Synthesis and Cytotoxicity Studies of Wood-Based Cationic Cellulose Nanocrystals as Potential Immunomodulators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
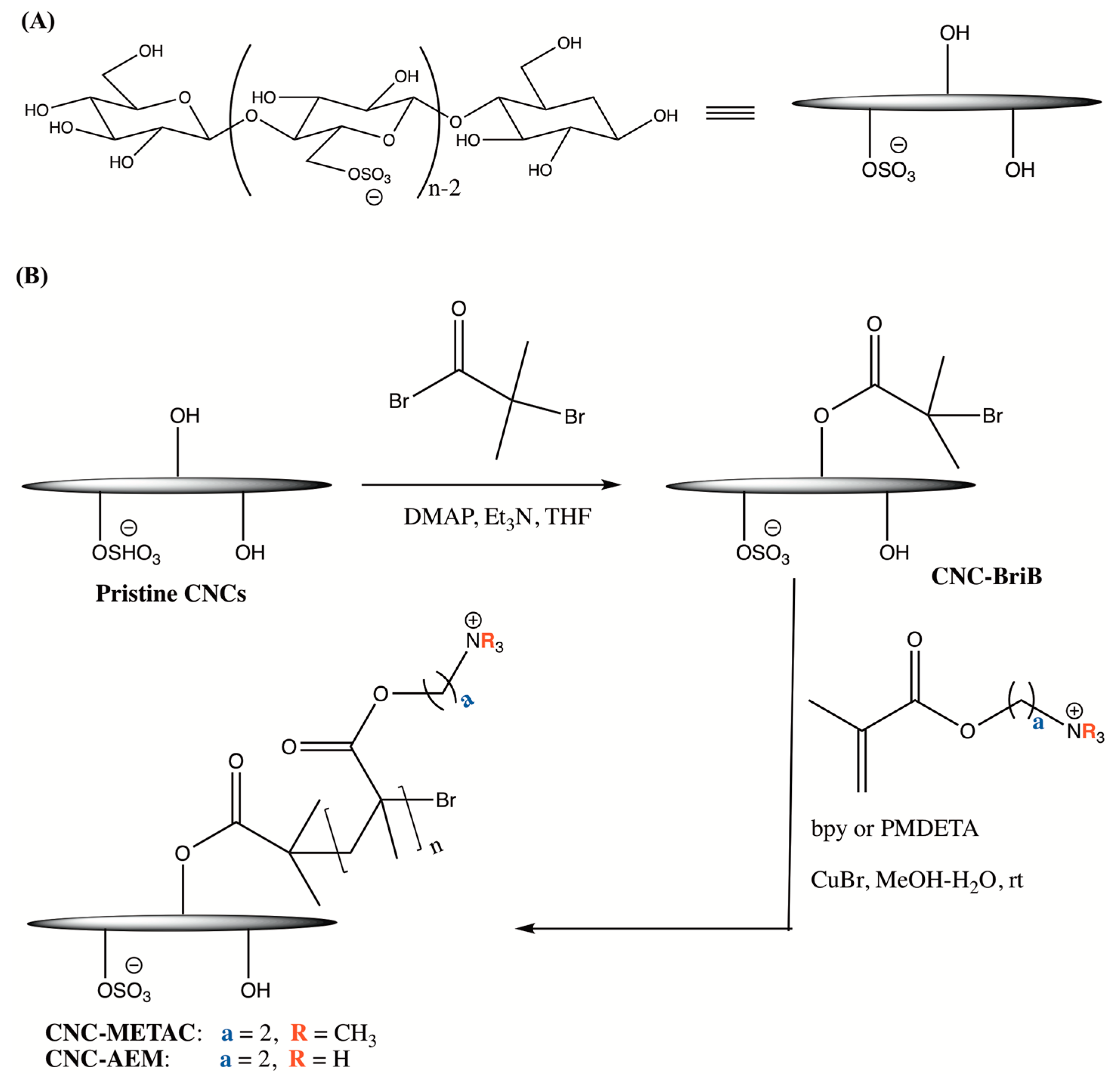
2.2. Synthesis of Wood-Based Cationic CNCs
2.3. Characterization of Pristine and Modified CNCs
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. Zeta Potential and Dynamic Light Scattering
2.3.3. Elemental Analysis
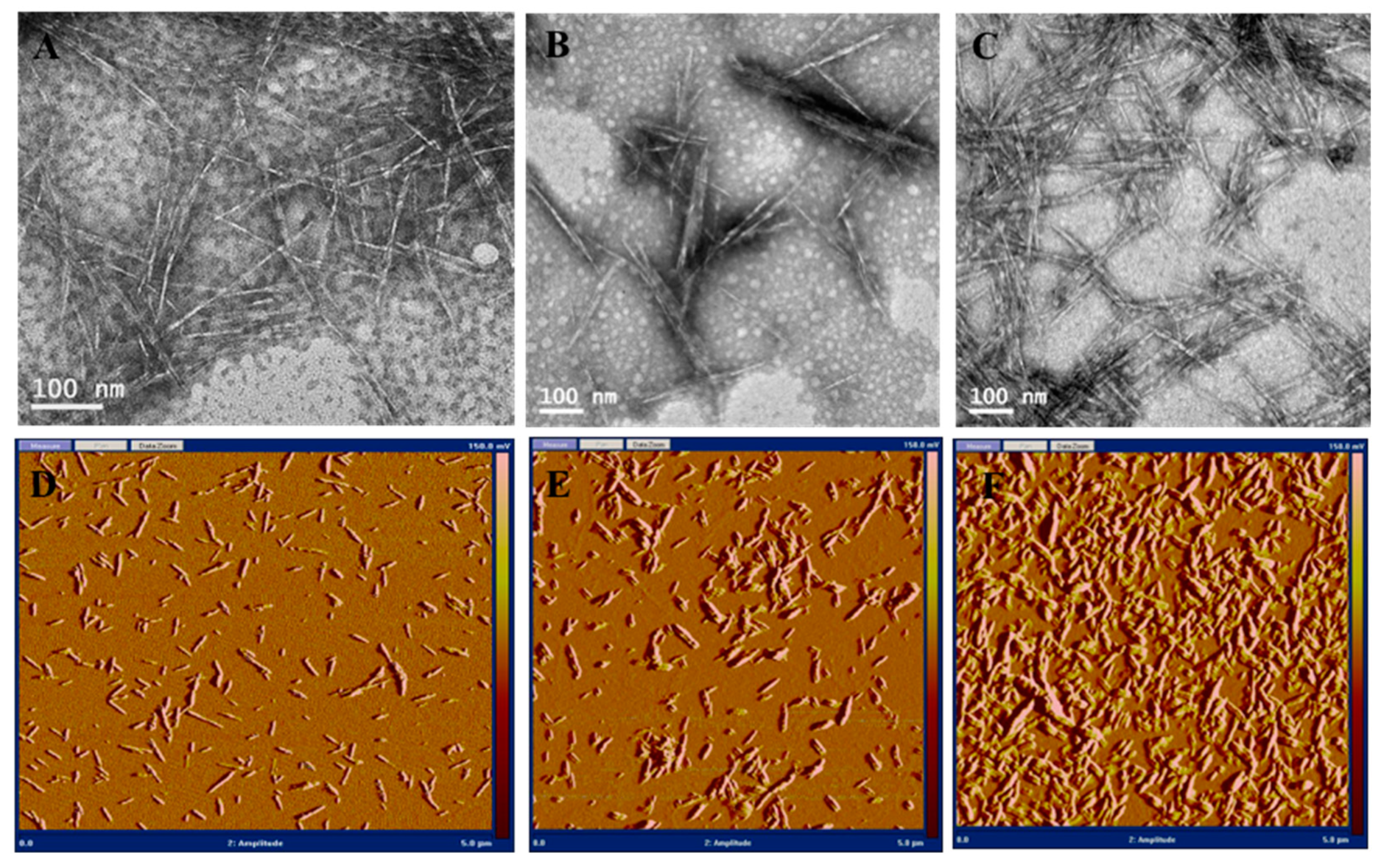
2.3.4. Transmission Electron Microscopy
2.3.5. Atomic Force Microscopy
2.4. Cytotoxicity Studies
2.4.1. Preparation of the Colloidal Suspension of CNCs for Cell-Based Assays
2.4.2. Cell Culture and Experimental Conditions
2.4.3. Cell Viability Assays
2.4.4. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Cationic CNCs
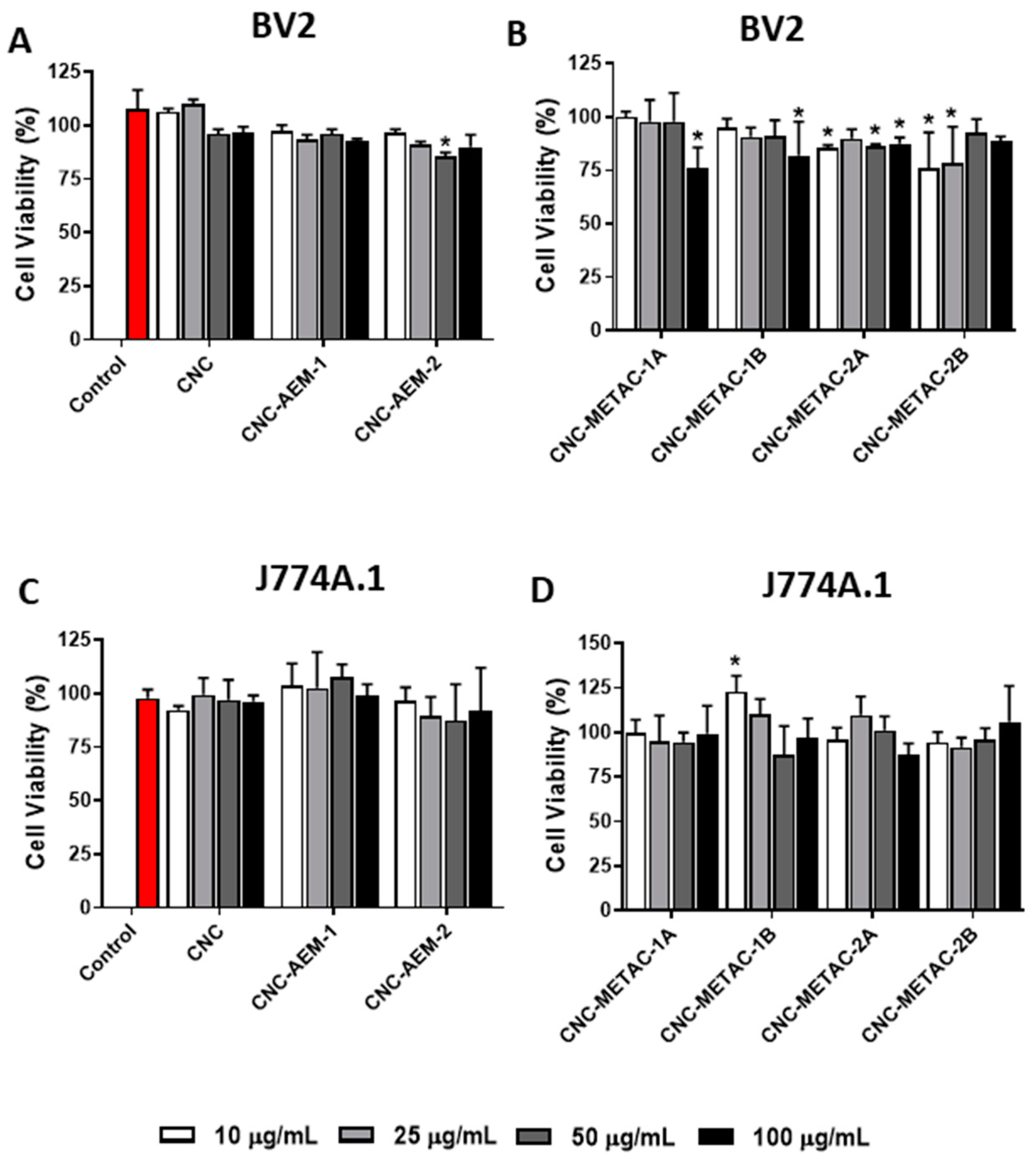
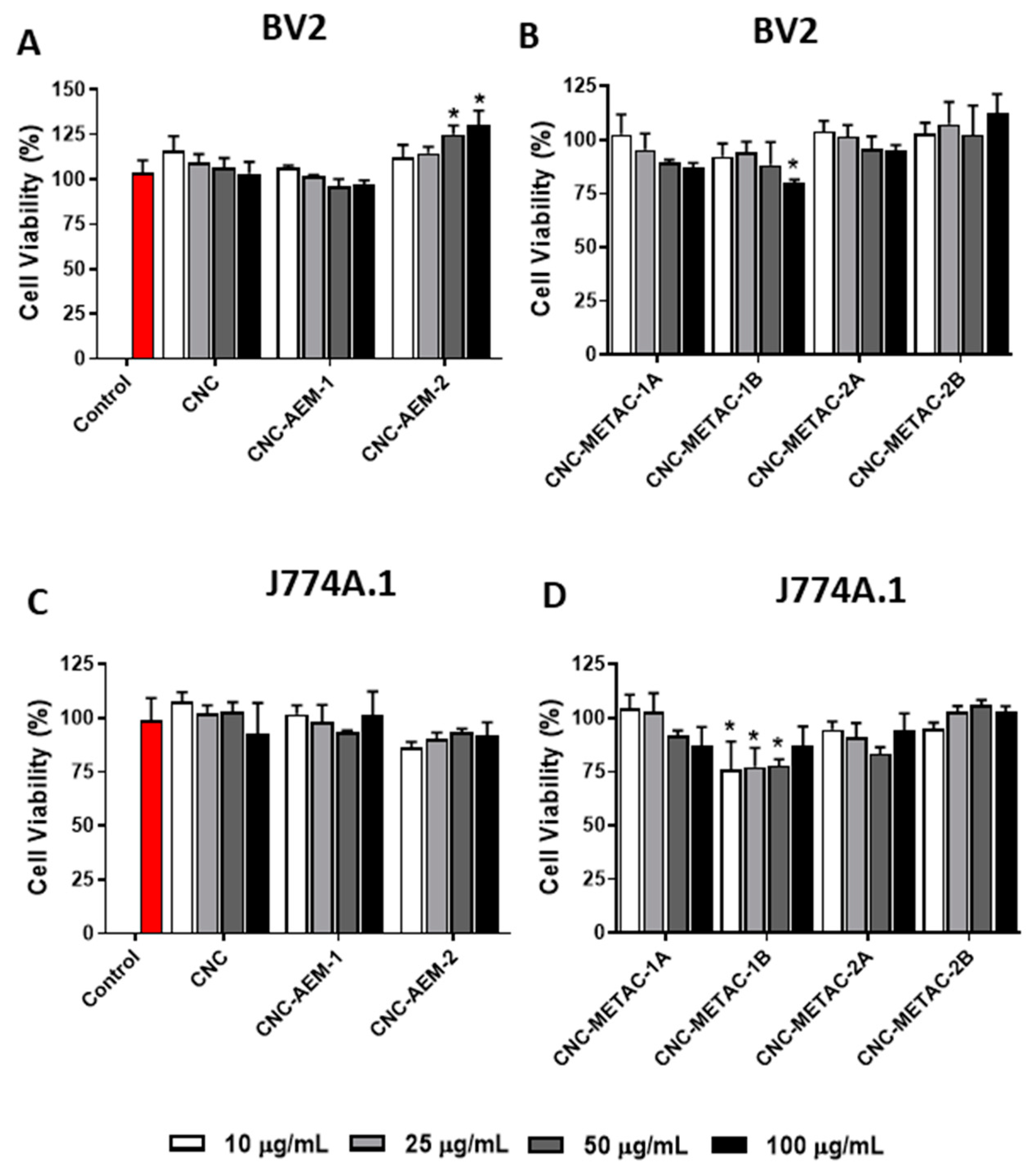
3.2. Cytotoxicity Studies of Cationic CNCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, T.B.; Huang, Y.P.; Yang, L.; Liu, T.T.; Gong, W.Y.; Wang, X.J.; Sheng, J.; Hu, J.M. Structural Characterization and Immunomodulating Activity of Polysaccharide from Dendrobium officinale. Int. J. Biol. Macromol. 2016, 83, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.P.; He, T.B.; Cuan, X.D.; Wang, X.J.; Hu, J.M.; Sheng, J. 1,4-Beta-d-Glucomannan from Dendrobium officinale Activates NF-small ka, CyrillicB via TLR4 to Regulate the Immune Response. Molecules 2018, 23, 2658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delves, P.J.; Roitt, I.M. The