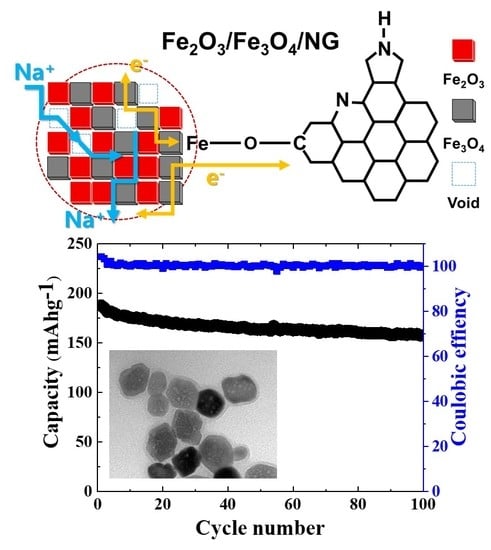
Plasma Enabled Fe2O3/Fe3O4 Nano-aggregates Anchored on Nitrogen-doped Graphene as Anode for Sodium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
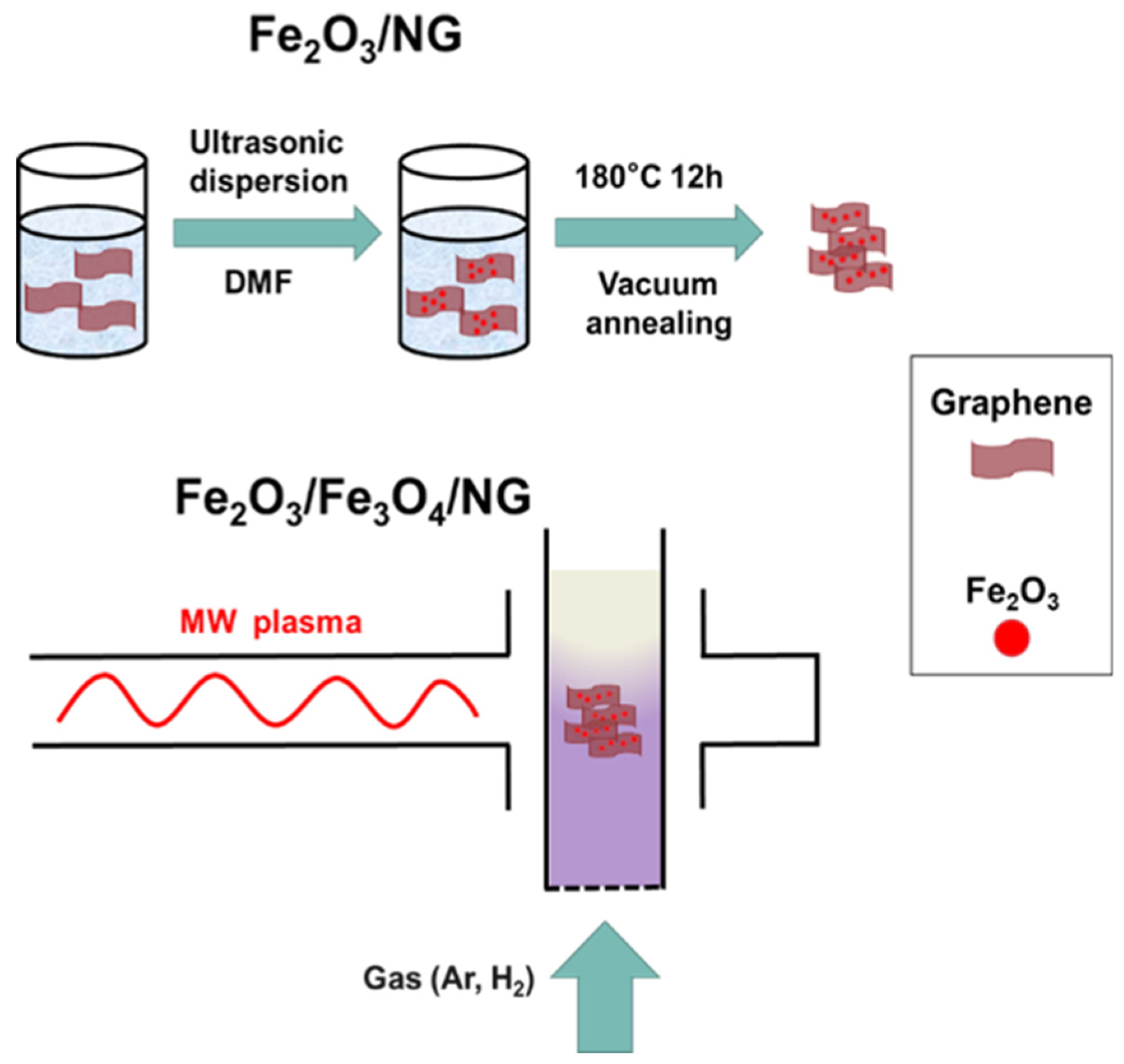
2.1. Materials Preparation
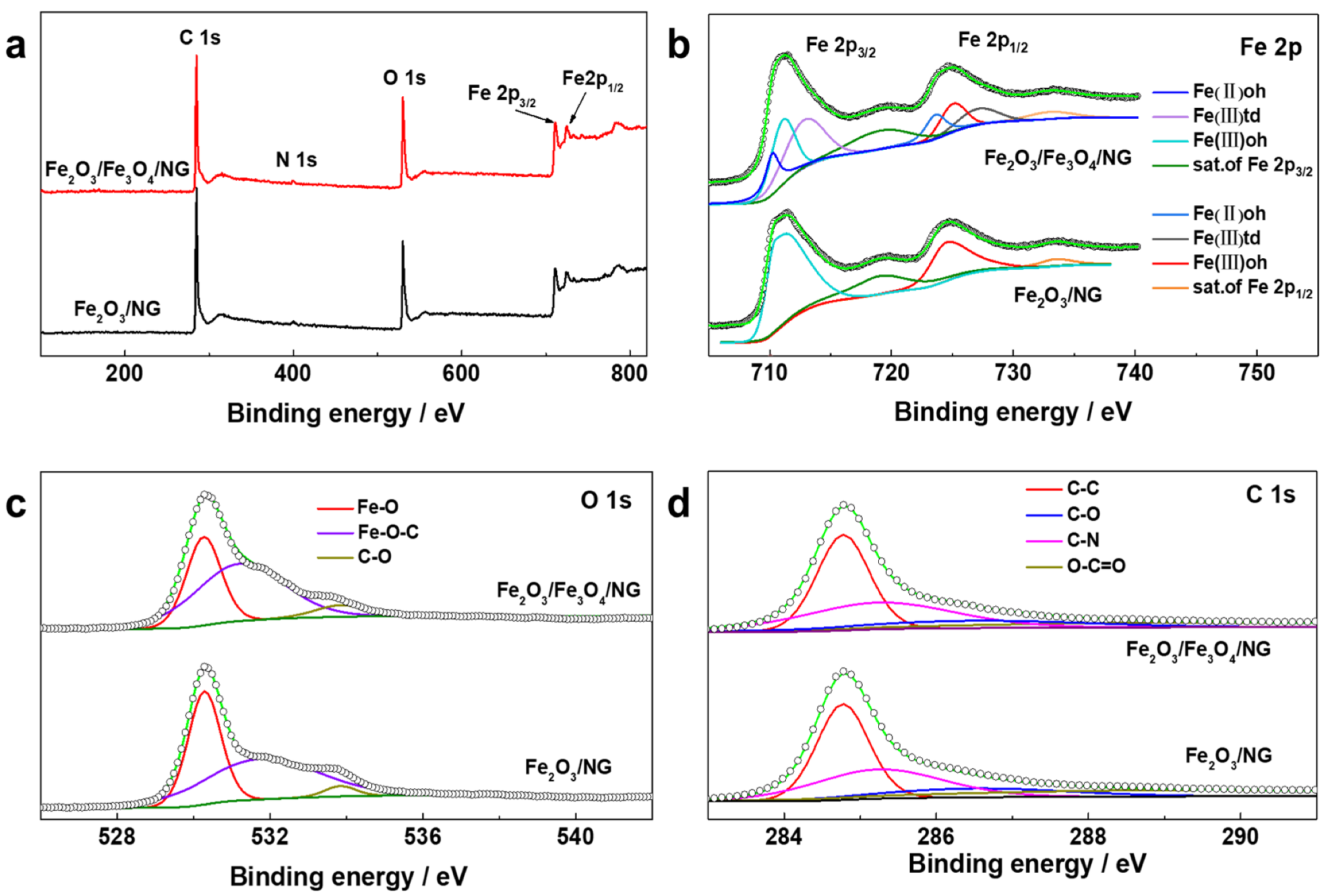
2.2. Materials Characterization
2.3. Electrochemical Measurements
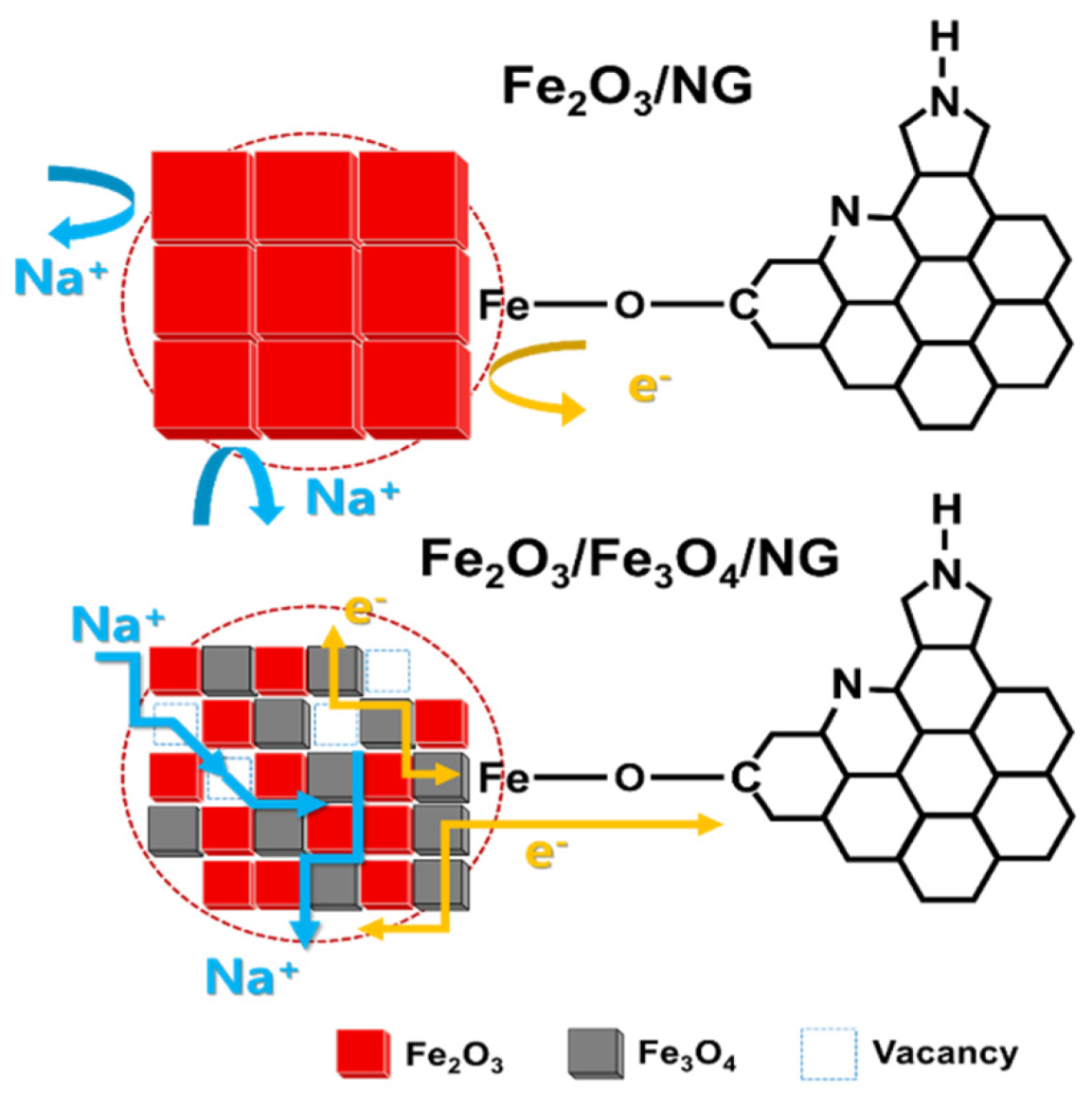
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiao, L.F.; Lu, H.Y.; Fang, Y.J.; Sushko, M.L.; Cao, Y.L.; Ai, X.P.; Yang, H.X.; Liu, J. Low-Defect and Low-Porosity Hard Carbon with High Coulombic Efficiency and High Capacity for Practical Sodium Ion Battery Anode. Adv. Energy Mater. 2018, 8, 7. [Google Scholar] [CrossRef]
- Sun, W.P.; Rui, X.H.; Zhang, D.; Jiang, Y.Z.; Sun, Z.Q.; Liu, H.K.; Dou, S.X. Bismuth sulfide: A high-capacity anode for sodium-ion batteries. J. Power Sources 2016, 309, 135–140. [Google Scholar] [CrossRef]
- Pan, H.L.; Hu, Y.S.; Chen, L.Q. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Ponrouch, A.; Marchante, E.; Courty, M.; Tarascon, J.M.; Palacin, M.R. In search of an optimized electrolyte for Na-ion batteries. Energy Environ. Sci. 2012, 5, 8572–8583. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [Green Version]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.N.; Li, W.Y.; Gou, X.L. alpha-Fe2O3 nanotubes in gas sensor and lithium-ion battery applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar] [CrossRef]
- Zhang, N.; Han, X.P.; Liu, Y.C.; Hu, X.F.; Zhao, Q.; Chen, J. 3D Porous gamma-Fe2O3@C Nanocomposite as High-Performance Anode Material of Na-Ion Batteries. Adv. Energy Mater. 2015, 5, 7. [Google Scholar] [CrossRef]
