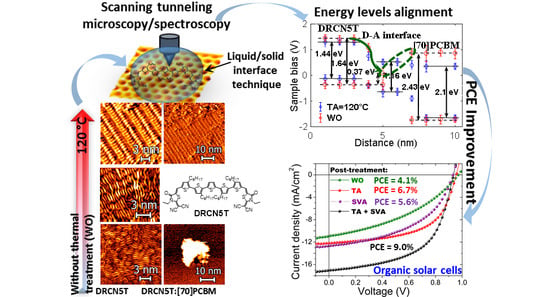
Interfacial Energetic Level Mapping and Nano-Ordering of Small Molecule/Fullerene Organic Solar Cells by Scanning Tunneling Microscopy and Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thin Films Fabrication for STM/STS Measurements
2.2. OSC Fabrication
2.3. Characterization and Measurements
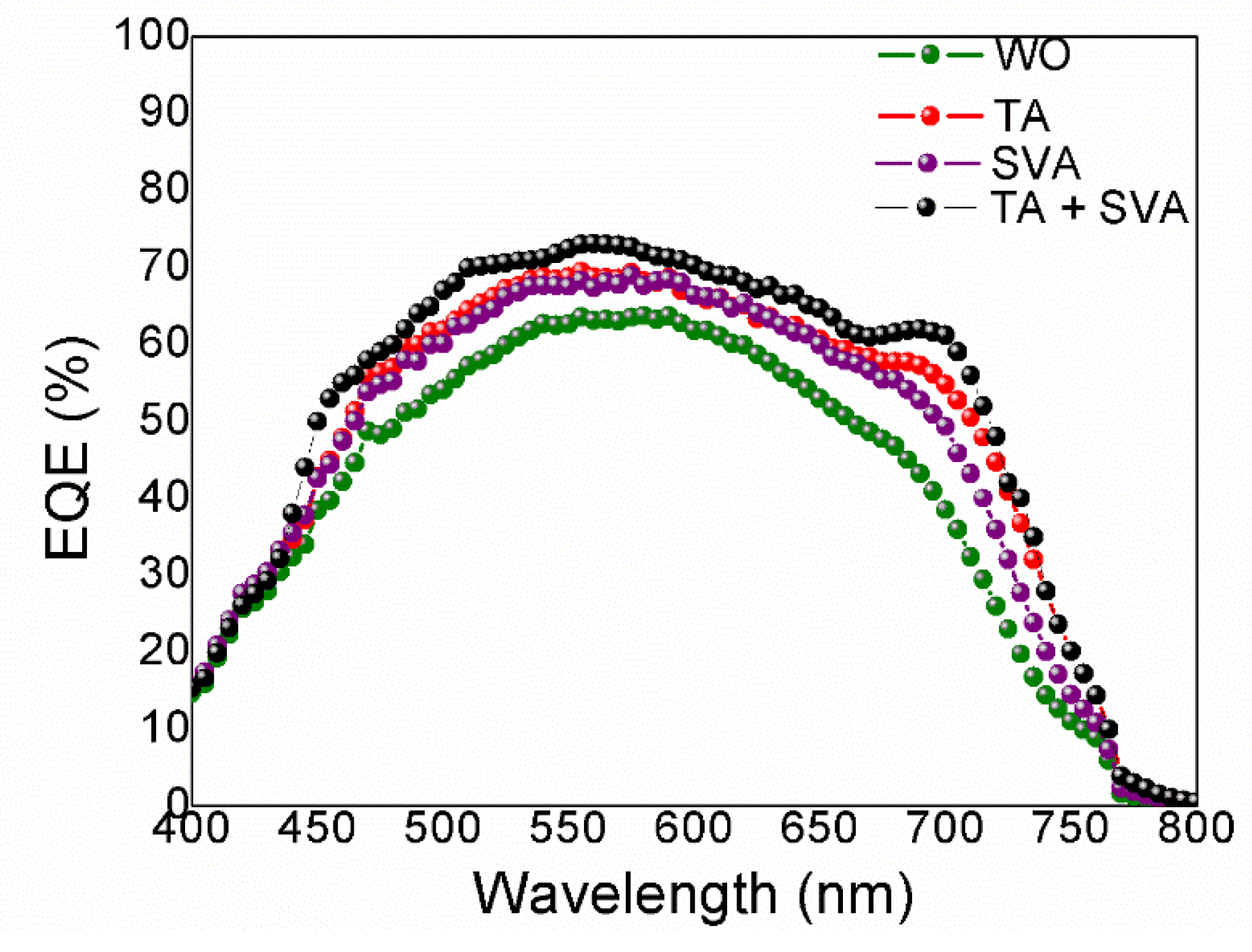
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Duan, L.; Elumalai, N.K.; Zhang, Y.; Uddin, A. Progress in non-fullerene acceptor based organic solar cells. Sol. Energy Mater. Sol. Cells 2019, 193, 22–65. [Google Scholar] [CrossRef]
- Zheng, J.-W.; Sun, Q.; Gao, C.; Chen, J.D.; Li, W.; Zhang, Y.X.; Wang, Y.; Ling, X.; Ma, W.; Li, Y.; et al. Toward ultra-low reflectance semi-transparent organic photovoltaic cells with biomimetic nanostructured transparent electrode. Org. Electron. 2018, 60, 38–44. [Google Scholar] [CrossRef]
- Zhan, L.; Li, S.; Lau, T.-K.; Cui, Y.; Lu, X.; Shi, M.; Li, C.-Z.; Li, H.; Hou, J.; Chen, H. Over 17% efficiency ternary organic solar cells enabled by two non-fullerene acceptors working in an alloy-like model. Energy Environ. Sci. 2020. [Google Scholar] [CrossRef]
- Love, J.A.; Proctor, C.M.; Liu, J.; Takacs, C.J.; Sharenko, A.; van der Poll, T.S.; Heeger, A.J.; Bazan, G.C.; Nguyen, T.Q. Film morphology of high efficiency solution-processed small-molecule solar cells. Adv. Funct. Mater. 2013, 23, 5019–5026. [Google Scholar] [CrossRef]
