Controlled Synthesis of Au Nanocrystals-Metal Selenide Hybrid Nanostructures toward Plasmon-Enhanced Photoelectrochemical Energy Conversion
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Materials
2.2. Growth of Au NRs
2.3. Growth of Au@Se NRs
2.4. Synthesis of Au–PbSe Hybrid NRs
2.5. Preparation of (PbSe NCs)@TiO2, (Au–PbSe Dumbbell NRs)@TiO2 and (Au–PbSe Yolk-Shell NRs)@TiO2 NWs Film Electrodes
2.6. Characterizations
2.7. Photocurrent Response and Incident Photon to Current Conversion Efficiency (IPCE) Measurements
3. Results and Discussion
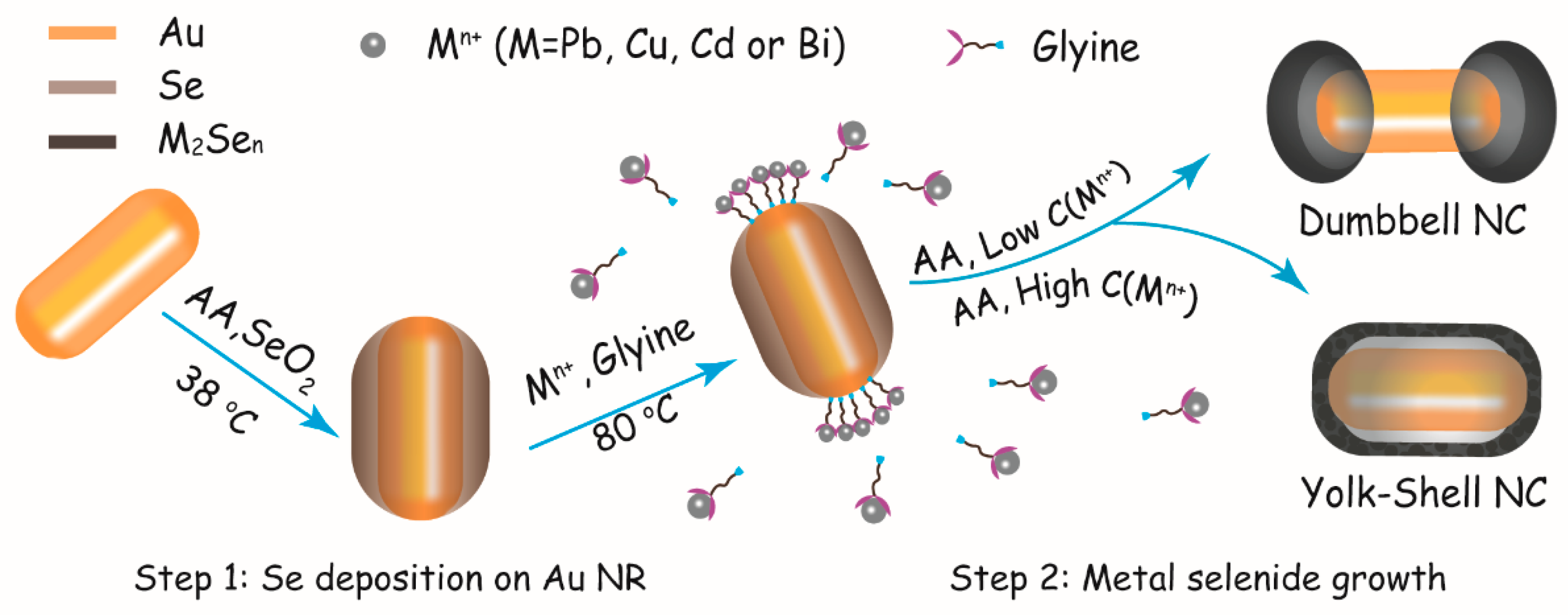
3.1. Controllable Synthesis of Au–PbSe Hybrid NRs
3.2. Growth Kinetics and Symmetry Controlling of Au–PbSe Hybrid NRs
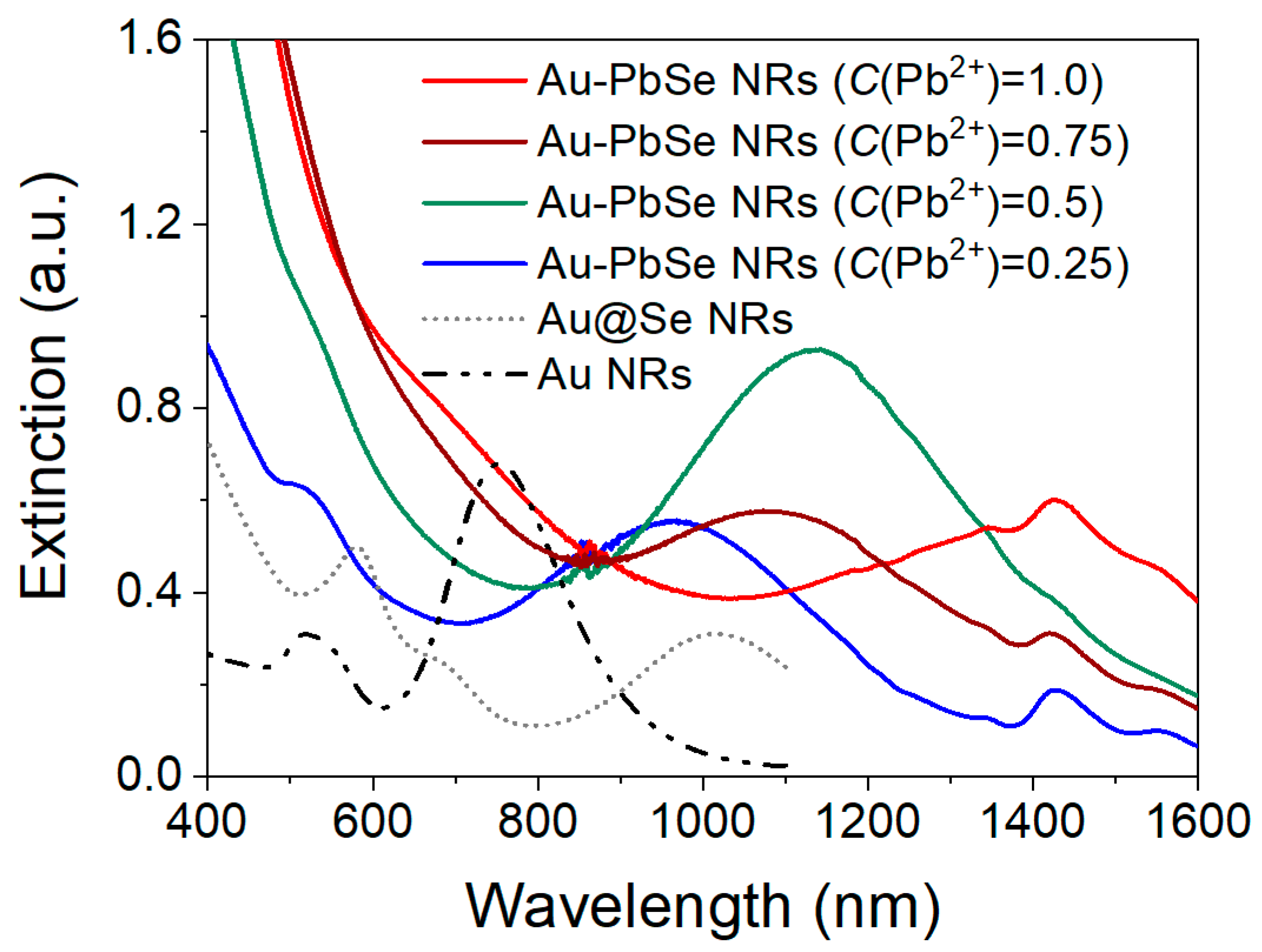
3.3. Optical Properties of Au–PbSe NRs with Different Shells Distributions
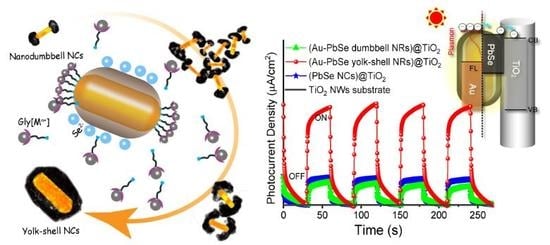
3.4. Photoelectrochemical Performances Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peng, M.; Xie, X.; Zheng, H.; Wang, Y.; Zhuo, Q.; Yuan, G.; Ma, W.; Shao, M.; Wen, Z.; Sun, X. PbS quantum dots/2D nonlayered CdSexS1−x nanosheet hybrid nanostructure for high-performance broadband photodetectors. ACS Appl. Mater. Inter. 2018, 10, 43887–43895. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Li, B.; Fang, C.; Wang, J. Metal/semiconductor hybrid nanostructures for plasmon-enhanced applications. Adv. Mater. 2014, 26, 5274–5309. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Huang, J.; Liu, C.; Wu, C.H.; Lin, Y.; Wang, X.; Zhang, S.; Hou, J.; Xia, Y. Gold-based hybrid nanocrystals through heterogeneous nucleation and growth. Adv. Mater. 2010, 22, 1936–1940. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Chen, J.S.; Lou, X.W.; Xing, X.; Lu, G.Q. Yolk/shell nanoparticles: New platforms for nanoreactors, drug delivery and lithium-ion batteries. Chem. Commun. 2011, 47, 12578–12591. [Google Scholar] [CrossRef]
- Kelestemur, Y.; Dede, D.; Gungor, K.; Usanmaz, C.F.; Erdem, O.; Demir, H.V. Alloyed heterostructures of CdSexS1−x nanoplatelets with highly tunable optical gain performance. Chem. Mater. 2017, 29, 4857–4865. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting-a critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Tan, C.; Chen, J.; Wu, X.-J.; Zhang, H. Epitaxial growth of hybrid nanostructures. Nat. Rev. Mater. 2018, 3, 2058–8437. [Google Scholar] [CrossRef]
- Zhou, Z.K.; Li, M.; Yang, Z.J.; Peng, X.N.; Su, X.R.; Zhang, Z.S.; Li, J.B.; Kim, N.C.; Yu, X.F.; Zhou, L.; et al. Plasmon-mediated radiative energy transfer across a silver nanowire array via resonant transmission and subwavelength imaging. ACS Nano 2010, 4, 5003–5010. [Google Scholar] [CrossRef]