Immune System. First of Two Parts. N. Engl. J. Med. 2000, 343, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key Roles of Adjuvants in Modern Vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Gause, K.T.; Wheatley, A.K.; Cui, J.; Yan, Y.; Kent, S.J.; Caruso, F. Immunological Principles Guiding the Rational Design of Particles for Vaccine Delivery. ACS Nano 2017, 11, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Kunisawa, J.; Hayashi, A.; Tsutsumi, Y.; Kubo, K.; Nakagawa, S.; Nakanishi, M.; Tanaka, K.; Mayumi, T. Positively Charged Liposome Functions as an Efficient Immunoadjuvant in Inducing Cell-Mediated Immune Response to Soluble Proteins. J. Control. Release 1999, 61, 233–240. [Google Scholar] [CrossRef]
- Sun, B.; Ji, Z.; Liao, Y.P.; Chang, C.H.; Wang, X.; Ku, J.; Xue, C.; Mirshafiee, V.; Xia, T. Enhanced Immune Adjuvant Activity of Aluminum Oxyhydroxide Nanorods through Cationic Surface Functionalization. ACS Appl. Mater. Interfaces 2017, 9, 21697–21705. [Google Scholar] [CrossRef]
- Sunasee, R.; Araoye, E.; Pyram, D.; Hemraz, U.D.; Boluk, Y.; Ckless, K. Cellulose Nanocrystal Cationic Derivative Induces NLRP3 Inflammasome-Dependent IL-1β Secretion Associated with Mitochondrial ROS Production. Biochem. Biophys. Rep. 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Guglielmo, A.; Sabra, A.; Elbery, M.; Cerveira, M.M.; Ghenov, F.; Sunasee, R.; Ckless, K. A Mechanistic Insight into Curcumin Modulation of the IL-1beta Secretion and NLRP3 S-glutathionylation Induced by Needle-like Cationic Cellulose Nanocrystals in Myeloid Cells. Chem. Biol. Interact. 