- Zhou, R.; Jiang, Z.; Yang, C.; Yu, J.; Feng, J.; Adil, M.A.; Deng, D.; Zou, W.; Zhang, J.; Lu, K.; et al. All-small-molecule organic solar cells with over 14% efficiency by optimizing hierarchical morphologies. Nat. Commun. 2019, 10, 5393. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Jiao, X.; Sgobba, V.; Kan, B.; Heumüller, T.; Rechberger, S.; Spiecker, E.; Guldi, D.M.; Wan, X.; Chen, Y.; et al. High efficiency and stability small molecule solar cells developed by bulk microstructure fine-tuning. Nano Energy 2016, 28, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Park, C.; Sin, D.H.; Park, J.H.; Cho, K. Recent advances in morphology optimization for organic photovoltaics. Adv. Mater. 2018, 30, e1800453. [Google Scholar] [CrossRef]
- Min, J.; Güldal, N.S.; Guo, J.; Fang, C.; Jiao, X.; Hu, H.; Heumüller, T.; Adec, H.; Brabec, C.J. Gaining further insight into the effects of thermal annealing and solvent vapor annealing on time morphological development and degradation in small molecule solar cells. J. Mater. Chem. A 2017, 5, 18101–18110. [Google Scholar] [CrossRef]
- Gu, X.; Gunkel, I.; Hexemer, A.; Russell, T.P. Controlling domain spacing and grain size in cylindrical block copolymer thin films by means of thermal and solvent vapor annealing. Macromolecules 2016, 49, 3373–3381. [Google Scholar] [CrossRef]
- Kan, B.; Li, M.; Zhang, Q.; Liu, F.; Wan, X.; Wang, Y.; Ni, W.; Long, G.; Yang, X.; Feng, H.; et al. A series of simple oligomer-like small molecules based on oligothiophenes for solution-processed solar cells with high efficiency. J. Am. Chem. Soc. 2015, 137, 3886–3893. [Google Scholar] [CrossRef]