- Meng, X.; Liu, L.; Ouyang, S.; Xu, H.; Wang, D.; Zhao, N.; Ye, J. Nanometals for solar-to-chemical energy conversion: From semiconductor-based photocatalysis to plasmon-mediated photocatalysis and photo-thermocatalysis. Adv. Mater. 2016, 28, 6781–6803. [Google Scholar] [CrossRef]
- Li, M.; Yu, X.-F.; Liang, S.; Peng, X.-N.; Yang, Z.-J.; Wang, Y.-L.; Wang, Q.-Q. Synthesis of Au-CdS core-shell hetero-nanorods with efficient exciton-plasmon interactions. Adv. Funct. Mater. 2011, 21, 1788–1794. [Google Scholar] [CrossRef]
- Dutta, S.K.; Mehetor, S.K.; Pradhan, N. Metal semiconductor heterostructures for photocatalytic conversion of light energy. J. Phys. Chem. Lett. 2015, 6, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Achermann, M. Exciton-plasmon interactions in metal-semiconductor nanostructures. J. Phys. Chem. Lett. 2010, 1, 2837–2843. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef]
- Liang, S.; Li, J.-B.; Li, M.; Tang, X. Tunable plasmon resonance and enhanced photocatalytic activity of Au-CdS core-shell nanodogbones. Plasmonics 2017, 13, 181–187. [Google Scholar] [CrossRef]
- Zhou, Z.-K.; Liu, J.; Bao, Y.; Wu, L.; Png, C.E.; Wang, X.-H.; Qiu, C.-W. Quantum plasmonics get applied. Prog. Quant. Electron. 2019, 65, 1–20. [Google Scholar] [CrossRef]
- Wu, K.; Chen, J.; McBride, J.R.; Lian, T. Efficient hot-electron transfer by a plasmon-induced interfacial charge-transfer transition. Science 2015, 349, 632–635. [Google Scholar] [CrossRef] [Green Version]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B. 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Shaviv, E.; Schubert, O.; Alves-Santos, M.; Goldoni, G.; Di Felice, R.; Vallée, F.; Del Fatti, N.; Banin, U.; Sönnichsen, C. Absorption properties of metal-semiconductor hybrid nanoparticles. ACS Nano 2011, 5, 4712–4719. [Google Scholar] [CrossRef]
- Ma, L.; Liang, S.; Liu, X.-L.; Yang, D.-J.; Zhou, L.; Wang, Q.-Q. Synthesis of dumbbell-like gold-metal sulfide core-shell nanorods with largely enhanced transverse plasmon resonance in visible region and efficiently improved photocatalytic activity. Adv. Funct. Mater. 2015, 25, 898–904. [Google Scholar] [CrossRef]
- Kahane, S.V.; Sudarsan, V.; Mahamuni, S. Anomalous photoluminescence enhancement due to hot electron transfer in core-shell Au-CdS nanocrystals. J. Lumin. 2017, 181, 91–95. [Google Scholar] [CrossRef]
