Continuous-Wave Laser-Induced Transfer of Metal Nanoparticles to Arbitrary Polymer Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation and CW Laser-Induced Metal NP Transfer Method
2.2. Optical Setup
2.3. Measurements
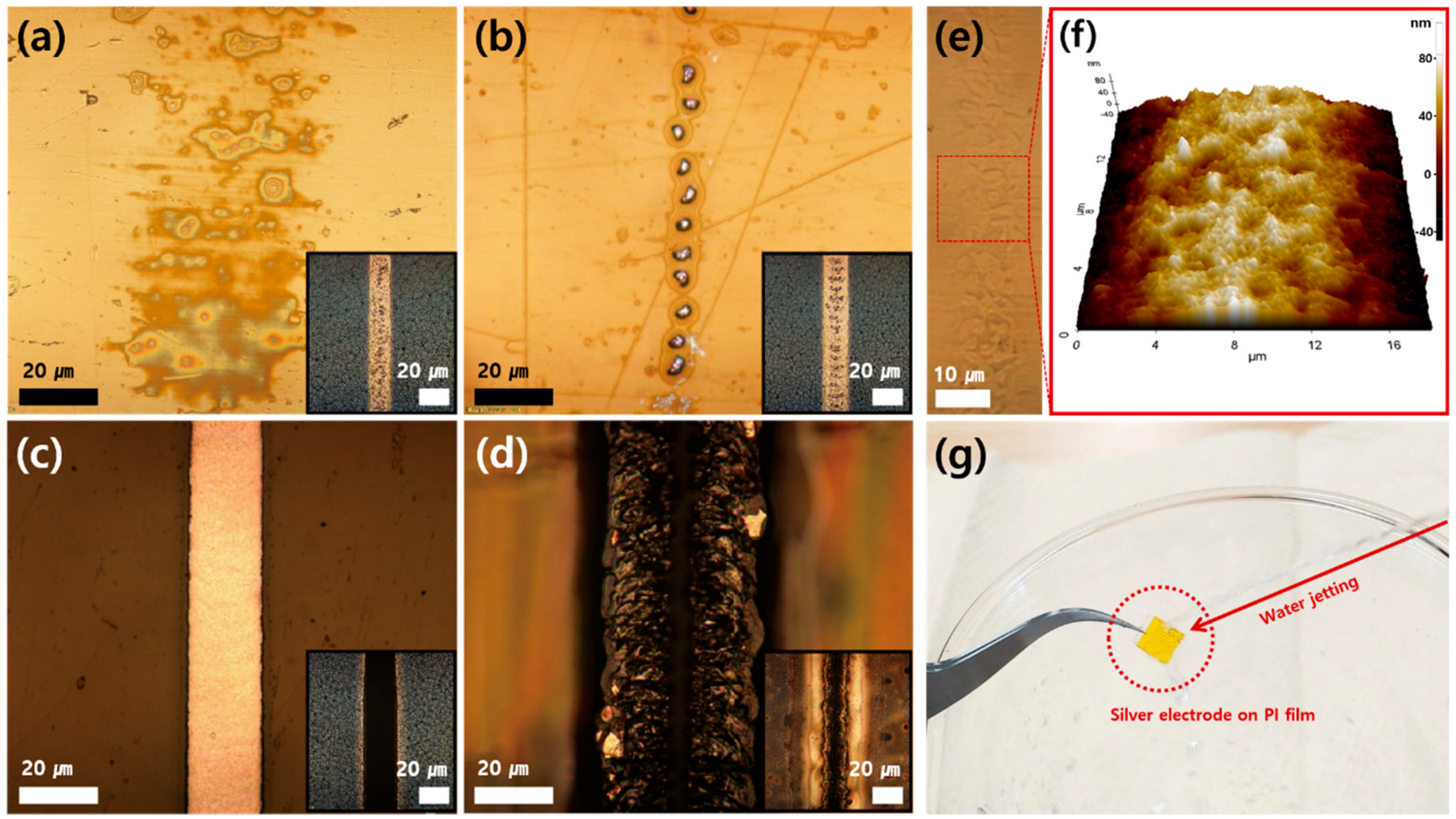
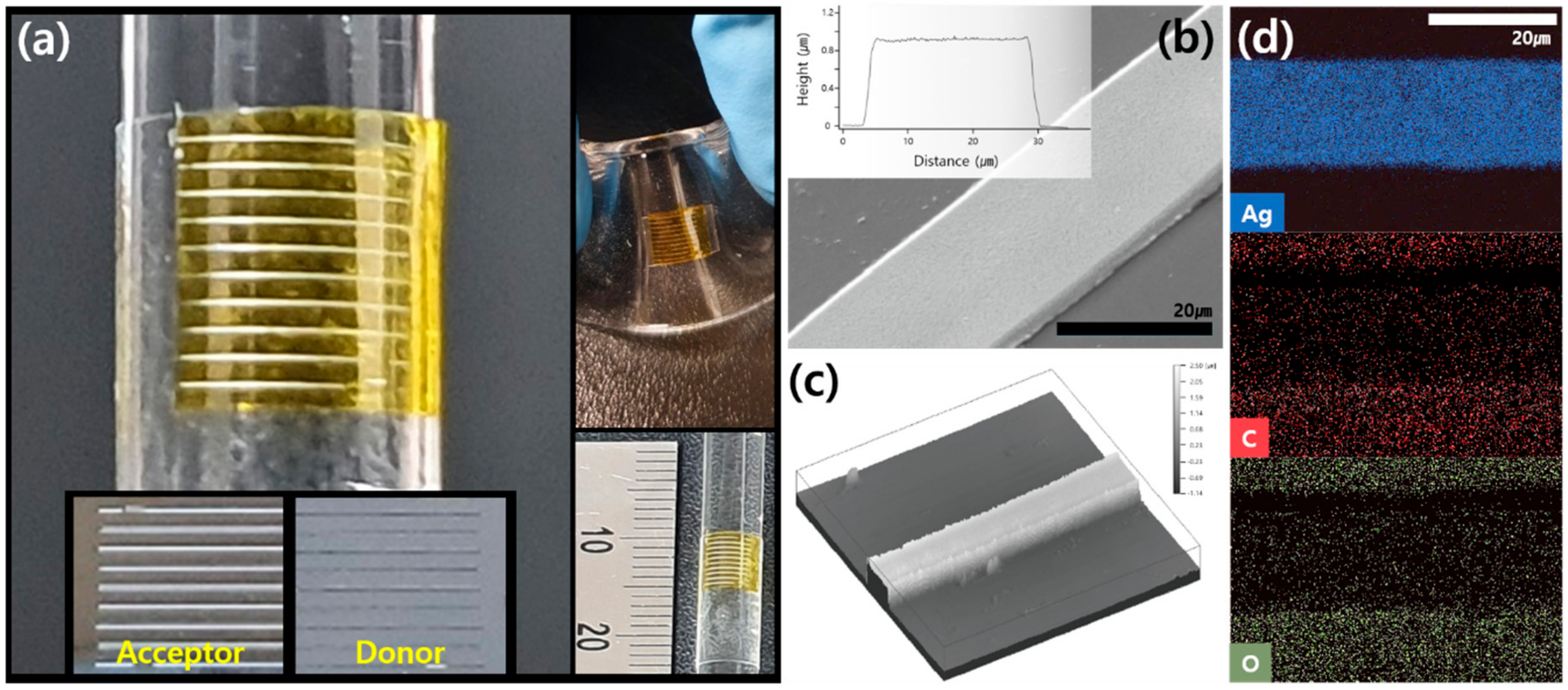
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, J.; Campbell, A.S.; de Avila, B.E.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Bao, Z.; Chen, X. Flexible and Stretchable Devices. Adv. Mater. 2016, 28, 4177–4179. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef] [PubMed]
- Jayathilaka, W.A.D.M.; Qi, K.; Qin, Y.; Chinnappan, A.; Serrano-García, W.; Baskar, C.; Wang, H.; He, J.; Cui, S.; Thomas, S.W.; et al. Significance of Nanomaterials in Wearables: A Review on Wearable Actuators and Sensors. Adv. Mater. 2019, 31, 1805921. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lee, H.; Lee, J.; Kwon, J.; Han, S.; Suh, Y.D.; Cho, H.; Shin, J.; Yeo, J.; Ko, S.H. Highly stretchable and transparent metal nanowire heater for wearable electronics applications. Adv. Mater. 2015, 27, 4744–4751. [Google Scholar] [CrossRef]
- Perelaer, J.; Schubert, U.S. Novel approaches for low temperature sintering of inkjet-printed inorganic nanoparticles for roll-to-roll (R2R) applications. J. Mater. Res. 2013, 28, 564–573. [Google Scholar] [CrossRef]
- Kumpulainen, T.; Pekkanen, J.; Valkama, J.; Laakso, J.; Tuokko, R.; Mäntysalo, M. Low temperature nanoparticle sintering with continuous wave and pulse lasers. Opt. Laser Technol. 2011, 43, 570–576. [Google Scholar] [CrossRef]
- Ko, S.H.; Pan, H.; Grigoropoulos, C.P.; Luscombe, C.K.; Fréchet, J.M.J.; Poulikakos, D. All-inkjet-printed flexible electronics fabrication on a polymer substrate by low-temperature high-resolution selective laser sintering of metal nanoparticles. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Hong, S.; Yeo, J.; Kim, G.; Kim, D.; Lee, H.; Kwon, J.; Lee, H.; Lee, P.; Ko, S.H. Nonvacuum, Maskless Fabrication of a Flexible Metal Grid Transparent Conductor by Low-Temperature Selective Laser Sintering of Nanoparticle Ink. ACS Nano 2013, 7, 5024–5031. [Google Scholar] [CrossRef]