- Diao, Y.; Shaw, L.; Bao, Z.; Mannsfeld, S.C.B. Morphology control strategies for solution-processed organic semiconductor thin films. Energy Environ. Sci. 2014, 7, 2145–2159. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.I.; Kim, M.; Park, C.W.; Kim, H.U.; Lee, H.-K.; Park, T. Morphological control of donor/acceptor interfaces in all-polymer solar cells using a pentafluorobenzene-based additive. Chem. Mater. 2017, 16, 6793–6798. [Google Scholar] [CrossRef]
- Saraswatula, V.G.; Sharada, D.; Saha, B.K. Stronger π-π interaction leads to a smaller thermal expansion in some charge transfer complexes. Cryst. Growth Des. 2017, 18, 52–56. [Google Scholar] [CrossRef]
- Bucher, L.; Tanguy, L.; Desbois, N.; Karsenti, P.L.; Harvey, P.D.; Gros, C.P.; Sharma, G.D. Photovoltaic properties of a porphyrin-containing polymer as donor in bulk heterojunction solar cells with low energy loss. Sol. RRL 2018. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Hsiao, Y.-C.; Hu, B.; Dadmun, M. Tuning the morphology and performance of low bandgap polymer: Fullerene heterojunctions via solvent annealing in selective solvents. Adv. Funct. Mater. 2014, 24, 5129–5136. [Google Scholar] [CrossRef]
- Shih, M.C.; Huang, B.C.; Lin, C.; Li, S.S.; Chen, H.A.; Chiu, Y.P.; Chen, C.W. Atomic-scale interfacial band mapping across vertically phased-separated polymer/fullerene hybrid solar cells. Nano Lett. 2013, 13, 2387–2392. [Google Scholar] [CrossRef]
- Caballero-Quintana, I.; Maldonado, J.-L.; Meneses-Nava, M.A.; Barbosa-García, O.; Valenzuela-Benavides, J.; Bousseksou, A. Semiconducting polymer thin films used in organic solar cells: A scanning tunneling microscopy study. Adv. Electron. Mater. 2019, 5, 1800499. [Google Scholar] [CrossRef]