- Kulakovich, O.; Strekal, N.; Yaroshevich, A.; Maskevich, S.; Gaponenko, S.; Nabiev, I.; Woggon, U.; Artemyev, M. Enhanced luminescence of CdSe quantum dots on gold colloids. Nano Lett. 2002, 2, 1449–1452. [Google Scholar] [CrossRef]
- Nan, F.; Xie, F.M.; Liang, S.; Ma, L.; Yang, D.J.; Liu, X.L.; Wang, J.H.; Cheng, Z.Q.; Yu, X.F.; Zhou, L.; et al. Growth of metal-semiconductor core-multishell nanorods with optimized field confinement and nonlinear enhancement. Nanoscale 2016, 8, 11969–11975. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Tan, X.L.; Ma, J.H.; Xu, S.Q.; Kuang, Z.W.; Liang, S.; Xiao, S.; He, M.D.; Kim, N.C.; Luo, J.H.; et al. Plasmon-modulated bistable four-wave mixing signals from a metal nanoparticle-monolayer MoS2 nanoresonator hybrid system. Nanotechnology 2018, 29, 255704. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Li, X.; Xin, N.; Gong, Y.; Guan, J.; Meng, L.; Meng, S.; Guo, X. Interface-engineered plasmonics in metal/semiconductor heterostructures. Adv. Energy Mater. 2016, 6, 1600431. [Google Scholar] [CrossRef]
- Ma, L.; Chen, K.; Nan, F.; Wang, J.-H.; Yang, D.-J.; Zhou, L.; Wang, Q.-Q. Improved hydrogen production of Au-Pt-CdS hetero-nanostructures by efficient plasmon-induced multipathway electron transfer. Adv. Funct. Mater. 2016, 26, 6076–6083. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Jang, Y.H.; Jang, Y.J.; Kim, S.; Quan, L.N.; Chung, K.; Kim, D.H. Plasmonic solar cells: From rational design to mechanism overview. Chem. Rev. 2016, 116, 14982–15034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-plasmon-driven hot electron photochemistry. Chem. Rev. 2018, 118, 2927–2954. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, J.; Gui, J.; Chen, T.; Xu, M.; Wang, H.; Dong, H.; Chen, H.; Li, X.; Wang, L.; et al. Metal@semiconductor core-shell nanocrystals with atomically organized interfaces for efficient hot electron-mediated photocatalysis. Nano Energy 2018, 48, 44–52. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Naghadeh, S.B.; Lindley, S.A.; Lai, T.-H.; Kuo, M.-Y.; Chang, K.-D.; Zhang, J.Z.; Hsu, Y.-J. Yolk-shell nanostructures as an emerging photocatalyst paradigm for solar hydrogen generation. Nano Energy 2019, 62, 289–298. [Google Scholar] [CrossRef]
