biological activity. J. Biol. Chem. 2011, 286, 24242–24252. [Google Scholar] [CrossRef]
- Csordas, G.; Santra, M.; Reed, C.C.; Eichstetter, I.; McQuillan, D.J.; Gross, D.; Nugent, M.A.; Hajnoczky, G.; Iozzo, R.V. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J. Biol. Chem. 2000, 275, 32879–32887. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Moscatello, D.K.; McQuillan, D.J.; Eichstetter, I. Decorin is a biological ligand for the epidermal growth factor receptor. J. Biol. Chem. 1999, 274, 4489–4492. [Google Scholar] [CrossRef] [PubMed]
- Moscatello, D.K.; Santra, M.; Mann, D.M.; McQuillan, D.J.; Wong, A.J.; Iozzo, R.V. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J. Clin. Invest. 1998, 101, 406–412. [Google Scholar] [CrossRef]
- Miura, T.; Kishioka, Y.; Wakamatsu, J.; Hattori, A.; Hennebry, A.; Berry, C.J.; Sharma, M.; Kambadur, R.; Nishimura, T. Decorin binds myostatin and modulates its activity to muscle cells. Biochem. Biophys. Res. Commun. 2006, 340, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, Y.; Shen, W.; Qiao, C.; Ambrosio, F.; Lavasani, M.; Nozaki, M.; Branca, M.F.; Huard, J. Relationships between transforming growth factor-beta1, myostatin, and decorin: implications for skeletal muscle fibrosis. J. Biol. Chem. 2007, 282, 25852–25863. [Google Scholar] [CrossRef] [PubMed]
- Goldoni, S.; Seidler, D.G.; Heath, J.; Fassan, M.; Baffa, R.; Thakur, M.L.; Owens, R.T.; McQuillan, D.J.; Iozzo, R.V. An antimetastatic role for decorin in breast cancer. Am. J. Pathol. 2008, 173, 844–855. [Google Scholar] [CrossRef]
- Seidler, D.G.; Goldoni, S.; Agnew, C.; Cardi, C.; Thakur, M.L.; Owens, R.T.; McQuillan, D.J.; Iozzo, R.V. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J. Biol. Chem. 2006, 281, 26408–26418. [Google Scholar] [CrossRef]
- Ma, H.I.; Hueng, D.Y.; Shui, H.A.; Han, J.M.; Wang, C.H.; Lai, Y.H.; Cheng, S.Y.; Xiao, X.; Chen, M.T.; Yang, Y.P. Intratumoral decorin gene delivery by AAV vector inhibits brain glioblastomas and prolongs survival of animals by inducing cell differentiation. Int. J. Mol. Sci. 2014, 15, 4393–4414. [Google Scholar] [CrossRef]
- Fiedler, L.R.; Schonherr, E.; Waddington, R.; Niland, S.; Seidler, D.G.; Aeschlimann, D.; Eble, J.A. Decorin regulates endothelial cell motility on collagen I through activation of insulin-like growth factor I receptor and modulation of alpha2beta1 integrin activity. J. Biol. Chem. 2008, 283, 17406–17415. [Google Scholar] [CrossRef]
- Järvinen, T.A.; Ruoslahti, E. Targeted Antiscarring Therapy for Tissue Injuries. Adv. Wound. Care. (New Rochelle) 2013, 2, 50–54. [Google Scholar]
- Iwao, K.; Inatani, M.; Matsumoto, Y.; Ogata-Iwao, M.; Takihara, Y.; Irie, F.; Yamaguchi, Y.; Okinami, S.; Tanihara, H. Heparan sulfate deficiency leads to Peters anomaly in mice by disturbing neural crest TGF-β2 signaling. J. Clin. Invest. 2009, 119, 1997–2008. [Google Scholar] [CrossRef][Green Version]
- Rider, C.C.; Mulloy, B. Heparin, Heparan Sulphate and the TGF-beta Cytokine Superfamily. Molecules 2017, 22. [Google Scholar] [CrossRef]
- Korf-Klingebiel, M.; Reboll, M.R.; Grote, K.; Schleiner, H.; Wang, Y.; Wu, X.; Klede, S.; Mikhed, Y.; Bauersachs, J.; Klintschar, M.; et al. Heparan Sulfate-Editing Extracellular Sulfatases Enhance VEGF Bioavailability for Ischemic Heart Repair. Circ. Res. 2019, 125, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Guc, E.; Park, A.J.; Julier, Z.; Briquez, P.S.; Kuhn, G.A.; Muller, R.; Swartz, M.A.; Hubbell, J.A.; Martino, M.M. Growth factors with enhanced syndecan binding generate tonic signalling and promote tissue healing. Nat. Biomed. Eng. 2019. Available online: https://europepmc.org/article/med/31685999 (accessed on 28 January 2020). [CrossRef] [PubMed]