- Barreiro-Argüelles, D.; Ramos-Ortiz, G.; Maldonado, J.-L.; Pérez-Gutiérrez, E.; Romero-Borja, D.; Meneses-Nava, M.-A.; Nolasco, J.C. Stability study in organic solar cells based on PTB7:PC71BM and the scaling effect of the active layer. Sol. Energy 2018, 163, 510–518. [Google Scholar] [CrossRef]
- Romero-Borja, D.; Maldonado, J.-L.; Barbosa-García, O.; Rodríguez, M.; de León, A.; Fernández, S.; Pérez-Gutiérrez, E. Organic solar cells based on graphene derivatives and eutectic alloys vacuum-free deposited as top electrodes. Carbon 2018, 134, 301–309. [Google Scholar] [CrossRef]
- Centore, R.; Causà, M. Translating microscopic molecular motion into macroscopic body motion: Reversible self-reshaping in the solid state transition of an organic crystal. Cryst. Growth Des. 2018, 18, 3535–3543. [Google Scholar] [CrossRef]
- Cao, H.; De Feyter, S. Amplification of chirality in surface-confined supramolecular bilayers. Nat. Commun. 2018, 9, 3416. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.Y.; Lin, C.H.; Tzeng, M.C.; Mahmood, A.; Saeed, M.; Chen, C.H.; Ong, C.W.; Lee, S.L. Superstructure manipulation and electronic measurement of monolayers comprising discotic liquid crystals with intrinsic dipole moment using STM/STS. Chem. Commun. 2018, 54, 8048–8051. [Google Scholar] [CrossRef]
- Breitwieser, R.; Hu, Y.C.; Chao, Y.C.; Tzeng, Y.R.; Liou, S.C.; Lin, K.C.; Chen, C.W.; Pai, W. Investigating ultraflexible freestanding graphene by scanning tunneling microscopy and spectroscopy. Phys. Rev. B 2017, 96, 8. [Google Scholar] [CrossRef]