2017, 274, 1–12. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstroem, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose Nanomaterials Review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Sunasee, R.; Hemraz, U.D.; Ckless, K. Cellulose Nanocrystals: A Versatile Nanoplatform for Emerging Biomedical Applications. Expert Opin. Drug Deliv. 2016, 13, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Sunasee, R. Nanocellulose: Preparation, Functionalization and Applications. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Reid, M.S.; Villalobos, M.; Cranston, E.D. Benchmarking Cellulose Nanocrystals: From the Laboratory to Industrial Production. Langmuir 2017, 33, 1583–1598. [Google Scholar] [CrossRef]
- Hasani, M.; Cranston, E.D.; Westman, G.; Gray, D.G. Cationic Surface Functionalization of Cellulose Nanocrystals. Soft Matter 2008, 4, 2238–2244. [Google Scholar] [CrossRef]
- Jasmani, L.; Eyley, S.; Wallbridge, R.; Thielemans, W. A Facile One-pot Route to Cationic Cellulose Nanocrystals. Nanoscale 2013, 5, 10207–10211. [Google Scholar] [CrossRef]
- Feese, E.; Sadeghifar, H.; Gracz, H.S.; Argyropoulos, D.S.; Ghiladi, R.A. Photobactericidal Porphyrin-Cellulose Nanocrystals: Synthesis, Characterization, and Antimicrobial properties. Biomacromolecules 2011, 12, 3528–3539. [Google Scholar] [CrossRef]
