Effects of Amorphous Silica Nanopowders on the Avoidance Behavior of Five Soil Species—A Screening Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Test Organisms
2.2. Test Materials
2.3. Test Soil and Spiking
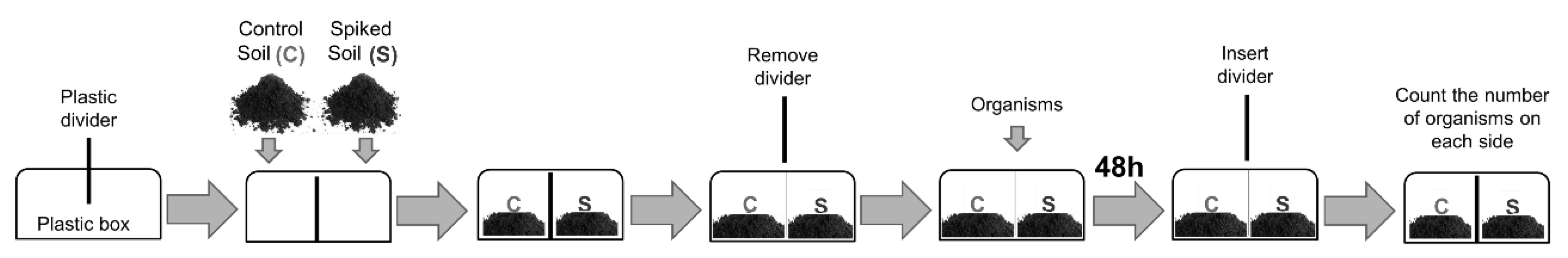
2.4. Avoidance Assays
2.5. Statistical Analysis
3. Results
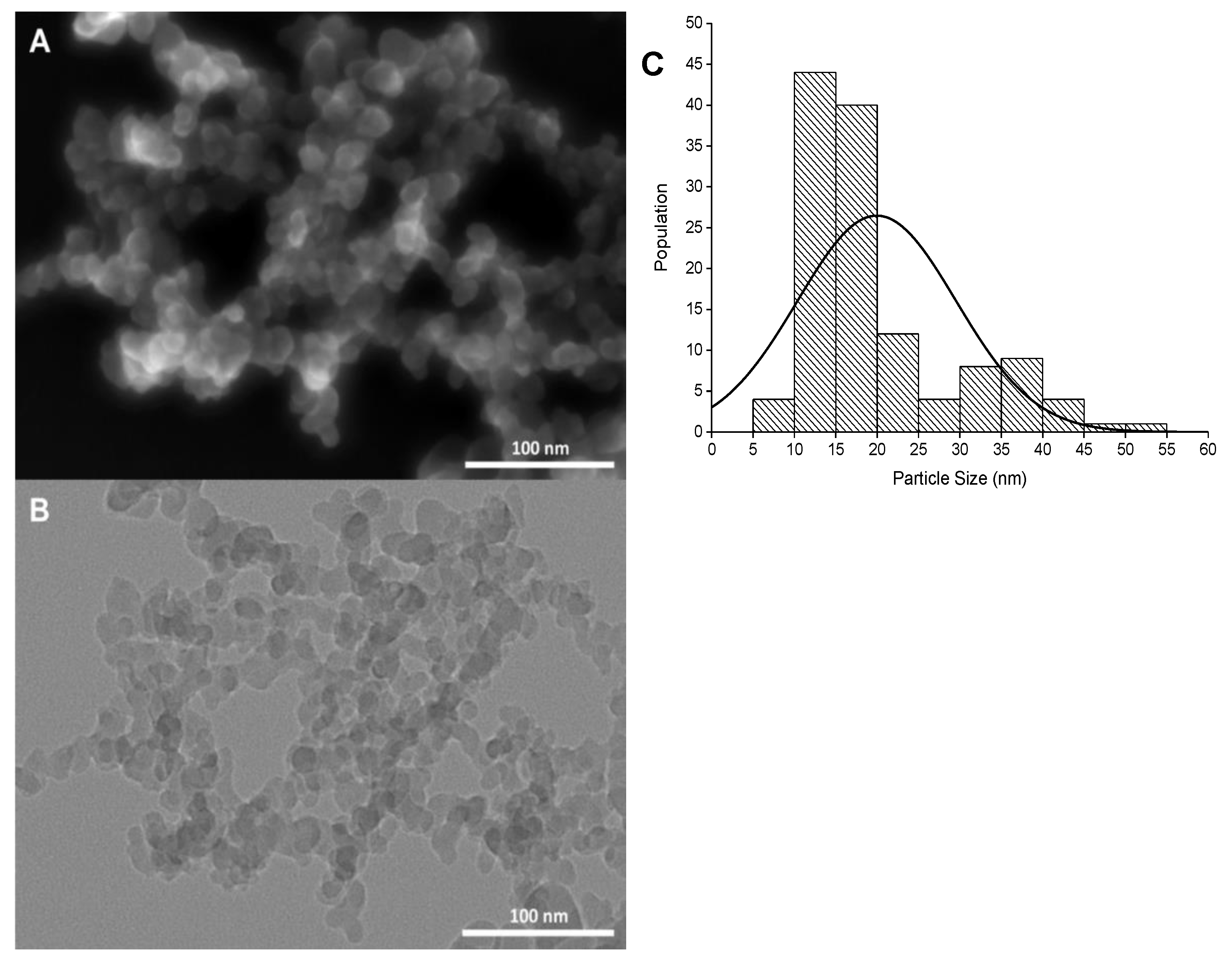
3.1. Characterization of SiO2NPs
3.2. Characterization of Soil
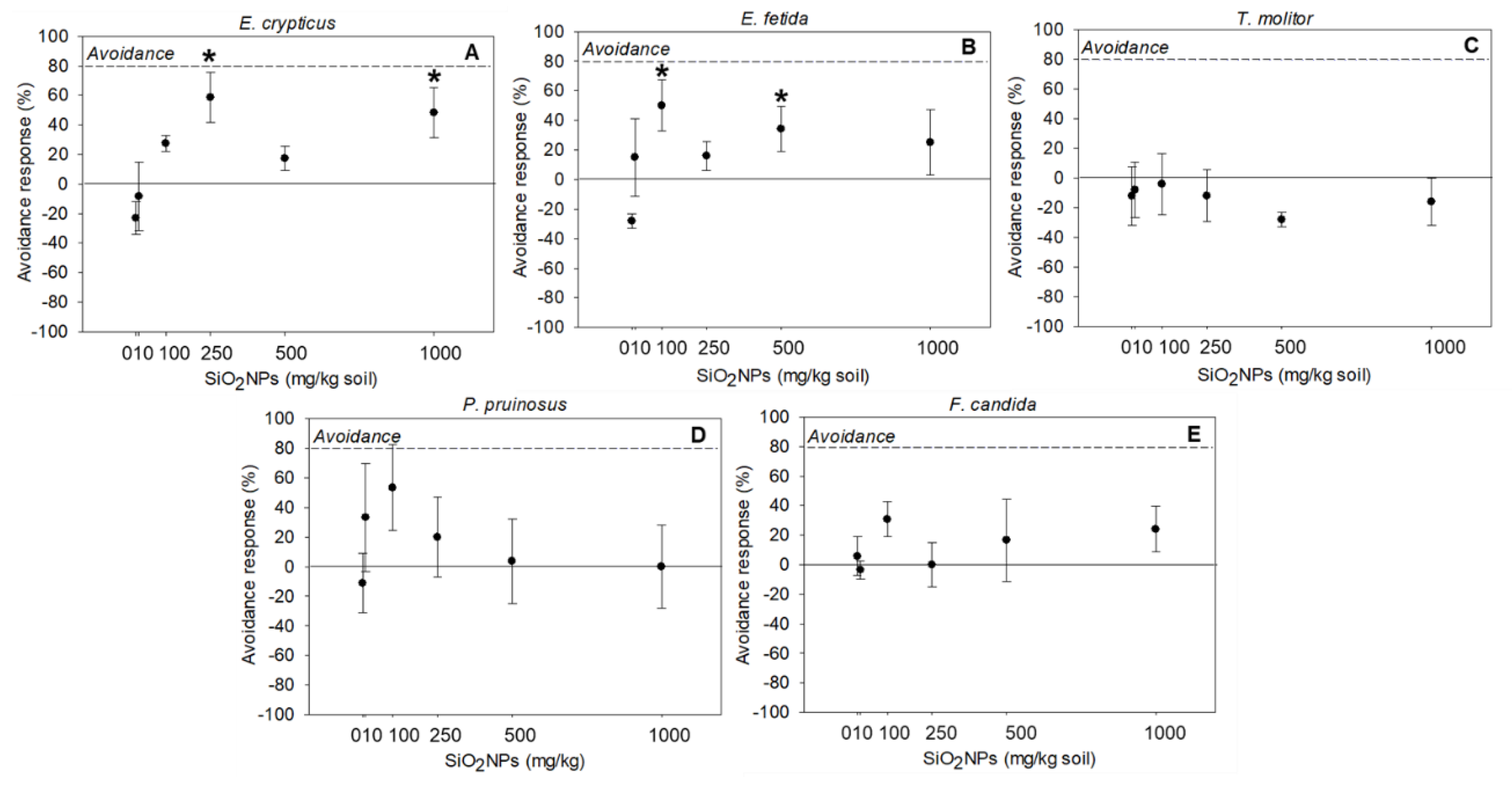
3.3. Avoidance Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sommer, M.; Kaczorek, D.; Kuzyakov, Y.; Breuer, J. Silicon pools and fluxes in soils and landscapes—A review. J. Plant Nutr. Soil Sci. 2006, 169, 310–329. [Google Scholar] [CrossRef]
- Duan, J.; Hu, H.; Feng, L.; Yang, X.; Sun, Z. Silica nanoparticles inhibit macrophage activity and angiogenesis via VEGFR2-mediated MAPK signaling pathway in zebrafish embryos. Chemosphere 2017, 183, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, B.; Li, X.-L.; Li, Y.-X.; Sun, M.-Z.; Chen, D.-Y.; Zhao, X.; Feng, X.-Z. SiO2 nanoparticles change colour preference and cause Parkinson’s-like behaviour in zebrafish. Sci. Rep. 2015, 4, 3810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, D.; De Roo, B.; Nguyen, X.-B.; Vervaele, M.; Kecskés, A.; Ny, A.; Copmans, D.; Vriens, H.; Locquet, J.; Hoet, P.; et al. Use of zebrafish larvae as a multi-endpoint platform to characterize the toxicity profile of silica nanoparticles. Sci. Rep. 2016, 6, 37145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugadoss, S.; Lison, D.; Godderis, L.; Van Den Brule, S.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of silica nanoparticles: an update. Arch. Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef] [PubMed]
- Bouguerra, S.; Gavina, A.; Ksibi, M.; da Graça Rasteiro, M.; Rocha-Santos, T.; Pereira, R. Ecotoxicity of titanium silicon oxide (TiSiO4) nanomaterial for terrestrial plants and soil invertebrate species. Ecotoxicol. Environ. Saf. 2016, 129, 291–301. [Google Scholar] [CrossRef]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted application of silica nanoparticles. A review. Silicon 2019. [Google Scholar] [CrossRef]