- Jian, Z.L.; Zhao, B.; Liu, P.; Li, F.J.; Zheng, M.B.; Chen, M.W.; Shi, Y.; Zhou, H.S. Fe2O3 nanocrystals anchored onto graphene nanosheets as the anode material for low-cost sodium-ion batteries. Chem. Commun. 2014, 50, 1215–1217. [Google Scholar] [CrossRef]
- Cui, Z.M.; Hang, L.Y.; Song, W.G.; Guo, Y.G. High-Yield Gas-Liquid Interfacial Synthesis of Highly Dispersed Fe3O4 Nanocrystals and Their Application in Lithium-Ion Batteries. Chem. Mater. 2009, 21, 1162–1166. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, N.; Tao, F.; Pan, Q.M. Improving the lithium storage properties of Fe2O3@C nanoparticles by superoleophilic and superhydrophobic polysiloxane coatings. J. Mater. Chem. 2012, 22, 15894–15900. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wang, Y.X.; Chou, S.L.; Li, H.J.; Liu, H.K.; Wang, J.Z. Rapid synthesis of alpha-Fe2O3/rGO nanocomposites by microwave autoclave as superior anodes for sodium-ion batteries. J. Power Sources 2015, 280, 107–113. [Google Scholar] [CrossRef]
- Liu, X.J.; Chen, T.Q.; Chu, H.P.; Niu, L.Y.; Sun, Z.; Pan, L.K.; Sun, C.Q. Fe2O3-reduced graphene oxide composites synthesized via microwave-assisted method for sodium ion batteries. Electrochim. Acta 2015, 166, 12–16. [Google Scholar] [CrossRef]
- Zhao, C.J.; Shao, X.X.; Zhang, Y.X.; Qian, X.Z. Fe2O3/Reduced Graphene Oxide/Fe3O4 Composite in Situ Grown on Fe Foil for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 30133–30142. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.J.; Li, S.X.; Xu, B.Y.; Zheng, F.Y.; Zhou, H.F.; Yu, H.W.; Lin, F.; Zhu, X.Q. Polycrystalline iron oxide nanoparticles prepared by C-dot-mediated aggregation and reduction for supercapacitor application. RSC Adv. 2016, 6, 45023–45030. [Google Scholar] [CrossRef]