- Yeo, J.; Hong, S.; Lee, D.; Hotz, N.; Lee, M.T.; Grigoropoulos, C.P.; Ko, S.H. Next generation non-vacuum, maskless, low temperature nanoparticle ink laser digital direct metal patterning for a large area flexible electronics. PLoS ONE 2012, 7, e42315. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.; Cho, H.; Eom, H.; Lee, H.; Suh, Y.D.; Moon, H.; Shin, J.; Hong, S.; Ko, S.H. Low-Temperature Oxidation-Free Selective Laser Sintering of Cu Nanoparticle Paste on a Polymer Substrate for the Flexible Touch Panel Applications. Acs Appl. Mater. Interfaces 2016, 8, 11575–11582. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Chen, J.K. Femtosecond laser sintering of copper nanoparticles. Appl. Phys. A 2016, 122. [Google Scholar] [CrossRef]
- Nam, V.B.; Shin, J.; Yoon, Y.; Giang, T.T.; Kwon, J.; Suh, Y.D.; Yeo, J.; Hong, S.; Ko, S.H.; Lee, D. Highly Stable Ni-Based Flexible Transparent Conducting Panels Fabricated by Laser Digital Patterning. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Shin, J.; Jeong, B.; Kim, J.; Nam, V.B.; Yoon, Y.; Jung, J.; Hong, S.; Lee, H.; Eom, H.; Yeo, J.; et al. Sensitive Wearable Temperature Sensor with Seamless Monolithic Integration. Adv. Mater. 2020, 32, 1905527. [Google Scholar] [CrossRef]
- Shin, W.; Lim, J.; Lee, Y.; Park, S.; Kim, H.; Cho, H.; Shin, J.; Yoon, Y.; Lee, H.; Kim, H.J.; et al. Shear-Assisted Laser Transfer of Metal Nanoparticle Ink to an Elastomer Substrate. Materials 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Boesel, L.F. Transfer printing processes. In Generating Micro- and Nanopatterns on Polymeric Materials; del Campo, A., Arzt, E., Eds.; Wiley-VCH: Weinheim, Germany, 2011; pp. 69–92. [Google Scholar] [CrossRef]
- Serra, P.; Piqué, A. Laser-Induced Forward Transfer: Fundamentals and Applications. Adv. Mater. Technol. 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- Papazoglou, S.; Zergioti, I. Laser Induced Forward Transfer (LIFT) of nano-micro patterns for sensor applications. Microelectron. Eng. 2017, 182, 25–34. [Google Scholar] [CrossRef]
- Puerto, D.; Biver, E.; Alloncle, A.P.; Delaporte, P. Single step high-speed printing of continuous silver lines by laser-induced forward transfer. Appl. Surf. Sci. 2016, 374, 183–189. [Google Scholar] [CrossRef]
- Florian, C.; Caballero-Lucas, F.; Fernández-Pradas, J.M.; Ogier, S.; Winchester, L.; Karnakis, D.; Geremia, R.; Artigas, R.; Serra, P. Printing of silver conductive lines through laser-induced forward transfer. Appl. Surf. Sci. 2016, 374, 265–270. [Google Scholar] [CrossRef]