- Meena, V.D.; Dotaniya, M.L.; Coumar, V.; Rajendiran, S.; Ajay; Kundu, S.; Subba Rao, A. A case for silicon fertilization to improve crop yields in tropical soils. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 505–518. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, P.; Abbasinia, M.; Assari, M.J.; Oliaei, M. The toxicology of silica nanoparticles: A review. Toxicol. Environ. Chem. 2018, 100, 285–316. [Google Scholar] [CrossRef]
- Bicho, R.C.; Soares, A.M.V.M.; Nogueira, H.I.S.; Amorim, M.J.B. Effects of europium polyoxometalate encapsulated in silica nanoparticles (nanocarriers) in soil invertebrates. J. Nanoparticle Res. 2016, 18, 360. [Google Scholar] [CrossRef]
- Pluskota, A.; Horzowski, E.; Bossinger, O.; von Mikecz, A. In Caenorhabditis elegans nanoparticle-bio-interactions become transparent: silica-nanoparticles induce reproductive senescence. PLoS ONE 2009, 4, e6622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, S.; Troeung, M.; Wang, R.; Draper, R.; Pantano, P.; Crawford, S.; Aravamudhan, S. Acute and chronic toxicity to Daphnia magna of colloidal silica nanoparticles in a chemical mechanical planarization slurry after polishing a gallium arsenide wafer. NanoImpact 2019, 13, 56–65. [Google Scholar] [CrossRef]
- Duan, J.; Yu, Y.; Shi, H.; Tian, L.; Guo, C.; Huang, P.; Zhou, X.; Peng, S.; Sun, Z. Toxic effects of silica nanoparticles on zebrafish embryos and larvae. PLoS ONE 2013, 8, e74606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clément, L.; Zenerino, A.; Hurel, C.; Amigoni, S.; Taffin de Givenchy, E.; Guittard, F.; Marmier, N. Toxicity assessment of silica nanoparticles, functionalised silica nanoparticles, and HASE-grafted silica nanoparticles. Sci. Total Environ. 2013, 450–451, 120–128. [Google Scholar] [CrossRef]
- Scharf, A.; Gührs, K.; von Mikecz, A. Anti-amyloid compounds protect from silica nanoparticle-induced neurotoxicity in the nematode C. elegans. Nanotoxicology 2016, 10, 426–435. [Google Scholar] [CrossRef] [Green Version]
- International Organization for Standardization (ISO). ISO Soil quality—Avoidance Test for Determining the Quality of Soils and Effects of Chemicals on Behaviour —Part 1: Test with Earthworms (Eisenia Fetida and Eisenia Andrei). 17512-1:2008; ISO: Geneva, Switzerland, 2008. [Google Scholar]
- International Organization for Standardization (ISO). ISO Soil Quality—Avoidance Test for Testing the Quality of Soils and Effects of Chemicals—part 2: Test with Collembolans (Folsomia Candida). 17512-2:2011; ISO: Geneva, Switzerland, 2011. [Google Scholar]