- Sunasee, R.; Hemraz, U.D. Synthetic Strategies for the Fabrication of Cationic Surface-Modified Cellulose Nanocrystals. Fibers 2018, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Rosilo, H.; McKee, J.R.; Kontturi, E.; Koho, T.; Hytönen, V.P.; Ikkala, O.; Kostiainen, M.A. Cationic Polymer Brush-modified Cellulose Nanocrystals for High-Affinity Virus Binding. Nanoscale 2014, 6, 11871–11881. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Dupire, A.V.M.; Lachat, T.G.G.; Lemal, P.; Rodriguez-Lorenzo, L.; Petri-Fink, A.; Weder, C.; Klok, H.A. Cellulose Nanocrystals with Tethered Polymer Chains: Chemically Patchy versus Uniform Decoration. ACS Macro Lett. 2017, 6, 892–897. [Google Scholar] [CrossRef]
- Hemraz, U.D.; Campbell, K.A.; Burdick, J.S.; Ckless, K.; Boluk, Y.; Sunasee, R. Cationic Poly(2-aminoethylmethacrylate) and Poly(N-(2-aminoethylmethacrylamide) Modified Cellulose Nanocrystals: Synthesis, Characterization, and Cytotoxicity. Biomacromolecules 2015, 16, 319–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [PubMed]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Habibi, Y.; Rojas, O.J.; Venditti, R.A.; Johansson, L.S.; Efimenko, K.; Osterberg, M.; Laine, J. Poly(N-isopropylacrylamide) Brushes Grafted from Cellulose Nanocrystals via Surface-Initiated Single-Electron Transfer Living Radical Polymerization. Biomacromolecules 2010, 11, 2683–2691. [Google Scholar] [CrossRef]
- Xu, Q.; Yi, J.; Zhang, X.; Zhang, H. A Novel Amphotropic Polymer Based on Cellulose Nanocrystals Grafted with Azo Polymers. Eur. Polym. J. 2008, 44, 2830–2837. [Google Scholar] [CrossRef]