- Tang, X.; Jia, R.Y.; Zhai, T.; Xia, H. Hierarchical Fe3O4@Fe2O3 Core-Shell Nanorod Arrays as High-Performance Anodes for Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 27518–27525. [Google Scholar] [CrossRef]
- Mallick, S.; Jana, P.P.; Raj, C.R. Asymmetric Supercapacitor Based on Chemically Coupled Hybrid Material of Fe2O3-Fe3O4 Heterostructure and Nitrogen-Doped Reduced Graphene Oxide. Chemelectrochem 2018, 5, 2348–2356. [Google Scholar] [CrossRef]
- Wang, X.L.; Qin, M.L.; Fang, F.; Jia, B.R.; Wu, H.Y.; Qu, X.H.; Volinsky, A.A. Solution combustion synthesis of nanostructured iron oxides with controllable morphology, composition and electrochemical performance. Ceram. Int. 2018, 44, 4237–4247. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.W.; Huang, R.S.; Yao, J.H. Ternary Fe2O3/Fe3O4/FeCO3 Composite as a High-Performance Anode Material for Lithium-Ion Batteries. J. Phys. Chem. C 2019, 123, 12614–12622. [Google Scholar] [CrossRef]
- Li, Y.F.; Fu, Y.Y.; Chen, S.H.; Huang, Z.Z.; Wang, L.; Song, Y.H. Porous Fe2O3/Fe3O4@Carbon octahedron arrayed on three-dimensional graphene foam for lithium-ion battery. Compos. Part B 2019, 171, 130–137. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Vasilev, K.; Ramiasa, M.M. Nanoengineered Interfaces, Coatings, and Structures by Plasma Techniques. Nanomaterials 2017, 7, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.; Cao, J.; Khattak, A.M.; Cai, F.; Jiang, B.; Yang, G.; Hu, S. High-performance tin oxide-nitrogen doped graphene aerogel hybrids as anode materials for lithium-ion batteries. J. Power Sources 2014, 270, 28–33. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.S.; Chen, X.H.; Song, H.H. Amorphous Fe2O3/Graphene Composite Nanosheets with Enhanced Electrochemical Performance for Sodium-Ion Battery. ACS Appl. Mater. Interfaces 2016, 8, 30899–30907. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.S.; Yang, C.; Chen, G.X.; Yan, X.Q.; Chen, S.N.; Su, B.W.; Liu, Z.B.; Tian, J.G. Preparation of high-quality graphene using triggered microwave reduction under an air atmosphere. J. Mater. Chem. C 2018, 6, 1829–1835. [Google Scholar] [CrossRef]
- Abe, T.; Miyazawa, A.; Kawanishi, Y.; Konno, H. Microwave-Assisted Synthesis of Metal Complexes. Mini-Rev. Org. Chem. 2011, 8, 315–333. [Google Scholar] [CrossRef]
- Zhou, K.; Zhen, Y.; Hong, Z.; Guo, J.; Huang, Z. Enhanced sodium ion batteries performance by the phase transition from hierarchical Fe2O3 to Fe3O4 hollow nanostructures. Mater. Lett. 2017, 190, 52–55. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.; Zhao, D.-L.; Wu, L.-L.; Ding, Z.-W.; Cheng, X.-W.; Hu, T. Fe2O3/nitrogen-doped graphene nanosheet nanocomposites as anode materials for sodium-ion batteries with enhanced electrochemical performance. J. Alloy. Compd. 2018, 737, 130–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Bakenov, Z.; Tan, T.; Huang, J. Synthesis of Core-Shell Carbon Encapsulated Fe2O3 Composite through a Facile Hydrothermal Approach and Their Application as Anode Materials for Sodium-Ion Batteries. Metals 2018, 8, 461. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Wei, Q.; Wang, X.; Zhang, G.; Shu, H.; Yang, X.; Tavares, A.C.; Sun, S. A facile synthesis of Fe3O4 nanoparticles/graphene for high-performance lithium/sodium-ion batteries. RSC Adv. 2016, 6, 16624–16633. [Google Scholar] [CrossRef]