- Loureiro, S.; Soares, A.M.V.M.; Nogueira, A.J.A. Terrestrial avoidance behaviour tests as screening tool to assess soil contamination. Environ. Pollut. 2016, 138, 121–131. [Google Scholar] [CrossRef]
- Kobetičová, K.; Hofman, J.; Holoubek, I. Ecotoxicity of wastes in avoidance tests with Enchytraeus albidus, Enchytraeus crypticus and Eisenia fetida (Oligochaeta). Waste Manag. 2010, 30, 558–564. [Google Scholar] [CrossRef]
- Gainer, A.; Hogan, N.; Siciliano, S.D. Soil invertebrate avoidance behavior identifies petroleum hydrocarbon contaminated soils toxic to sensitive plant species. J. Hazard. Mater. 2019, 361, 338–347. [Google Scholar] [CrossRef]
- Amorim, M.J.B.; Römbke, J.; Soares, A.M.V.M. Avoidance behaviour of Enchytraeus albidus: Effects of Benomyl, Carbendazim, phenmedipham and different soil types. Chemosphere 2005, 59, 501–510. [Google Scholar] [CrossRef]
- Pereira, C.M.S.; Novais, S.C.; Soares, A.M.V.M.; Amorim, M.J.B. Dimethoate affects cholinesterases in Folsomia candida and their locomotion--false negative results of an avoidance behaviour test. Sci. Total Environ. 2013, 443, 821–827. [Google Scholar] [CrossRef]
- Morgado, R.; Ferreira, N.G.C.; Cardoso, D.N.; Soares, A.M.V.M.; Loureiro, S. Abiotic factors affect the performance of the terrestrial isopod Porcellionides pruinosus. Appl. Soil Ecol. 2015, 95, 161–170. [Google Scholar] [CrossRef]
- Vijver, M.; Jager, T.; Posthuma, L.; Peijnenburg, W. Metal uptake from soils and soil-sediment mixtures by larvae of Tenebrio molitor (L.) (Coleoptera). Ecotoxicol. Environ. Saf. 2003, 54, 277–289. [Google Scholar] [CrossRef]
- Natal-da-Luz, T.; Amorim, M.J.B.; Römbke, J.; Paulo Sousa, J. Avoidance tests with earthworms and springtails: Defining the minimum exposure time to observe a significant response. Ecotoxicol. Environ. Saf. 2008, 71, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, P.S.; Van Gestel, C.A.M.; Jurkschat, K.; Soares, A.M.V.M.; Loureiro, S. Effects of soil and dietary exposures to Ag nanoparticles and AgNO3 in the terrestrial isopod Porcellionides pruinosus. Environ. Pollut. 2015, 205, 170–177. [Google Scholar] [CrossRef]