- Krenner, W.; Kuhne, D.; Klappenberger, F.; Barth, J.V. Assessment of scanning tunneling spectroscopy modes inspecting electron confinement in surface-confined supramolecular networks. Sci. Rep. 2013, 3, 1454. [Google Scholar] [CrossRef] [Green Version]
- Nirmalraj, P.; Thompson, D.; Ontoria, A.M.; Sousa, M.; Martín, N.; Gotsmann, B.; Riel, H. Nanoelectrical analysis of single molecules and atomic-scale materials at the liquid/solid interface. Nat. Mater. 2014, 13, 947–953. [Google Scholar] [CrossRef] [Green Version]
- Le Quang, T.; Cherkez, V.; Nogajewski, K.; Potemski, M.; Dau, M.T.; Jamet, M.; Mallet, P.; Veuillen, J.-Y. Scanning tunneling spectroscopy of van der Waals graphene/semiconductor interfaces: Absence of Fermi level pinning. 2D Mater. 2017, 4, 035019. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.L.; Chen, Y.; Zhang, W.; Quek, S.Y.; Chen, C.H.; Li, L.J.; Hsu, W.T.; Chang, W.H.; Zheng, Y.J.; Chen, W.; et al. Bandgap tunability at single-layer molybdenum disulphide grain boundaries. Nat. Commun. 2015, 6, 6298. [Google Scholar] [CrossRef] [Green Version]
- Scifo, L.; Dubois, M.; Brun, M.; Rannou, P.; Latil, S.; Rubio, A.; Grévin, B. Probing the electronic properties of self-organized poly(3-dodecylthiophene) monolayers by two-dimensional scanning tunneling spectroscopy imaging at the single chain scale. Nano Lett. 2006, 6, 1711–1718. [Google Scholar] [CrossRef]
