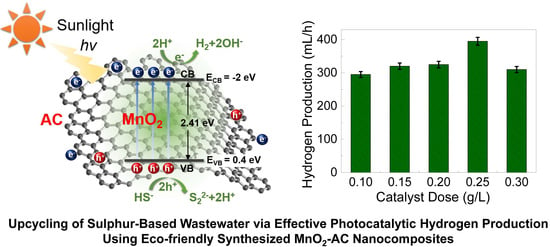
Upcycling of Wastewater via Effective Photocatalytic Hydrogen Production Using MnO2 Nanoparticles—Decorated Activated Carbon Nanoflakes
Abstract
:1. Introduction
2. Experimental Details
2.1. Synthesis of MnO2 Nanoparticles
2.2. Derivation of Biomass AC
2.3. Synthesis of MnO2-AC Nanocomposites
2.4. Characterization of Material Properties
2.5. Measurement of Photocatalytic Performances
3. Results and Discussion
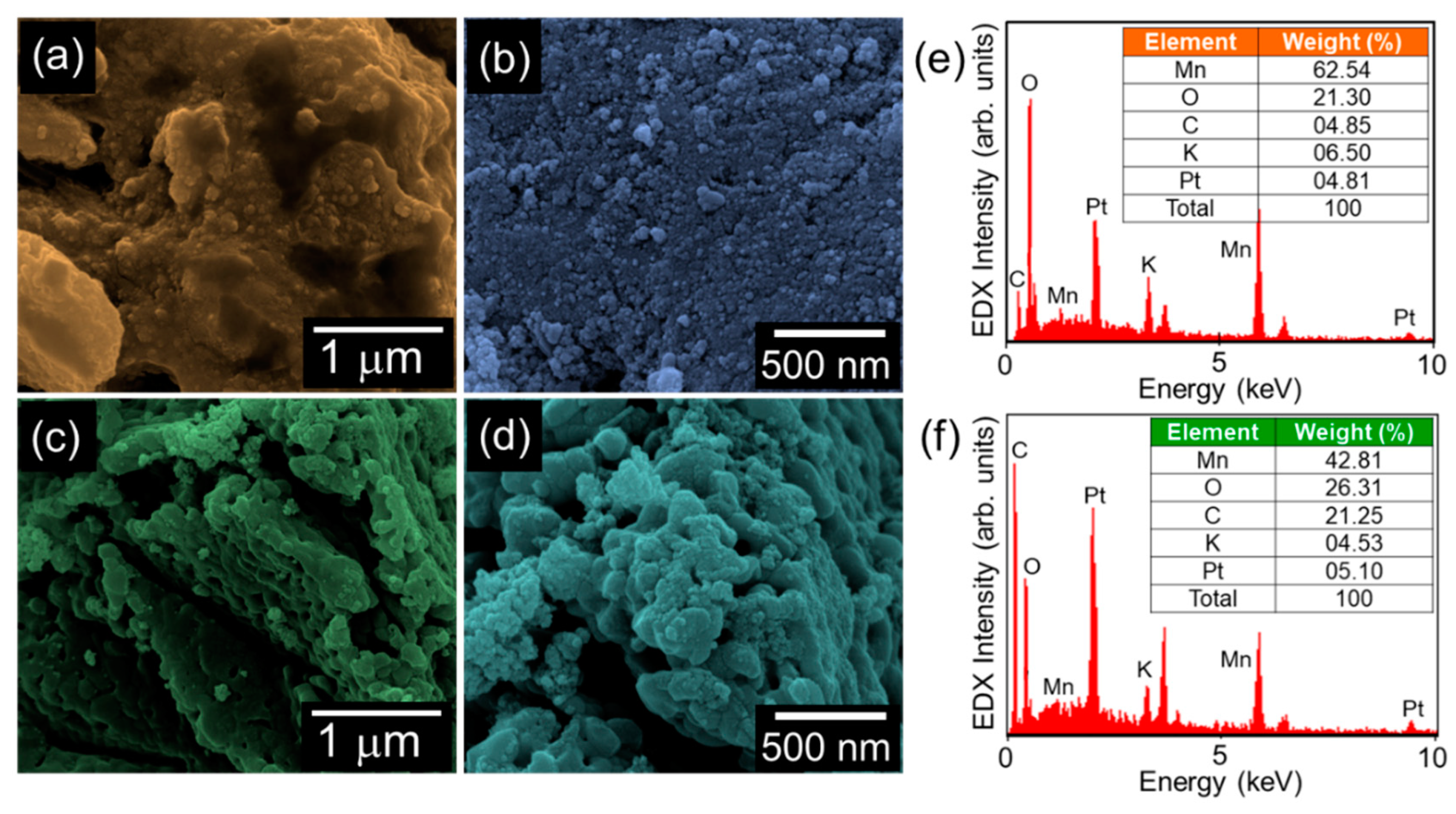
3.1. Topographical and Compositional Properties
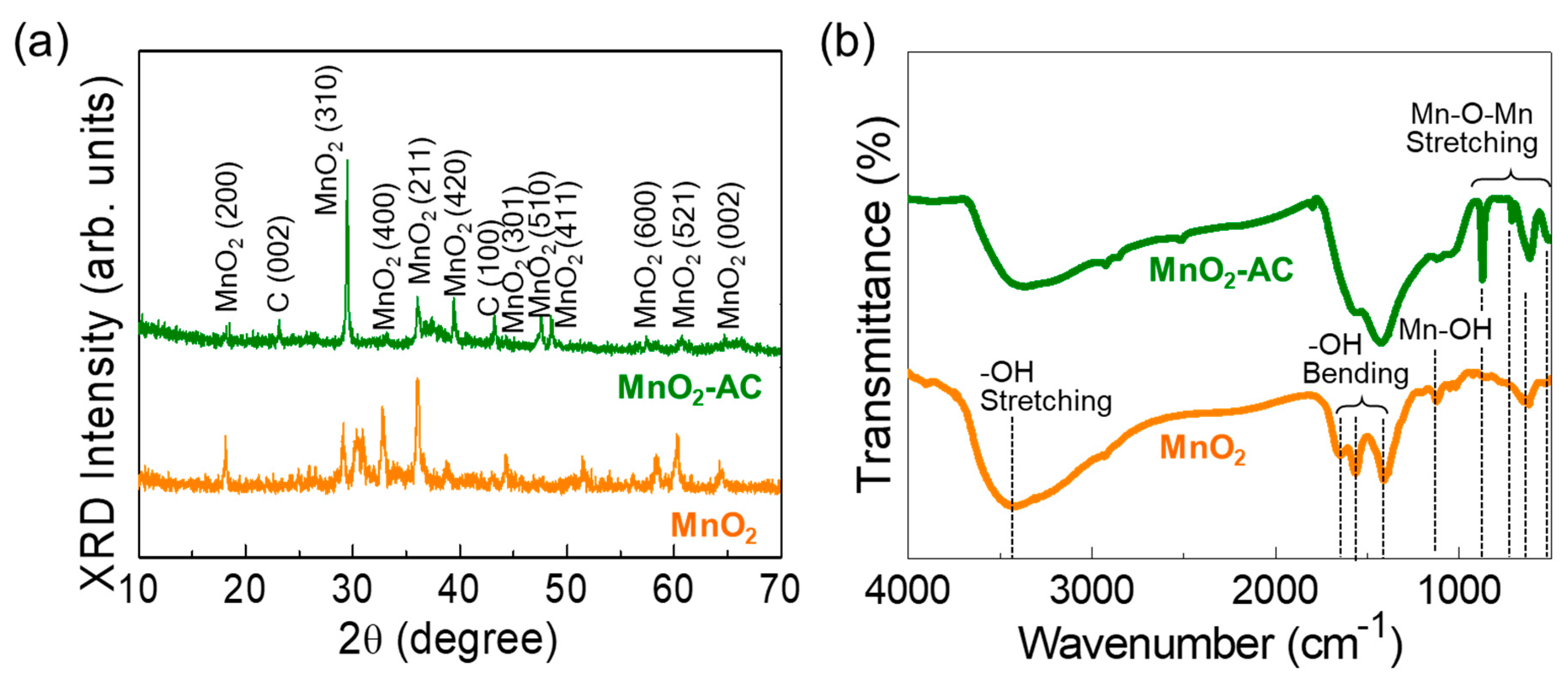