- Ishihara, J.; Ishihara, A.; Starke, R.D.; Peghaire, C.R.; Smith, K.E.; McKinnon, T.A.J.; Tabata, Y.; Sasaki, K.; White, M.J.V.; Fukunaga, K.; et al. The heparin binding domain of von Willebrand factor binds to growth factors and promotes angiogenesis in wound healing. Blood 2019, 133, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, J.; Ishihara, A.; Fukunaga, K.; Sasaki, K.; White, M.J.V.; Briquez, P.S.; Hubbell, J.A. Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat. Commun. 2018, 9, 2163. [Google Scholar] [CrossRef]
- Zhao, L.; Niu, J.; Lin, H.; Zhao, J.; Liu, Y.; Song, Z.; Xiang, C.; Wang, X.; Yang, Y.; Li, X.; et al. Paracrine-endocrine FGF chimeras as potent therapeutics for metabolic diseases. EBioMedicine 2019, 48, 462–477. [Google Scholar] [CrossRef]
- Flippo, K.H.; Potthoff, M.J. Chronicles of an FGF chimera: The odyssey continues. EBioMedicine 2019, 49, 15–16. [Google Scholar] [CrossRef]
- Schmid, A.S.; Neri, D. Advances in antibody engineering for rheumatic diseases. Nat. Rev. Rheumatol. 2019, 15, 197–207. [Google Scholar] [CrossRef]
- Yeow, Y.L.; Kotamraju, V.R.; Wang, X.; Chopra, M.; Azme, N.; Wu, J.; Schoep, T.D.; Delaney, D.S.; Feindel, K.; Li, J.; et al. Immune-mediated ECM depletion improves tumour perfusion and payload delivery. EMBO Mol. Med. 2019, e10923. [Google Scholar] [CrossRef]
- Sasaki, K.; Ishihara, J.; Ishihara, A.; Miura, R.; Mansurov, A.; Fukunaga, K.; Hubbell, J.A. Engineered collagen-binding serum albumin as a drug conjugate carrier for cancer therapy. Sci. Adv. 2019, 5, eaaw6081. [Google Scholar] [CrossRef]
- Schwager, K.; Kaspar, M.; Bootz, F.; Marcolongo, R.; Paresce, E.; Neri, D.; Trachsel, E. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res. Ther. 2009, 11, R142. [Google Scholar] [CrossRef] [PubMed]
- Gutbrodt, K.L.; Schliemann, C.; Giovannoni, L.; Frey, K.; Pabst, T.; Klapper, W.; Berdel, W.E.; Neri, D. Antibody-based delivery of interleukin-2 to neovasculature has potent activity against acute myeloid leukemia. Sci. Transl. Med. 2013, 5, 201ra118. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.R.; Yarovinsky, T.O.; Young, B.D.; Moraes, F.; Ross, T.D.; Ceneri, N.; Zhang, J.; Zhuang, Z.W.; Sinusas, A.J.; Pardi, R.; et al. Chemokine-coupled beta2 integrin-induced macrophage Rac2-Myosin IIA interaction regulates VEGF-A mRNA stability and arteriogenesis. J. Exp. Med. 2014, 211, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.; Doll, F.; Grun, K.; Richter, P.; Kose, N.; Ziffels, B.; Schubert, H.; Figulla, H.R.; Jung, C.; Gummert, J.; et al. Targeted delivery of interleukin-10 to chronic cardiac allograft rejection using a human antibody specific to the extra domain A of fibronectin. Int. J. Cardiol. 2015, 195, 311–322. [Google Scholar] [CrossRef]
- Huyghe, L.; Van Parys, A.; Cauwels, A.; Van Lint, S.; De Munter, S.; Bultinck, J.; Zabeau, L.; Hostens, J.; Goethals, A.; Vanderroost, N.; et al. Safe eradication of large established tumors using neovasculature-targeted tumor necrosis factor-based therapies. EMBO Mol. Med. 2020, e11223. [Google Scholar] [CrossRef]
- Kammertoens, T.; Kemna, J.; Leisegang, M. “Designer cytokines” targeting the tumor vasculature-think global and act local. EMBO Mol. Med. 2020. Epub ahead of print. Available online: https://www.embopress.org/doi/full/10.15252/emmm.201911801 (accessed on 28 January 2020).