- Qi, H.; Cao, L.; Li, J.; Huang, J.; Xu, Z.; Jie, Y.; Wang, C. Thin Carbon Layer Coated Porous Fe3O4 Particles Supported by rGO Sheets for Improved Stable Sodium Storage. Chemistryselect 2019, 4, 2668–2675. [Google Scholar] [CrossRef]
- Guo, T.X.; Liao, H.X.; Ge, P.; Zhang, Y.; Tian, Y.Q.; Hong, W.W.; Shi, Z.D.; Shao, C.S.; Hou, H.S.; Ji, X.B. Fe2O3 embedded in the nitrogen-doped carbon matrix with strong C-O-Fe oxygen-bridge bonds for enhanced sodium storages. Mater. Chem. Phys. 2018, 216, 58–63. [Google Scholar] [CrossRef]
- Liu, H.; Jia, M.Q.; Zhu, Q.Z.; Cao, B.; Chen, R.J.; Wang, Y.; Wu, F.; Xu, B. 3D-0D Graphene-Fe3O4 Quantum Dot Hybrids as High-Performance Anode Materials for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 26878–26885. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, Q.; Li, Y.; Chen, J.; Gu, J.; Zhang, W.; Zhang, D. Self-crosslink assisted synthesis of 3D porous branch-like Fe3O4/C hybrids for high-performance lithium/sodium-ion batteries. RSC Adv. 2017, 7, 50307–50316. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.J.; Wang, F.X.; Wang, C.; Wang, S.; Wang, C.Y.; Zhao, Z.W.; Duan, L.L.; Liu, Y.P.; Wu, Y.P.; Li, W.; et al. Encapsulating highly crystallized mesoporous Fe3O4 in hollow N-doped carbon nanospheres for high-capacity long-life sodium-ion batteries. Nano Energy 2019, 56, 426–433. [Google Scholar] [CrossRef]





| Electrode | Rs (Ω) | Rct (Ω) | DNa+ (cm2 s−1) |
|---|---|---|---|
| Fe2O3/NG | 6 | 210.6 | 1.65 × 10−12 |
| Fe2O3/Fe3O4/NG | 8.9 | 134.7 | 1.34 × 10−11 |
| Anodes | Current Density (mA g−1) | References | |||
|---|---|---|---|---|---|