- Park, J.H.; Vishwanath, S.; Liu, X.; Zhou, H.; Eichfeld, S.M.; Fullerton-Shirey, S.K.; Robinson, J.A.; Feenstra, R.M.; Furdyna, J.; Jena, D.; et al. Scanning tunneling microscopy and spectroscopy of air exposure effects on molecular beam epitaxy grown WSe2 monolayers and bilayers. ACS Nano 2016, 10, 4258–4267. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Dey, S.; Pal, A.J. Orientation of organic molecules in a monolayer vis-à-vis their molecular orbitals and transport gap. Appl. Phys. Lett. 2011, 99, 053308. [Google Scholar] [CrossRef]
- Tskipuri, L.; Shao, Q.; Reutt-Robey, J. Molecular ordering in PCBM-Au(111) interface formation. J. Phys. Chem. C 2012, 116, 21874–21879. [Google Scholar] [CrossRef]
- Dasgupta, U.; Bera, A.; Pal, A.J. Band diagram of heterojunction solar cells through scanning tunneling spectroscopy. ACS Energy Lett. 2017, 2, 582–591. [Google Scholar] [CrossRef]
- Cochrane, K.A.; Roussy, T.S.; Yuan, B.; Tom, G.; Mårsell, E.; Burke, S.A. Molecularly resolved electronic landscapes of differing acceptor-donor interface geometries. J. Phys. Chem. C 2018, 122, 8437–8444. [Google Scholar] [CrossRef]
- Stefanou, M.; Chandler, H.J.; Mignolet, B.; Williams, E.; Nanoh, S.A.; Thompson, J.O.F.; Remacle, F.; Schaub, R.; Campbell, E.E.B. Angle-resolved photoelectron spectroscopy and scanning tunnelling spectroscopy studies of the endohedral fullerene Li@C60. Nanoscale 2019, 11, 2668–2678. [Google Scholar] [CrossRef] [Green Version]
- Nakano, K.; Suzuki, K.; Chen, Y.; Tajima, K. Roles of energy/charge cascades and intermixed layers at donor/acceptor interfaces in organic solar cells. Sci. Rep. 2016, 6, 29529. [Google Scholar] [CrossRef] [Green Version]
- Morvillo, P.; Ricciardi, R.; Nenna, G.; Bobeico, E.; Diana, R.; Minarini, C. Elucidating the origin of the improved current output in inverted polymer solar cells. Sol. Energy Mater. Sol. Cells 2016, 152, 51–58. [Google Scholar] [CrossRef]