- Johansson-Percival, A.; Li, Z.J.; Lakhiani, D.D.; He, B.; Wang, X.; Hamzah, J.; Ganss, R. Intratumoral LIGHT Restores Pericyte Contractile Properties and Vessel Integrity. Cell. Rep. 2015, 13, 2687–2698. [Google Scholar] [CrossRef]
- He, B.; Jabouille, A.; Steri, V.; Johansson-Percival, A.; Michael, I.P.; Kotamraju, V.R.; Junckerstorff, R.; Nowak, A.K.; Hamzah, J.; Lee, G.; et al. Vascular targeting of LIGHT normalizes blood vessels in primary brain cancer and induces intratumoural high endothelial venules. J. Pathol. 2018, 245, 209–221. [Google Scholar] [CrossRef]
- Järvinen, T.A. Neovascularisation in tendinopathy: from eradication to stabilisation? Br. J. Sports Med. 2020, 54, 1–2. [Google Scholar] [CrossRef]
- Lingasamy, P.; Tobi, A.; Haugas, M.; Hunt, H.; Paiste, P.; Asser, T.; Ratsep, T.; Kotamraju, V.R.; Bjerkvig, R.; Teesalu, T. Bi-specific tenascin-C and fibronectin targeted peptide for solid tumor delivery. Biomaterials 2019, 219, 119373. [Google Scholar] [CrossRef]
- Torrieri, G.; Fontana, F.; Figueiredo, P.; Liu, Z.; Ferreira, M.P.A.; Talman, V.; Martins, J.P.; Fusciello, M.; Moslova, K.; Teesalu, T.; et al. Dual-peptide functionalized acetalated dextran-based nanoparticles for sequential targeting of macrophages during myocardial infarction. Nanoscale 2020. Available online: https://pubs.rsc.org/en/content/articlelanding/2020/NR/C9NR09934D#!divAbstract (accessed on 28 January 2020). [CrossRef]
- Järvinen, T.A.; Kannus, P.; Järvinen, T.L.; Jozsa, L.; Kalimo, H.; Järvinen, M. Tenascin-C in the pathobiology and healing process of musculoskeletal tissue injury. Scand. J. Med. Sci. Sports 2000, 10, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Greenwald, D.R.; Ruoslahti, E. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science 2010, 328, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, P.; Perrett, I.; Lin, J.; Liao, Y.P.; Chang, C.H.; Jiang, J.; Wu, N.; Donahue, T.; Wainberg, Z.; et al. Tumor-penetrating peptide enhances transcytosis of silicasome-based chemotherapy for pancreatic cancer. J. Clin. Invest. 2017, 127, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.B.; Braun, G.B.; Friman, T.; Aza-Blanc, P.; Ruidiaz, M.E.; Sugahara, K.N.; Teesalu, T.; Ruoslahti, E. An endocytosis pathway initiated through neuropilin-1 and regulated by nutrient availability. Nat. Commun. 2014, 5, 4904. [Google Scholar] [CrossRef]
- Teesalu, T.; Sugahara, K.N.; Kotamraju, V.R.; Ruoslahti, E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA 2009, 106, 16157–16162. [Google Scholar] [CrossRef]
- Nel, A.; Ruoslahti, E.; Meng, H. New Insights into "Permeability" as in the Enhanced Permeability and Retention Effect of Cancer Nanotherapeutics. ACS Nano 2017, 11, 9567–9569. [Google Scholar] [CrossRef]
- Pang, H.B.; Braun, G.B.; Ruoslahti, E. Neuropilin-1 and heparan sulfate proteoglycans cooperate in cellular uptake of nanoparticles functionalized by cationic cell-penetrating peptides. Sci. Adv. 2015, 1, e1500821. [Google Scholar] [CrossRef]
- Oh, P.; Testa, J.E.; Borgstrom, P.; Witkiewicz, H.; Li, Y.; Schnitzer, J.E. In vivo proteomic imaging analysis of caveolae reveals pumping system to penetrate solid tumors. Nat. Med. 2014, 20, 1062–1068. [Google Scholar] [CrossRef]
- Oh, P.; Borgstrom, P.; Witkiewicz, H.; Li, Y.; Borgstrom, B.J.; Chrastina, A.; Iwata, K.; Zinn, K.R.; Baldwin, R.; Testa, J.E.; et al. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat. Biotechnol. 2007, 25, 327–337. [Google Scholar] [CrossRef]