- Zidar, P.; Kos, M.; Ilič, E.; Marolt, G.; Drobne, D.; Jemec Kokalj, A. Avoidance behaviour of isopods (Porcellio scaber) exposed to food or soil contaminated with Ag- and CeO2 - nanoparticles. Appl. Soil Ecol. 2019, 141, 69–78. [Google Scholar] [CrossRef]
- Amorim, M.J.B.; Novais, S.; Römbke, J.; Soares, A.M.V.M. Avoidance test with Enchytraeus albidus (Enchytraeidae): Effects of different exposure time and soil properties. Environ. Pollut. 2008, 155, 112–116. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). Determination of the Specific Surface Area of Solids by Gas Adsorption — BET Method, ISO 9277:2010(en); ISO: Geneva, Switzerland, 2010. [Google Scholar]
- Drees, R.L.; Wilding, L.P.; Dixon, J.B.; Smeck, N.E.; Senkayi, A.L. Silica in Soils: Quartz and Disordered Silica Polymorphs. In Minerals in Soil Environments; Soil Science Society of America: Madison, WI, USA, 1989. [Google Scholar]
- Bicho, R.C.; Gomes, S.I.L.; Soares, A.M.V.M.; Amorim, M.J.B. Non-avoidance behaviour in enchytraeids to boric acid is related to the GABAergic mechanism. Environ. Sci. Pollut. Res. 2015, 22, 6898–6903. [Google Scholar] [CrossRef]
- Guimarães, B.; Bandow, C.; Amorim, M.J.B.; Kehrer, A.; Coors, A. Mixture toxicity assessment of a biocidal product based on reproduction and avoidance behaviour of the collembolan Folsomia candida. Ecotoxicol. Environ. Saf. 2018, 165, 284–290. [Google Scholar] [CrossRef]
- Daniel-da-Silva, A.L.; Pinto, F.; Lopes-da-Silva, J.A.; Trindade, T.; Goodfellow, B.J.; Gil, A.M. Rheological behavior of thermoreversible κ-carrageenan/nanosilica gels. J. Colloid Interface Sci. 2008, 320, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Barata, J.F.B.; Daniel-da-Silva, A.L.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Trindade, T. Corrole-silica hybrid particles: synthesis and effects on singlet oxygen generation. RSC Adv. 2013, 3, 274–280. [Google Scholar] [CrossRef]
- Soares, S.F.; Trindade, T.; Daniel-da-Silva, A.L. Carrageenan-Silica Hybrid Nanoparticles Prepared by a Non-Emulsion Method. Eur. J. Inorg. Chem. 2015, 27, 4588–4594. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential – What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- International Centre for Diffraction Data Powder Diffraction; ICDD: Newtown Square, PA, USA, 2013; File 04-012-0490 2013.
- International Centre for Diffraction Data Powder Diffraction; ICDD: Newtown Square, PA, USA, 2018; File 04-022-1040 2018.