- Florian, C.; Caballero-Lucas, F.; Fernández-Pradas, J.M.; Artigas, R.; Ogier, S.; Karnakis, D.; Serra, P. Conductive silver ink printing through the laser-induced forward transfer technique. Appl. Surf. Sci. 2015, 336, 304–308. [Google Scholar] [CrossRef]
- Fernández-Pradas, J.M.; Sopeña, P.; González-Torres, S.; Arrese, J.; Cirera, A.; Serra, P. Laser-induced forward transfer for printed electronics applications. Appl. Phys. A 2018, 124. [Google Scholar] [CrossRef]
- Sopena, P.; Arrese, J.; Gonzalez-Torres, S.; Fernandez-Pradas, J.M.; Cirera, A.; Serra, P. Low-Cost Fabrication of Printed Electronics Devices through Continuous Wave Laser-Induced Forward Transfer. ACS Appl. Mater. Interfaces 2017, 9, 29412–29417. [Google Scholar] [CrossRef] [PubMed]
- Sopena, P.; Gonzalez-Torres, S.; Fernandez-Pradas, J.M.; Serra, P. Spraying dynamics in continuous wave laser printing of conductive inks. Sci. Rep. 2018, 8, 7999. [Google Scholar] [CrossRef] [PubMed]
- Zacharatos, F.; Theodorakos, I.; Karvounis, P.; Tuohy, S.; Braz, N.; Melamed, S.; Kabla, A.; de la Vega, F.; Andritsos, K.; Hatziapostolou, A.; et al. Selective Laser Sintering of Laser Printed Ag Nanoparticle Micropatterns at High Repetition Rates. Materials 2018, 11, 2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalaal, M.; Li, S.; Klein Schaarsberg, M.; Qin, Y.; Lohse, D. Destructive mechanisms in laser induced forward transfer. Appl. Phys. Lett. 2019, 114, 213703. [Google Scholar] [CrossRef]
- Mathews, S.A.; Auyeung, R.C.Y.; Kim, H.; Charipar, N.A.; Piqué, A. High-speed video study of laser-induced forward transfer of silver nano-suspensions. J. Appl. Phys. 2013, 114. [Google Scholar] [CrossRef]
- Park, S.; Kwon, J.; Lim, J.; Shin, W.; Lee, Y.; Lee, H.; Kim, H.J.; Han, S.; Yeo, J.; Ko, S.H.; et al. Micropatterning of Metal Nanoparticle Ink by Laser-Induced Thermocapillary Flow. Nanomaterials 2018, 8, 645. [Google Scholar] [CrossRef] [Green Version]
- Raynor, P.C.; Leith, D. Evaporation of accumulated multicomponent liquids from fibrous filters. Ann. Occup. Hyg. 1999, 43, 181–192. [Google Scholar] [CrossRef]
- Shen, Y.F.; Gu, D.D.; Pan, Y.F. Balling Process in Selective Laser Sintering 316 Stainless Steel Powder. Key Eng. Mater. 2006, 315–316, 357–360. [Google Scholar] [CrossRef]
- Oyar, P. Laser Sintering Technology and Balling Phenomenon. Photomed. Laser Surg. 2018, 36, 72–77. [Google Scholar] [CrossRef]
- Tiliakos, A.; Ceaus, C.; Iordache, S.M.; Vasile, E.; Stamatin, I. Morphic transitions of nanocarbons via laser pyrolysis of polyimide films. J. Anal. Appl. Pyrolysis 2016, 121, 275–286. [Google Scholar] [CrossRef]