- Barreiro-Argüelles, D.; Ramos-Ortiz, G.; Maldonado, J.-L.; Pérez-Gutiérrez, E.; Romero-Borja, D.; Álvarez-Fernández, A. PTB7:PC71BM-based solar cells fabricated with the eutectic alloy field’s metal as an alternative cathode and the influence of an electron extraction layer. IEEE J. Photovolt. 2017, 7, 191–198. [Google Scholar] [CrossRef]
- Azzouz, M.I.; Kirchartz, T.; Nelson, J. Factors controlling open-circuit voltage losses in organic solar cells. Trends Chem. 2019, 1, 49–62. [Google Scholar] [CrossRef]
- Elumalai, N.K.; Uddin, A. Open circuit voltage of organic solar cells: An in-depth review. Energy Environ. Sci. 2016, 9, 391–410. [Google Scholar] [CrossRef]
- Amargós-Reyes, O.; Maldonado, J.-L.; Romero-Borja, D.; Barreiro-Argüelles, D.; Caballero-Quintana, I.; Barbosa-García, O.; Gaspar, J.A. Organic photovoltaic cell analysis through quantum efficiency and scanning tunneling microscopy of the donor/blend as an active film. J. Mater. Sci. 2019, 54, 2427–2445. [Google Scholar] [CrossRef]


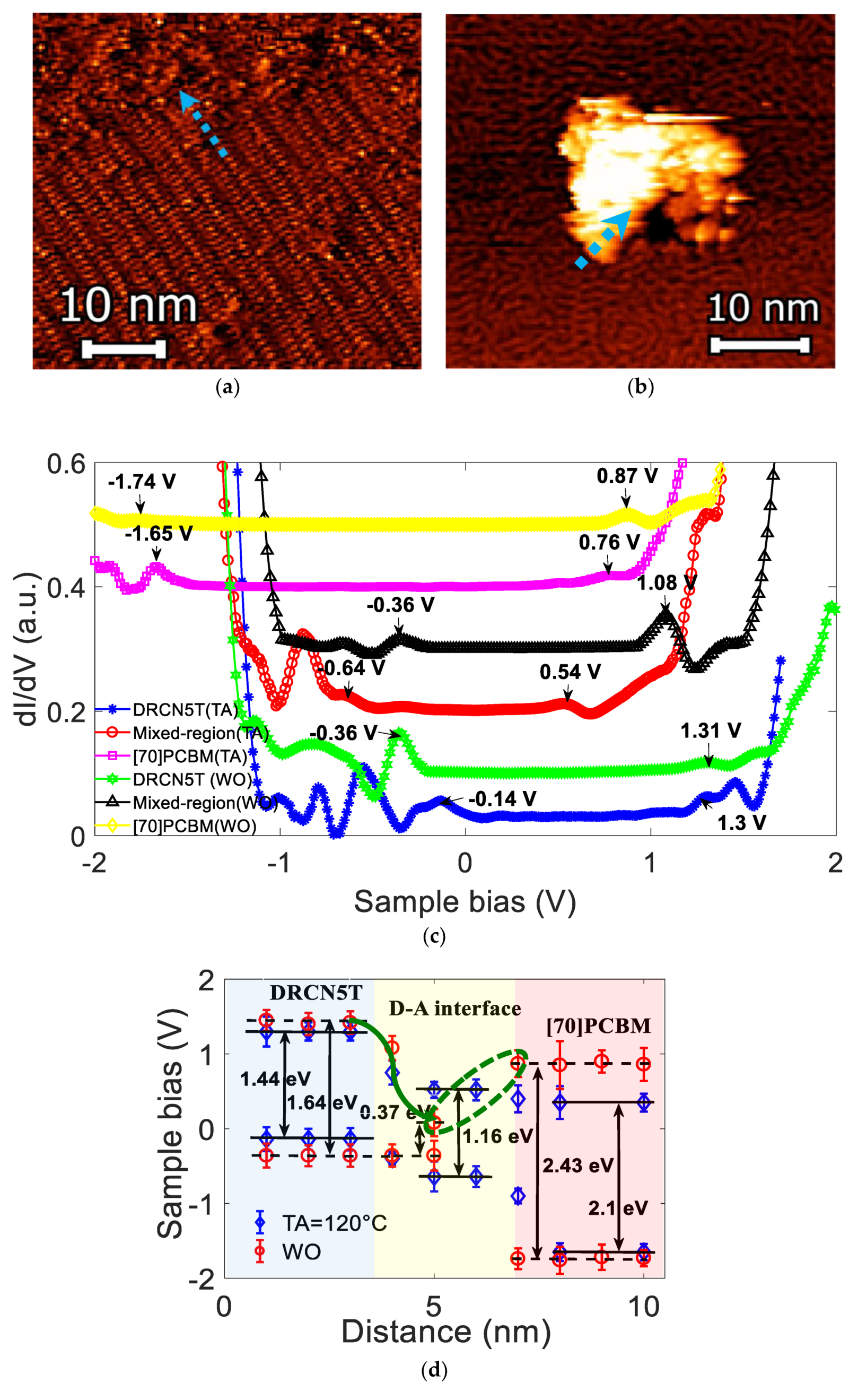

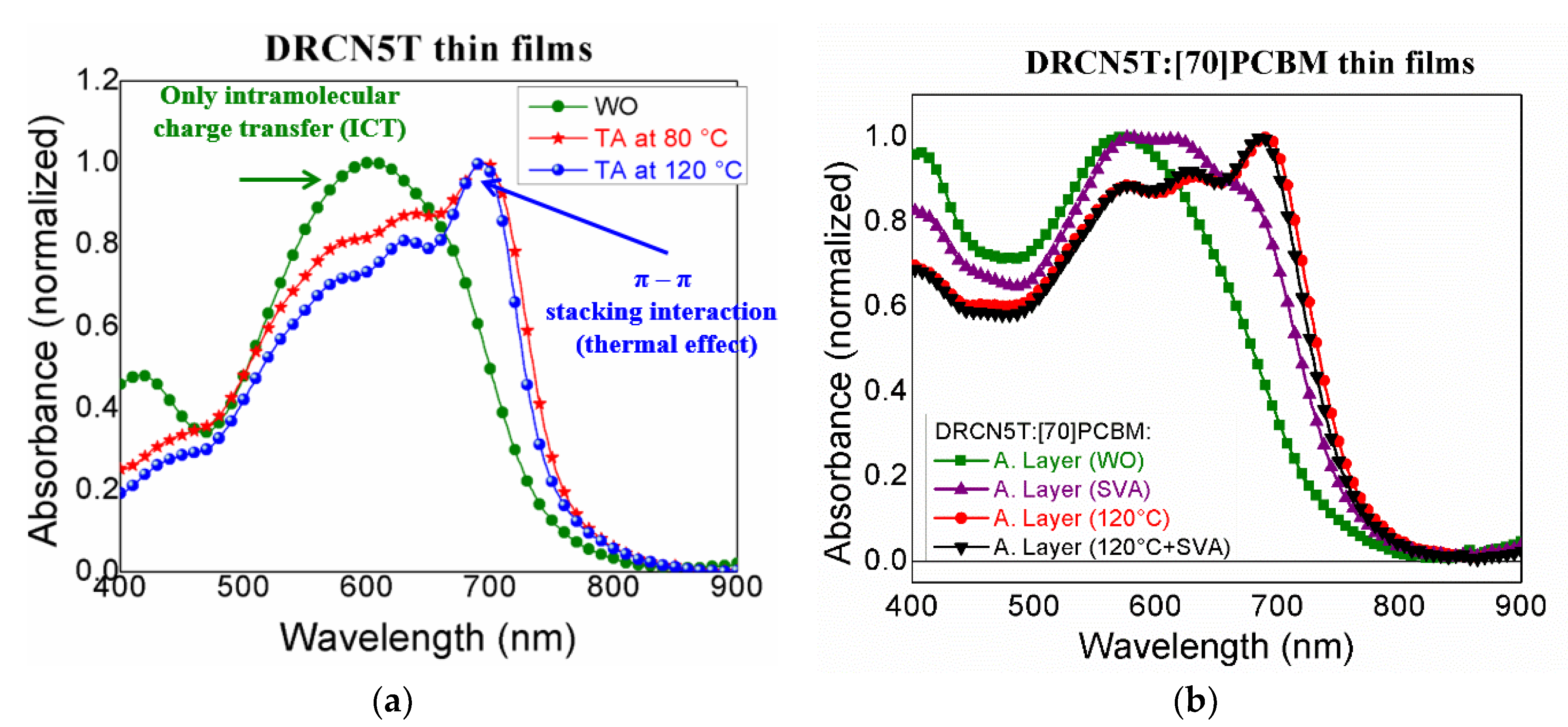
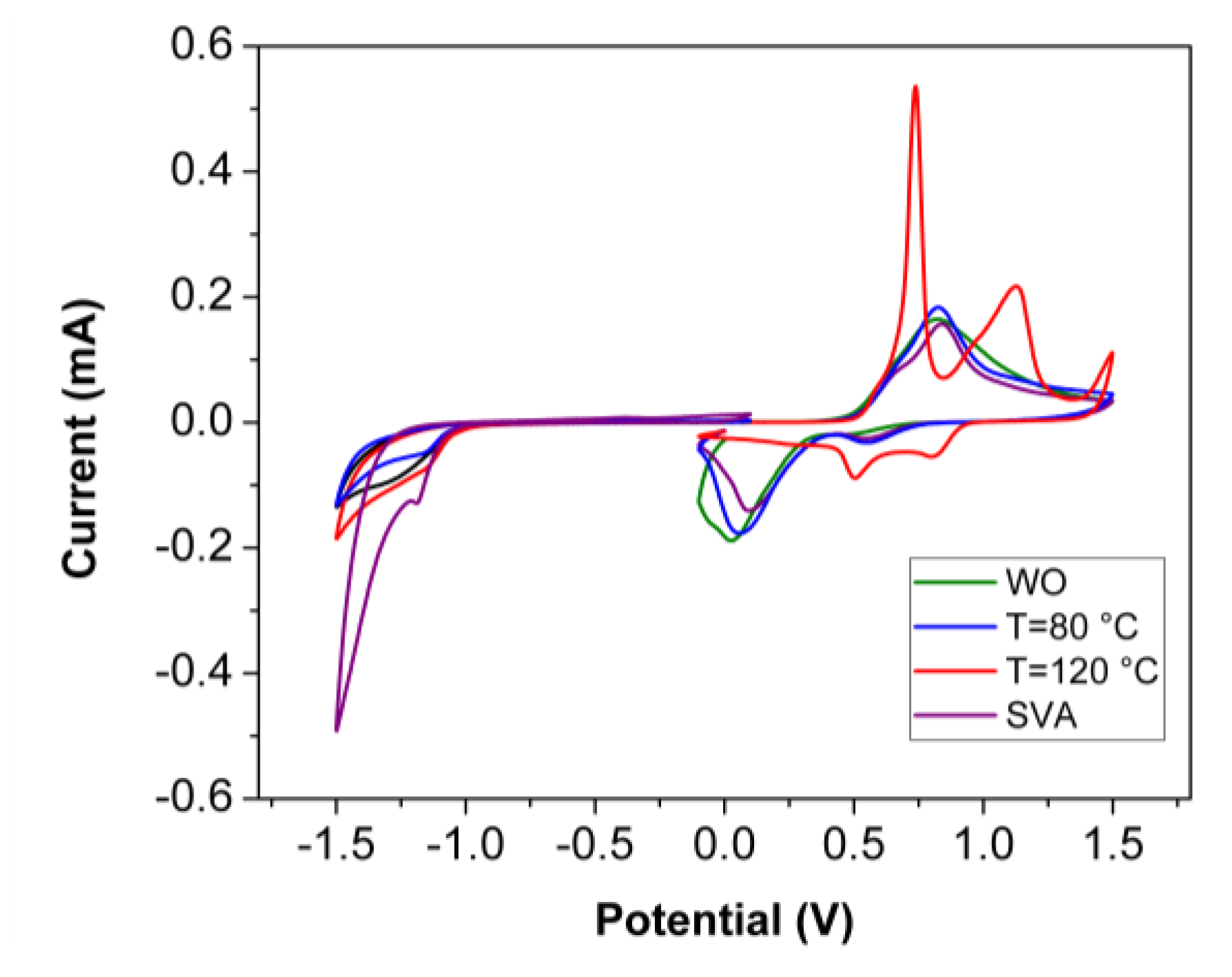
| DRCN5T Post-Treatment | Eox, onset (eV) | HOMO (eV) | Ered, onset (eV) | LUMO (eV) | Eg (eV) by CV | Eg (eV) by STS |
|---|---|---|---|---|---|---|
| WO | 0.49 | −5.09 | −1.02 | −3.58 | 1.51 | 1.69 |
| TA80 | 0.51 | −5.11 | −1.04 | −3.56 | 1.55 | 1.57 |
| TA120 | 0.52 | −5.12 | −1.08 | −3.52 | 1.60 | 1.52 |
| SVA | 0.50 | −5.10 | −1.03 | −3.57 | 1.53 | - |