- Gupta, V.; Gupta, N.; Shaik, I.H.; Mehvar, R.; Nozik-Grayck, E.; McMurtry, I.F.; Oka, M.; Komatsu, M.; Ahsan, F. Inhaled PLGA particles of prostaglandin E(1) ameliorate symptoms and progression of pulmonary hypertension at a reduced dosing frequency. Mol. Pharm. 2013, 10, 1655–1667. [Google Scholar] [CrossRef]
- Gupta, N.; Ibrahim, H.M.; Ahsan, F. Peptide-micelle hybrids containing fasudil for targeted delivery to the pulmonary arteries and arterioles to treat pulmonary arterial hypertension. J. Pharm. Sci. 2014, 103, 3743–3753. [Google Scholar] [CrossRef]
- Nahar, K.; Absar, S.; Gupta, N.; Kotamraju, V.R.; McMurtry, I.F.; Oka, M.; Komatsu, M.; Nozik-Grayck, E.; Ahsan, F. Peptide-coated liposomal fasudil enhances site specific vasodilation in pulmonary arterial hypertension. Mol. Pharm. 2014, 11, 4374–4384. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Al-Saikhan, F.I.; Patel, B.; Rashid, J.; Ahsan, F. Fasudil and SOD packaged in peptide-studded-liposomes: Properties, pharmacokinetics and ex-vivo targeting to isolated perfused rat lungs. Int. J. Pharm. 2015, 488, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Patel, B.; Nahar, K.; Ahsan, F. Cell permeable peptide conjugated nanoerythrosomes of fasudil prolong pulmonary arterial vasodilation in PAH rats. Eur. J. Pharm. Biopharm. 2014, 88, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, H.; Xu, H.; Zhao, Y.; Li, Z.; Li, J.; Wang, H.; Zhuge, D.; Guo, X.; Xu, H.; et al. Novel multi-drug delivery hydrogel using scar-homing liposomes improves spinal cord injury repair. Theranostics 2018, 8, 4429–4446. [Google Scholar] [CrossRef]
- Keshavarz, A.; Alobaida, A.; McMurtry, I.F.; Nozik-Grayck, E.; Stenmark, K.R.; Ahsan, F. CAR, a Homing Peptide, Prolongs Pulmonary Preferential Vasodilation by Increasing Pulmonary Retention and Reducing Systemic Absorption of Liposomal Fasudil. Mol. Pharm. 2019, 16, 3414–3429. [Google Scholar] [CrossRef]
- Rashid, J.; Nahar, K.; Raut, S.; Keshavarz, A.; Ahsan, F. Fasudil and DETA NONOate, Loaded in a Peptide-Modified Liposomal Carrier, Slow PAH Progression upon Pulmonary Delivery. Mol. Pharm. 2018, 15, 1755–1765. [Google Scholar] [CrossRef]
- Agemy, L.; Sugahara, K.N.; Kotamraju, V.R.; Gujraty, K.; Girard, O.M.; Kono, Y.; Mattrey, R.F.; Park, J.H.; Sailor, M.J.; Jimenez, A.I.; et al. Nanoparticle-induced vascular blockade in human prostate cancer. Blood 2010, 116, 2847–2856. [Google Scholar] [CrossRef]
- Gupta, N.; Rashid, J.; Nozik-Grayck, E.; McMurtry, I.F.; Stenmark, K.R.; Ahsan, F. Cocktail of Superoxide Dismutase and Fasudil Encapsulated in Targeted Liposomes Slows PAH Progression at a Reduced Dosing Frequency. Mol. Pharm. 2017, 14, 830–841. [Google Scholar] [CrossRef]
- Gupta, N.; Patel, B.; Ahsan, F. Nano-engineered erythrocyte ghosts as inhalational carriers for delivery of fasudil: preparation and characterization. Pharm. Res. 2014, 31, 1553–1565. [Google Scholar] [CrossRef]
- Kim, B.; Pang, H.B.; Kang, J.; Park, J.H.; Ruoslahti, E.; Sailor, M.J. Immunogene therapy with fusogenic nanoparticles modulates macrophage response to Staphylococcus aureus. Nat. Commun. 2018, 9, 1969-018-04390-7. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Ndifon, C.; Lui, S.; Widdows, K.; Kotamraju, V.R.; Agemy, L.; Teesalu, T.; Glazier, J.D.; Cellesi, F.; Tirelli, N.; et al. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta. Sci. Adv. 2016, 2, e1600349. [Google Scholar] [CrossRef] [PubMed]