- Mamleyev, E.R.; Heissler, S.; Nefedov, A.; Weidler, P.G.; Nordin, N.; Kudryashov, V.V.; Länge, K.; MacKinnon, N.; Sharma, S. Laser-induced hierarchical carbon patterns on polyimide substrates for flexible urea sensors. NPJ Flex. Electron. 2019, 3, 2. [Google Scholar] [CrossRef]
- Lee, LH. (Ed.) The chemistry and physics of solid adhesion. In Fundamentals of Adhesion; Springer: Boston, MA, USA, 1991; pp. 1–86. [Google Scholar] [CrossRef]
- Benjamin, P.; Weaver, C.; Mott, N.F. The adhesion of evaporated metal films on glass. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1961, 261, 516–531. [Google Scholar] [CrossRef]
- Blanchet, G.B.; Loo, Y.-L.; Rogers, J.A.; Gao, F.; Fincher, C.R. Large area, high resolution, dry printing of conducting polymers for organic electronics. Appl. Phys. Lett. 2003, 82, 463–465. [Google Scholar] [CrossRef] [Green Version]
- Makrygianni, M.; Kalpyris, I.; Boutopoulos, C.; Zergioti, I. Laser induced forward transfer of Ag nanoparticles ink deposition and characterization. Appl. Surf. Sci. 2014, 297, 40–44. [Google Scholar] [CrossRef]
- Sopena, P.; Fernández-Pradas, J.M.; Serra, P. Laser-induced forward transfer of low viscosity inks. Appl. Surf. Sci. 2017, 418, 530–535. [Google Scholar] [CrossRef]
- Theodorakos, I.; Kalaitzis, A.; Makrygianni, M.; Hatziapostolou, A.; Kabla, A.; Melamed, S.; de la Vega, F.; Zergioti, I. Laser-Induced Forward Transfer of High Viscous, Non-Newtonian Silver Nanoparticle Inks: Jet Dynamics and Temporal Evolution of the Printed Droplet Study. Adv. Eng. Mater. 2019, 21. [Google Scholar] [CrossRef] [Green Version]
- Koritsoglou, O.; Theodorakos, I.; Zacharatos, F.; Makrygianni, M.; Kariyapperuma, D.; Price, R.; Cobb, B.; Melamed, S.; Kabla, A.; de la Vega, F.; et al. Copper micro-electrode fabrication using laser printing and laser sintering processes for on-chip antennas on flexible integrated circuits. Opt. Mater. Express 2019, 9, 3046–3058. [Google Scholar] [CrossRef]
- Wang, X.S.; Xu, B.; Huang, Y.K.; Zhang, J.; Liu, Q. Laser-induced forward transfer of silver nanoparticle ink using burst technique. Appl. Phys. A Mater. 2019, 125, 845. [Google Scholar] [CrossRef]


| Laser | Laser Condition | Donor | Acceptor | Gap distance | Year | Material | Method | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Pulsed laser (ns) | 266 nm 40–100 mJ/cm2 | Quartz | SiO2 | 300 µm | 2014 | Silver | LIFT Additional Heat treatment | [37] |