- Anastasaki, A.; Nikolaou, V.; Nurumbetov, G.; Wilson, P.; Kempe, K.; Quinn, J.F.; Davis, T.P.; Whittaker, M.R.; Haddleton, D.M. Cu(0)-Mediated Living Radical Polymerization: A Versatile Tool for Materials Synthesis. Chem. Rev. 2016, 116, 835–877. [Google Scholar] [CrossRef]
- Jimenez, A.S.; Jaramillo, F.; Hemraz, U.D.; Boluk, Y.; Ckless, K.; Sunasee, R. Effect of Surface Organic Coatings of Cellulose Nanocrystals on the Viability of Mammalian Cell Lines. Nanotechnol. Sci. Appl. 2017, 10, 123–136. [Google Scholar] [CrossRef] [Green Version]
- Morandi, G.; Heath, L.; Thielemans, W. Cellulose Nanocrystals Grafted with Polystyrene Chains through Surface-Initiated Atom Transfer Radical Polymerization (Si-Atrp). Langmuir 2009, 25, 8280–8286. [Google Scholar] [CrossRef]
- Hu, Z.; Berry, R.M.; Pelton, R.; Cranston, E.D. One-Pot Water-Based Hydrophobic Surface Modification of Cellulose Nanocrystals Using Plant Polyphenols. ACS Sustain. Chem. Eng. 2017, 5, 5018–5026. [Google Scholar] [CrossRef]
- Gallagher, Z.J.; Fleetwood, S.; Kirley, T.L.; Shaw, M.A.; Mullins, E.S.; Ayres, N.; Foster, E.J. Heparin Mimic Material Derived from Cellulose Nanocrystals. Biomacromolecules 2020, 21, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Gauche, C.; Felisberti, M.I. Colloidal Behavior of Cellulose Nanocrystals Grafted with Poly(2-alkyl-2-oxazoline)s. ACS Omega 2019, 4, 11893–11905. [Google Scholar] [CrossRef] [PubMed]