- Lim, S.C.; Lo, W.F.; Yang, P.Y.; Lu, S.C.; Joplin, A.; Link, S.; Chang, W.S.; Tuan, H.Y. Au@CdSe heteroepitaxial nanorods: An example of metal nanorods fully covered by a semiconductor shell with strong photo-induced interfacial charge transfer effects. J. Colloid Interf. Sci. 2018, 532, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, D.-J.; Song, X.-P.; Li, H.-X.; Ding, S.-J.; Xiong, L.; Qin, P.-L.; Chen, X.-B. Pt decorated (Au nanosphere)/(CuSe ultrathin nanoplate) tangential hybrids for efficient photocatalytic hydrogen generation via dual-plasmon-induced strong Vis-Nir light absorption and interfacial electric field coupling. Solar RRL. 2019, 9, 1900376. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Y.; Liu, J.; Li, X.; Ji, M.; Zhang, E.; Cheng, X.; Xu, M.; Liu, J.; Rong, H.; et al. Efficient plasmonic Au/CdSe nanodumbbell for photoelectrochemical hydrogen generation beyond visible region. Adv. Energy Mater. 2019, 9, 1803889. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Y.; Chen, C.; Ma, M.; Zeng, J.; Li, S.; Xia, Y.; Gao, M. Monodisperse dual plasmonic Au@Cu2−xE (E= S, Se) core@shell supraparticles: Aqueous fabrication, multimodal imaging, and tumor therapy at in vivo level. ACS Nano 2017, 11, 8273–8281. [Google Scholar] [CrossRef]
- Huang, Z.; Gong, J.; Nie, Z. Symmetry-breaking synthesis of multicomponent nanoparticles. Acc. Chem. Res. 2019, 52, 1125–1133. [Google Scholar] [CrossRef]
- Wang, M.; Ye, M.; Iocozzia, J.; Lin, C.; Lin, Z. Plasmon-mediated solar energy conversion via photocatalysis in noble metal/semiconductor composites. Adv. Sci. 2016, 3, 1600024. [Google Scholar] [CrossRef] [Green Version]
- Wu, N. Plasmonic metal-semiconductor photocatalysts and photoelectrochemical cells: A review. Nanoscale 2018, 10, 2679–2696. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, W.; Xu, C.-H.; Chen, H.-Y.; Xu, J.-J. Electrochemical synthesis of Au@semiconductor core-shell nanocrystals guided by single particle plasmonic imaging. Chem. Sci. 2019, 10, 9308–9314. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.S.; Li, J.; Li, J. Metal/semiconductor nanocomposites for photocatalysis: Fundamentals, structures, applications and properties. Nanomaterials 2019, 9, 359. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wang, P.; Li, H.; Wang, M.; Zhang, J.; Qi, G.; Jin, Y. Plasmon-driven water splitting enhancement on plasmonic metal-insulator-semiconductor hetero-nanostructures: Unraveling the crucial role of interfacial engineering. Nanoscale 2018, 10, 14290–14297. [Google Scholar] [CrossRef]
- Banin, U.; Ben-Shahar, Y.; Vinokurov, K. Hybrid semiconductor-metal nanoparticles: From architecture to function. Chem. Mater. 2013, 26, 97–110. [Google Scholar] [CrossRef]