- Mendes, L.; Maria, V.L.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Ag Nanoparticles (Ag NM300K) in the Terrestrial Environment: Effects at Population and Cellular Level in Folsomia candida (Collembola). Int. J. Environ. Res. Public Health 2015, 12, 12530–12542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, M.J.; Maria, V.L.; Soares, A.M.V.M.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Fate and Effect of Nano Tungsten Carbide Cobalt (WCCo) in the Soil Environment: Observing a Nanoparticle Specific Toxicity in Enchytraeus crypticus. Environ. Sci. Technol. 2018, 52, 11394–11401. [Google Scholar] [CrossRef]
- Maria, V.L.; Licha, D.; Scott-Fordsmand, J.J.; Huber, C.G.; Amorim, M.J.B. The Proteome of Enchytraeus crypticus—Exposure to CuO Nanomaterial and CuCl2—In Pursue of a Mechanistic Interpretation. Proteomics 2018, 18, 1800091. [Google Scholar] [CrossRef]
- Gonçalves, M.F.M.; Gomes, S.I.L.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Shorter lifetime of a soil invertebrate species when exposed to copper oxide nanoparticles in a full lifespan exposure test. Sci. Rep. 2017, 7, 1355. [Google Scholar] [CrossRef] [Green Version]
- Gomes, S.I.L.; Roca, C.P.; Scott-Fordsmand, J.J.; Amorim, M.J.B. High-throughput transcriptomics: Insights into the pathways involved in (nano) nickel toxicity in a key invertebrate test species. Environ. Pollut. 2019, 245, 131–140. [Google Scholar] [CrossRef]
- Tourinho, P.S.; van Gestel, C.A.M.; Lofts, S.; Svendsen, C.; Soares, A.M.V.M.; Loureiro, S. Metal-based nanoparticles in soil: Fate, behavior, and effects on soil invertebrates. Environ. Toxicol. Chem. 2012, 31, 1679–1692. [Google Scholar] [CrossRef]
- Hassellöv, M.; Readman, J.W.; Ranville, J.F.; Tiede, K. Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles. Ecotoxicology 2008, 17, 344–361. [Google Scholar] [CrossRef]
- Saccone, L.; Conley, D.J.; Koning, E.; Sauer, D.; Sommer, M.; Kaczorek, D.; Blecker, S.W.; Kelly, E.F. Assessing the extraction and quantification of amorphous silica in soils of forest and grassland ecosystems. Eur. J. Soil Sci. 2007, 58, 1446–1459. [Google Scholar] [CrossRef]