| 2 | Pulsed laser (fs) | 1027 nm 530 mJ/cm2 | Glass | Glass | 200 µm | 2015 | Silver | LIFT Additional Heat treatment | [21] |
| 3 | Pulsed laser (fs) | 1027 nm 530 mJ/cm2 | Glass | Glass with ablated channel | 160 µm | 2016 | Silver | LIFT Additional Heat treatment | [20] |
| 4 | Pulsed laser (ps) | 343 nm 35–64 mJ/cm2 | Quartz | Glass | 230 µm | 2016 | Silver | LIFT Additional Heat treatment | [19] |
| 5 | Pulsed laser (ns) | 1064 nm 2–6 J/cm2 | Glass | Glass | 150 µm | 2017 | Silver | LIFT | [38] |
| 6 | Continuous wave laser | 1064 nm 1W 600mm/s | Glass | Glass | 150 µm | 2017 | Silver | CW-LIFT Additional Heat treatment | [23] |
| 7 | Pulsed laser (ns) | 1064 nm 11000 mJ/cm2 | Glass | Paper | 150 µm | 2018 | Silver | LIFT Additional Heat treatment | [22] |
| 8 | Continuous wave laser | 532 nm 0.54 W 140 mm/s | Glass | PDMS | Contact | 2018 | Silver | Shear-assisted | [15] |
| 9 | Pulsed laser (ns) | 532 nm 320 mJ/cm2 | Glass | Glass SU-8 film Organic layer | 150 µm 500 µm 3000 µm | 2019 | Silver | LIFT Additional Heat treatment | [39] |
| 10 | Pulsed laser (ns) | 532 nm 360 mJ/cm2 | Quartz | SU8 on glass | 100 µm | 2019 | Copper | LIFT Post sintering | [40] |
| 11 | Pulsed laser (ps) | 532 nm 80–240 nJ (burst mode) | Glass | Glass | 200 µm | 2019 | Silver | LIFT(burst) | [41] |
| Current Study | Continuous wave laser | 532 nm 0.27 W 5 mm/s | Glass | Polymer Substrates | Contact | Silver | Enhanced adhesion by CW laser |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.; Kim, Y.; Shin, J.; Lee, Y.; Shin, W.; Qu, W.; Hwang, E.; Park, S.; Hong, S. Continuous-Wave Laser-Induced Transfer of Metal Nanoparticles to Arbitrary Polymer Substrates. Nanomaterials 2020, 10, 701. https://doi.org/10.3390/nano10040701
Lim J, Kim Y, Shin J, Lee Y, Shin W, Qu W, Hwang E, Park S, Hong S. Continuous-Wave Laser-Induced Transfer of Metal Nanoparticles to Arbitrary Polymer Substrates. Nanomaterials. 2020; 10(4):701. https://doi.org/10.3390/nano10040701
Chicago/Turabian StyleLim, Jaemook, Youngchan Kim, Jaeho Shin, Younggeun Lee, Wooseop Shin, Weihao Qu, Eunseung Hwang, Seongje Park, and Sukjoon Hong. 2020. "Continuous-Wave Laser-Induced Transfer of Metal Nanoparticles to Arbitrary Polymer Substrates" Nanomaterials 10, no. 4: 701. https://doi.org/10.3390/nano10040701