- Cureton, N.; Korotkova, I.; Baker, B.; Greenwood, S.; Wareing, M.; Kotamraju, V.R.; Teesalu, T.; Cellesi, F.; Tirelli, N.; Ruoslahti, E.; et al. Selective Targeting of a Novel Vasodilator to the Uterine Vasculature to Treat Impaired Uteroplacental Perfusion in Pregnancy. Theranostics 2017, 7, 3715–3731. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.A.H.J.; Lin, P.; Caplan, A.I.; Dennis, J.E. MSCs: Delivery Routes and Engraftment, Cell-Targeting Strategies, and Immune Modulation. Stem Cells Int. 2013, 2013, 732742. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Jiang, X.C.; Zhang, T.Y.; Hu, Y.L.; Tabata, Y.; Chen, Z.; Pluchino, S.; Gao, J.Q. Peptide modified mesenchymal stem cells as targeting delivery system transfected with miR-133b for the treatment of cerebral ischemia. Int. J. Pharm. 2017, 531, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Zhao, Z.; Meng, Q.; Yu, Y.; Sun, J.; Yang, Z.; Chen, Y.; Li, J.; Ma, T.; et al. Engineered Exosomes With Ischemic Myocardium-Targeting Peptide for Targeted Therapy in Myocardial Infarction. J. Am. Heart Assoc. 2018, 7, e008737. [Google Scholar] [CrossRef]





| Peptide | Sequence | Target | Receptor | Therapeutic application | Reference |
|---|---|---|---|---|---|
| CAR | CARSKNKDC | angiogenesis (early state) and reactive vasculature in vast number of inflammatory diseases | heparan sulfate proteoglycans | recombinant proteins | [26,30,47] |
| stem cells | [29] | ||||
| unconjugated drug delivery (bystander effect) | [28] | ||||
| nanoparticles | [26,27,28,96,97,98,99,100,102,103,105,106] | ||||
| CARG | CARGGLKSC | Staphylococcus aureus infected tissue | nanoparticles | [44] | |
| c-RGDfK | CRGDFC | Angiogenesis | αvβ3 integrin | nanoparticles | [34] |
| CAQK | CAQK | acute brain injury | chondroitin sulfate | siRNA | [39,40] |
| CH6 | see ref. [40] | osteoblasts | lipid nanoparticle | [42] | |
| CLT1 | CGLIIQKNEC | blood clots | chloride intracellular channel 3 and integrin α5 (β1) | [33] | |
| CRK | CRKDKC | angiogenesis (late stage) | stem cells | [29] | |
| nanoparticles | [26,104] | ||||
| Np41 | NTQTLAKAPEHT | nerves | laminins α2, α4 | identification of nerves during surgery | [45,46] |
| poly-Asp | DDDDDDDC | bone | hydroxyapatite | nanoparticles | [43] |
| RTL | RTLAFVRFK | ruptured blood vessel | tissue factor (TF) | nanofibers | [35] |
| TAxI | SACQSQSQMRCGGG | transected nerves | recombinant proteins | [36] | |
| TBP | TPLSYLKGLVTVG | bone, fracture | tartrate-resistant acid phosphatase (TRAP) | nanoparticles | [41] |
| 4-11 | SFKPSGLPAQSL | burn-injured intestine | [37,38] | ||
| CBP | LRELHLNNNC | Inflammation | Collagen | recombinant proteins | [32] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Järvinen, T.A.H.; Pemmari, T. Systemically Administered, Target-Specific, Multi-Functional Therapeutic Recombinant Proteins in Regenerative Medicine. Nanomaterials 2020, 10, 226. https://doi.org/10.3390/nano10020226
Järvinen TAH, Pemmari T. Systemically Administered, Target-Specific, Multi-Functional Therapeutic Recombinant Proteins in Regenerative Medicine. Nanomaterials. 2020; 10(2):226. https://doi.org/10.3390/nano10020226
Chicago/Turabian StyleJärvinen, Tero A.H., and Toini Pemmari. 2020. "Systemically Administered, Target-Specific, Multi-Functional Therapeutic Recombinant Proteins in Regenerative Medicine" Nanomaterials 10, no. 2: 226. https://doi.org/10.3390/nano10020226
APA StyleJärvinen, T. A. H., & Pemmari, T. (2020). Systemically Administered, Target-Specific, Multi-Functional Therapeutic Recombinant Proteins in Regenerative Medicine. Nanomaterials, 10(2), 226. https://doi.org/10.3390/nano10020226