- Jia, H.; Du, A.; Zhang, H.; Yang, J.; Jiang, R.; Wang, J.; Zhang, C.Y. Site-selective growth of crystalline ceria with oxygen vacancies on gold nanocrystals for near-Infrared nitrogen photofixation. J. Am. Chem. Soc. 2019, 141, 5083–5086. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhao, Z.J.; Zhang, Q.; Han, L.; Jiang, X.; Li, C.; Cardenas, M.T.P.; Huang, P.; Yin, J.J.; Luo, J.; et al. A welding phenomenon of dissimilar nanoparticles in dispersion. Nat. Commun. 2019, 10, 219. [Google Scholar] [CrossRef]
- Zou, Y.; Sun, C.; Gong, W.; Yang, X.; Huang, X.; Yang, T.; Lu, W.; Jiang, J. Morphology-controlled synthesis of hybrid nanocrystals via a selenium-mediated strategy with ligand shielding effect: The case of dual plasmonic Au-Cu2−xSe. ACS Nano 2017, 11, 3776–3785. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, Z.; Lu, Q.; Zeng, F.; Su, D. One-pot synthesis of (Au nanorod)-(metal sulfide) core-shell nanostructures with enhanced gas-sensing property. Small 2012, 8, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, Y.; Lee, K.; Ouyang, M. Nonepitaxial growth of hybrid core-shell nanostructures with large lattice mismatches. Science 2010, 327, 1634–1638. [Google Scholar] [CrossRef]
- Lou, Z.; Kim, S.; Fujitsuka, M.; Yang, X.; Li, B.; Majima, T. Anisotropic Ag2S-Au triangular nanoprisms with desired configuration for plasmonic photocatalytic hydrogen generation in visible/near-infrared region. Adv. Funct. Mater. 2018, 28, 1706969. [Google Scholar] [CrossRef]
- Liang, S.; Liu, X.L.; Yang, Y.Z.; Wang, Y.L.; Wang, J.H.; Yang, Z.J.; Wang, L.B.; Jia, S.F.; Yu, X.F.; Zhou, L.; et al. Symmetric and asymmetric Au-AgCdSe hybrid nanorods. Nano Lett. 2012, 12, 5281–5286. [Google Scholar] [CrossRef]
- Huang, X.; Neretina, S.; El-Sayed, M.A. Gold nanorods: From synthesis and properties to biological and biomedical applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Shan, B.; Zhao, Y.; Li, Y.; Wang, H.; Chen, R.; Li, M. High-quality dual-plasmonic Au@Cu2−xSe nanocrescents with precise Cu2−xSe domain size control and tunable optical properties in the second near-infrared biowindow. Chem. Mater. 2019, 31, 9875–9886. [Google Scholar] [CrossRef]
- Pakiari, A.H.; Jamshidi, Z. Interaction of amino acids with gold and silver clusters. J. Phys. Chem. A 2007, 111, 4391–4396. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, M.; Yang, K.; Lu, J.; Chen, L. Simple synthesis of luminescent CdSe quantum dots from ascorbic acid and selenium dioxide. Luminescence 2015, 30, 1375–1379. [Google Scholar] [CrossRef]