- Zoppe, J.O.; Osterberg, M.; Venditti, R.A.; Laine, J.; Rojas, O.J. Surface Interaction Forces of Cellulose Nanocrystals Grafted with Thermoresponsive Polymer Brushes. Biomacromolecules 2011, 12, 2788–2796. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Meng, H.; Wang, X.; Lin, S.; Ji, Z.; Zhang, H. Nanomaterial Toxicity Testing in the 21st Century: Use of a Predictive Toxicological Approach and High-Throughput Screening. Acc. Chem. Res. 2013, 46, 604–621. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, B.; Liu, S.; Xia, T. Structure Activity Relationships of Engineered Nanomaterials in inducing NLRP3 Inflammasome Activation and Chronic Lung Fibrosis. NanoImpact 2017, 6, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murdock, R.C.; Braydich-Stolle, L.; Schrand, A.M.; Schlager, J.J.; Hussain, S.M. Characterization of Nanomaterial Dispersion in Solution Prior to In Vitro Exposure Using Dynamic Light Scattering Technique. Toxicol. Sci. 2008, 101, 239–253. [Google Scholar] [CrossRef] [Green Version]



| Sample | [Br]/[AGU] | [Monomer]/[AGU] |
|---|---|---|
| CNC-METAC-1A | 5:3 | 50:3 |
| CNC-METAC-1B | 5:3 | 60:3 |
| CNC-METAC-2A | 5:12 | 50:3 |
| CNC-METAC-2B | 5:12 | 60:3 |
| CNC-AEM-1A | 5:3 | 50:3 |
| CNC-AEM-2A | 5:12 | 50:3 |
| Sample | Apparent Particle Size (nm) | Polydispersity Index (PdI) | Zeta Potential (mV) |
|---|---|---|---|
| Pristine CNCs | 101.6 ± 0.72 | 0.23 | −34.8 ± 2.16 |
| CNC-BriB-1 | 96.3 ± 1.65 | 0.18 | −28.9 ± 1.47 |
| CNC-BriB-2 | 99.9 ± 1.80 | 0.22 | −30.2 ± 1.69 |
| CNC-METAC-1A | 123.4 ± 1.32 | 0.20 | +31.8 ± 2.89 |
| CNC-METAC-1B | 136.2 ± 1.50 | 0.22 | +44.9 ± 3.93 |
| CNC-METAC-2A | 178.1 ± 2.53 | 0.24 | +32.0 ± 0.94 |