| Reference | Thermal Treatment | Voc (V) | Jsc (mA/cm2) | FF | PCEbest (PCEav) a (%) |
|---|---|---|---|---|---|
| [6] | WO | 0.98 | 7.5 | 0.48 | 3.6 |
| TA | 0.93 | 12.3 | 0.58 | 6.7 | |
| SVA | 0.95 | 12.6 | 0.55 | 6.6 | |
| TA + SVA | 0.93 | 14.4 | 0.66 | 8.3 | |
| [10] | TA + SVA | 0.92 | 15.7 | 0.68 | 10.1 |
| This work | WO | 0.97 | 10.9 | 0.39 | 4.1 (3.4) |
| TA | 0.95 | 12.2 | 0.58 | 6.7 (6.1) | |
| SVA | 0.93 | 12.6 | 0.48 | 5.6 (5.6) | |
| TA + SVA | 0.93 | 17.0 | 0.57 | 9.0 (8.6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballero-Quintana, I.; Romero-Borja, D.; Maldonado, J.-L.; Nicasio-Collazo, J.; Amargós-Reyes, O.; Jiménez-González, A. Interfacial Energetic Level Mapping and Nano-Ordering of Small Molecule/Fullerene Organic Solar Cells by Scanning Tunneling Microscopy and Spectroscopy. Nanomaterials 2020, 10, 427. https://doi.org/10.3390/nano10030427
Caballero-Quintana I, Romero-Borja D, Maldonado J-L, Nicasio-Collazo J, Amargós-Reyes O, Jiménez-González A. Interfacial Energetic Level Mapping and Nano-Ordering of Small Molecule/Fullerene Organic Solar Cells by Scanning Tunneling Microscopy and Spectroscopy. Nanomaterials. 2020; 10(3):427. https://doi.org/10.3390/nano10030427
Chicago/Turabian StyleCaballero-Quintana, Irving, Daniel Romero-Borja, José-Luis Maldonado, Juan Nicasio-Collazo, Olivia Amargós-Reyes, and Antonio Jiménez-González. 2020. "Interfacial Energetic Level Mapping and Nano-Ordering of Small Molecule/Fullerene Organic Solar Cells by Scanning Tunneling Microscopy and Spectroscopy" Nanomaterials 10, no. 3: 427. https://doi.org/10.3390/nano10030427