| Capacity (mAh g−1) | |||||
| Fe2O3/Fe3O4/NG | 100 | 200 | 1000 | 1200 | This work |
| 362 | 300 | 185 | 174 | ||
| Fe2O3/NG | 50 | 100 | 200 | 1000 | Meng 2017 [28] |
| 343 | 285 | 230 | 132 | ||
| Fe2O3/C | 50 | 100 | 200 | 1000 | Zhang 2018 [29] |
| 364 | 291 | 245 | 150 | ||
| Fe3O4/G | 100 | 200 | 500 | 1000 | Fu 2016 [30] |
| 310 | 225 | 180 | 140 | ||
| Fe3O4@C/G | 100 | 200 | 500 | 1000 | Qi 2019 [31] |
| 375 | 300 | 254 | 200 | ||
| Fe2O3@NC | 200 | 500 | 1000 | 4000 | Guo 2018 [32] |
| 289 | 253.7 | 221.5 | 167.8 | ||
| Fe3O4/G/QD | 100 | 200 | 500 | 1000 | Liu 2016 [33] |
| 316 | 273 | 216 | 113 | ||
| Fe3O4/C | 100 | 200 | 500 | 1000 | Wang 2017 [34] |
| 293 | 262 | 223 | 195 | ||
| Fe3O4@N–C | 80 | 240 | 400 | 800 | Zhao 2019 [35] |
| 386 | 315 | 277 | 248 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Ma, Y.; Liu, L.; Yao, S.; Wu, W.; Wang, Z.; Lv, P.; Zheng, J.; Yu, K.; Wei, W.; et al. Plasma Enabled Fe2O3/Fe3O4 Nano-aggregates Anchored on Nitrogen-doped Graphene as Anode for Sodium-Ion Batteries. Nanomaterials 2020, 10, 782. https://doi.org/10.3390/nano10040782
Wang Q, Ma Y, Liu L, Yao S, Wu W, Wang Z, Lv P, Zheng J, Yu K, Wei W, et al. Plasma Enabled Fe2O3/Fe3O4 Nano-aggregates Anchored on Nitrogen-doped Graphene as Anode for Sodium-Ion Batteries. Nanomaterials. 2020; 10(4):782. https://doi.org/10.3390/nano10040782
Chicago/Turabian StyleWang, Qianqian, Yujie Ma, Li Liu, Shuyue Yao, Wenjie Wu, Zhongyue Wang, Peng Lv, Jiajin Zheng, Kehan Yu, Wei Wei, and et al. 2020. "Plasma Enabled Fe2O3/Fe3O4 Nano-aggregates Anchored on Nitrogen-doped Graphene as Anode for Sodium-Ion Batteries" Nanomaterials 10, no. 4: 782. https://doi.org/10.3390/nano10040782