- Kim, C.; Kim, D.H.; Kim, J.T.; Han, Y.S.; Kim, H. Investigation of reaction mechanisms of bismuth tellurium selenide nanomaterials for simple reaction manipulation causing effective adjustment of thermoelectric properties. ACS Appl. Mater. Inter. 2014, 6, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.J.; Knez, M.; Scholz, R.; Hesse, D.; Nielsch, K.; Zacharias, M.; Gosele, U. Influence of surface diffusion on the formation of hollow nanostructures induced by the Kirkendall effect: The basic concept. Nano Lett. 2007, 7, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Rioux, R.M.; Erdonmez, C.K.; Hughes, S.; Somorjai, G.A.; Alivisatos, A.P. Formation of hollow nanocrystals through the nanoscale Kirkendall effect. Science 2004, 304, 711–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guijarro, N.; Lana-Villarreal, T.; Lutz, T.; Haque, S.A.; Gómez, R. Sensitization of TiO2 with PbSe quantum dots by silar: How mercaptophenol improves charge separation. J. Phys. Chem. Lett. 2012, 3, 3367–3372. [Google Scholar] [CrossRef]
- Sushil, K.; Khana, M.A.M. Schottky barrier junctions of gold with lead chalcogenides: Growth and characteristics. Chalcogenide Lett. 2012, 9, 99–103. [Google Scholar]
- Choi, J.J.; Lim, Y.F.; Santiago-Berrios, M.B.; Oh, M.; Hyun, B.R.; Sun, L.; Bartnik, A.C.; Goedhart, A.; Malliaras, G.G.; Abruna, H.D.; et al. PbSe nanocrystal excitonic solar cells. Nano Lett. 2009, 9, 3749–3755. [Google Scholar] [CrossRef]
- Law, M.; Luther, J.M.; Song, Q.; Hughes, B.K.; Perkins, C.L.; Nozik, A.J. Structural, optical, and electrical properties of PbSe nanocrystal solids treated thermally or with simple amines. J. Am. Chem. Soc. 2008, 130, 5974–5985. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Liang, S.; Li, J.-B.; Zhang, D.; Chen, W.-B.; Yang, Z.-J.; Xiao, S.; Wang, Q.-Q. Controlled Synthesis of Au Nanocrystals-Metal Selenide Hybrid Nanostructures toward Plasmon-Enhanced Photoelectrochemical Energy Conversion. Nanomaterials 2020, 10, 564. https://doi.org/10.3390/nano10030564
Tang L, Liang S, Li J-B, Zhang D, Chen W-B, Yang Z-J, Xiao S, Wang Q-Q. Controlled Synthesis of Au Nanocrystals-Metal Selenide Hybrid Nanostructures toward Plasmon-Enhanced Photoelectrochemical Energy Conversion. Nanomaterials. 2020; 10(3):564. https://doi.org/10.3390/nano10030564
Chicago/Turabian StyleTang, Ling, Shan Liang, Jian-Bo Li, Dou Zhang, Wen-Bo Chen, Zhong-Jian Yang, Si Xiao, and Qu-Quan Wang. 2020. "Controlled Synthesis of Au Nanocrystals-Metal Selenide Hybrid Nanostructures toward Plasmon-Enhanced Photoelectrochemical Energy Conversion" Nanomaterials 10, no. 3: 564. https://doi.org/10.3390/nano10030564