- Matichenkov, V.V.; Bocharnikova, E.A. Chapter 13 The relationship between silicon and soil physical and chemical properties. In Silicon in Agriculture; Datnoff, L.E., Snyder, G.H., Korndörfer, G.H.B.T.-S., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 209–219. ISBN 0928-3420. [Google Scholar]
- Wang, H.; Wick, R.L.; Xing, B. Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Environ. Pollut. 2009, 157, 1171–1177. [Google Scholar] [CrossRef]
- Hu, C.W.; Li, M.; Cui, Y.B.; Li, D.S.; Chen, J.; Yang, L.Y. Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil Biol. Biochem. 2010, 42, 586–591. [Google Scholar] [CrossRef]
- Tierney, K.B. Chemical avoidance responses of fishes. Aquat. Toxicol. 2016, 174, 228–241. [Google Scholar] [CrossRef]
- Ockleford, C.; Adriaanse, P.; Berny, P.; Brock, T.; Duquesne, S.; Grilli, S.; Hernandez-Jerez, A.F.; Bennekou, S.H.; Klein, M.; Kuhl, T.; et al. Scientific Opinion addressing the state of the science on risk assessment of plant protection products for in-soil organisms. EFSA J. 2017, 15, e04690. [Google Scholar]
- Guimarães, B.; Maria, V.L.; Römbke, J.; Amorim, M.J.B. Multigenerational exposure of Folsomia candida to ivermectin – Using avoidance, survival, reproduction, size and cellular markers as endpoints. Geoderma 2019, 337, 273–279. [Google Scholar] [CrossRef]


| Concentration (mg/L) | pH | Zeta Potential (mV) |
|---|---|---|
| 10 | 5.3 | −30.2 ± 0.3 |
| 100 | 5.5 | −40.6 ± 0.5 |
| 250 | 5.4 | −24.2 ± 0.7 |
| 500 | 5.4 | −30.2 ± 1.5 |
| 1000 | 5.4 | −32.4 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.; Barreto, Â.; Nogueira, J.; Daniel-da-Silva, A.L.; Trindade, T.; Amorim, M.J.B.; Maria, V.L. Effects of Amorphous Silica Nanopowders on the Avoidance Behavior of Five Soil Species—A Screening Study. Nanomaterials 2020, 10, 402. https://doi.org/10.3390/nano10030402
Santos J, Barreto Â, Nogueira J, Daniel-da-Silva AL, Trindade T, Amorim MJB, Maria VL. Effects of Amorphous Silica Nanopowders on the Avoidance Behavior of Five Soil Species—A Screening Study. Nanomaterials. 2020; 10(3):402. https://doi.org/10.3390/nano10030402
Chicago/Turabian StyleSantos, Joana, Ângela Barreto, João Nogueira, Ana Luísa Daniel-da-Silva, Tito Trindade, Mónica J. B. Amorim, and Vera L. Maria. 2020. "Effects of Amorphous Silica Nanopowders on the Avoidance Behavior of Five Soil Species—A Screening Study" Nanomaterials 10, no. 3: 402. https://doi.org/10.3390/nano10030402