| CNC-METAC-2B | 203.2 ± 2.66 | 0.31 | +38.2 ± 0.94 |
| CNC-AEM-1A | 172.0 ± 9.08 | 0.38 | +45.0 ± 1.44 |
| CNC-AEM-2A | 215.3 ± 2.86 | 0.31 | +41.4 ± 3.15 |
| Sample | % Carbon | % Hydrogen | % Nitrogen | % Sulfur |
|---|---|---|---|---|
| Pristine CNCs | 40.92 | 6.07 | 0.00 | 0.30 |
| CNC-METAC-1A | 44.32 | 6.94 | 5.90 | <0.20 |
| CNC-METAC-1B | 44.38 | 7.47 | 7.68 | <0.20 |
| CNC-METAC-2A | 42.58 | 6.56 | 1.70 | <0.20 |
| CNC-METAC-2B | 43.10 | 6.59 | 1.82 | <0.20 |
| CNC-AEM-1A | 41.97 | 6.67 | 5.80 | 0.22 |
| CNC-AEM-2A | 40.45 | 6.35 | 4.83 | <0.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imtiaz, Y.; Tuga, B.; Smith, C.W.; Rabideau, A.; Nguyen, L.; Liu, Y.; Hrapovic, S.; Ckless, K.; Sunasee, R. Synthesis and Cytotoxicity Studies of Wood-Based Cationic Cellulose Nanocrystals as Potential Immunomodulators. Nanomaterials 2020, 10, 1603. https://doi.org/10.3390/nano10081603
Imtiaz Y, Tuga B, Smith CW, Rabideau A, Nguyen L, Liu Y, Hrapovic S, Ckless K, Sunasee R. Synthesis and Cytotoxicity Studies of Wood-Based Cationic Cellulose Nanocrystals as Potential Immunomodulators. Nanomaterials. 2020; 10(8):1603. https://doi.org/10.3390/nano10081603
Chicago/Turabian StyleImtiaz, Yusha, Beza Tuga, Christopher W. Smith, Alexander Rabideau, Long Nguyen, Yali Liu, Sabahudin Hrapovic, Karina Ckless, and Rajesh Sunasee. 2020. "Synthesis and Cytotoxicity Studies of Wood-Based Cationic Cellulose Nanocrystals as Potential Immunomodulators" Nanomaterials 10, no. 8: 1603. https://doi.org/10.3390/nano10081603