3.2. Crystallographic Properties
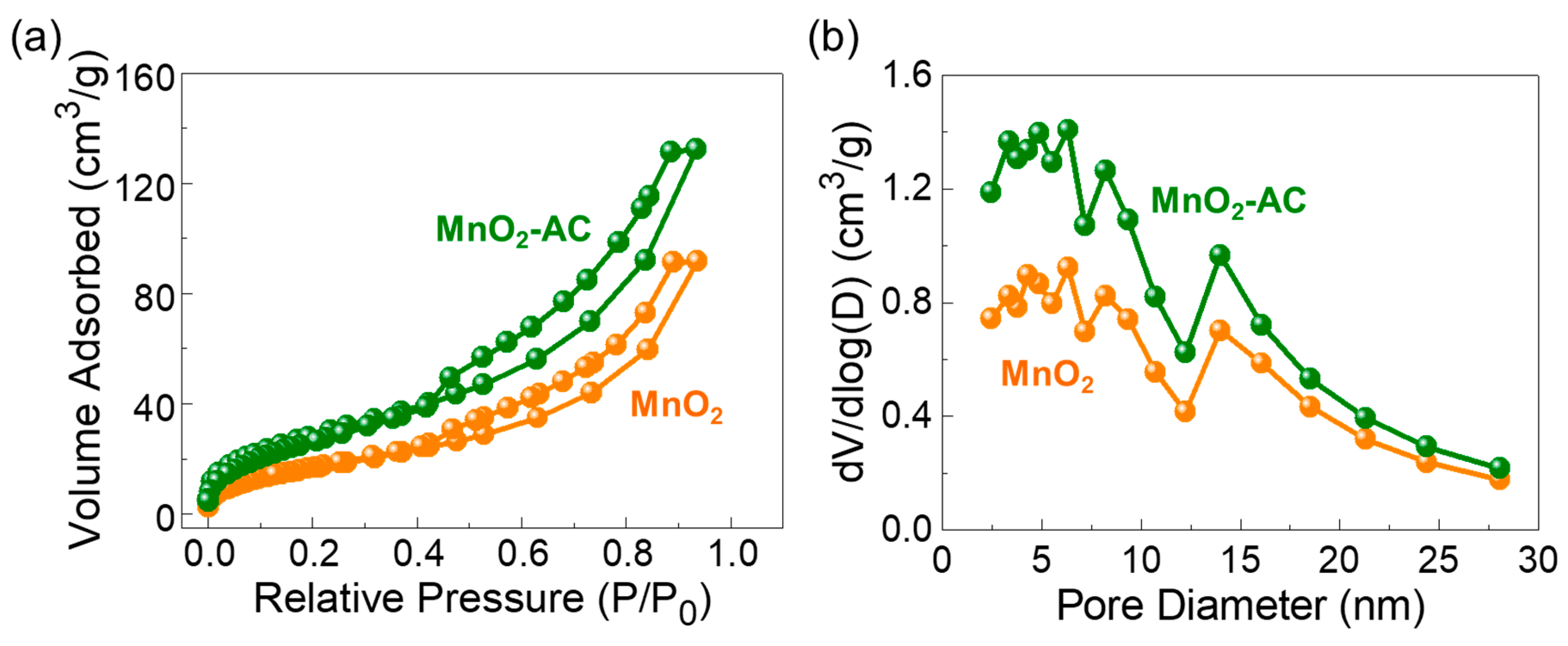
3.3. Textural Characteristics
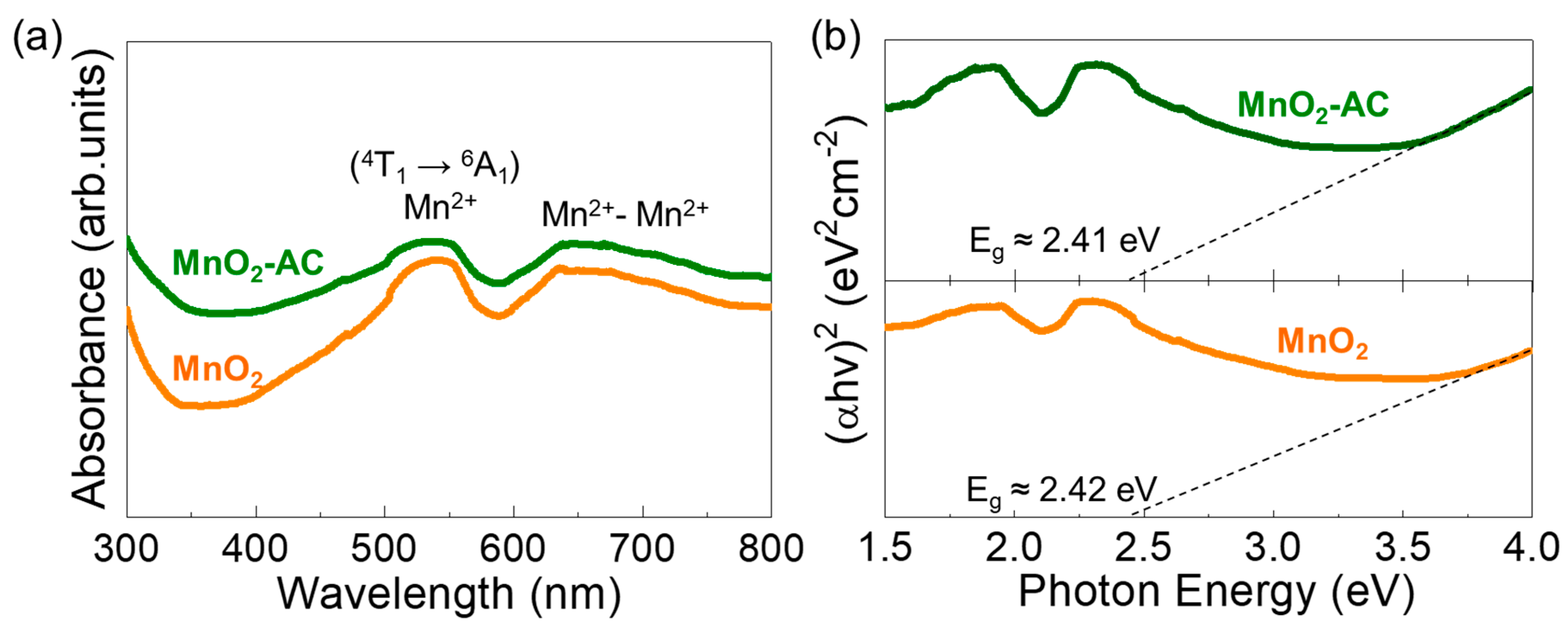
3.4. Optical Properties
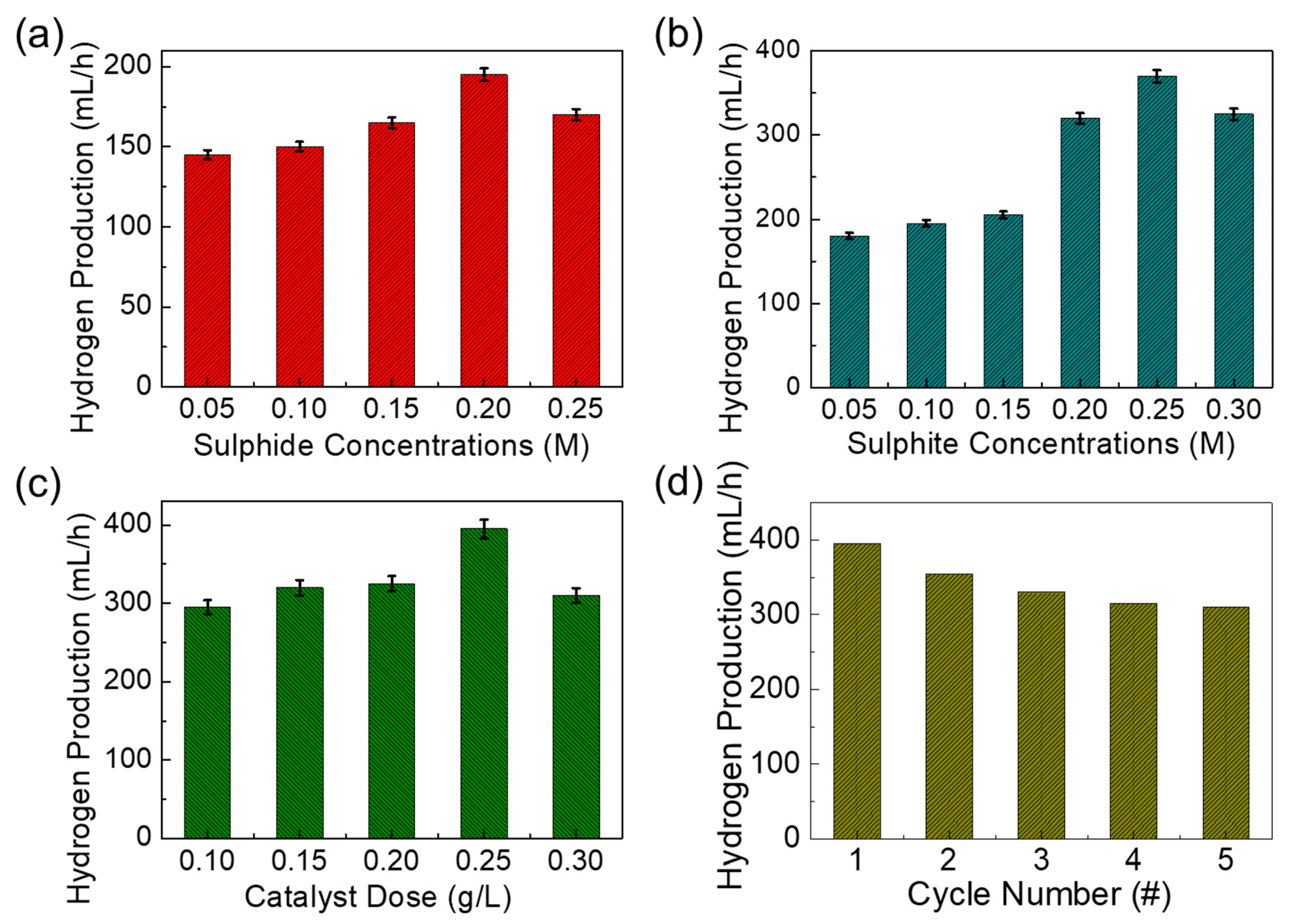
3.5. Photocatalytic Hydrogen Production Efficiencies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tsuji, I.; Kato, H.; Kudo, A. Visible-Light-Induced H2 Evolution from an Aqueous Solution Containing Sulfide and Sulfite over a ZnS–CuInS2–AgInS2 Solid-Solution Photocatalyst. Angew. Chem. Int. Ed. 2005, 44, 3565–3568. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Samanta, A.; Jana, S. Light-Assisted Synthesis of Hierarchical Flower-Like MnO2 Nanocomposites with Solar Light Induced Enhanced Photocatalytic Activity. ACS Sustain. Chem. Eng. 2017, 5, 9086–9094. [Google Scholar] [CrossRef]
- Preethi, V.; Kanmani, S. Photocatalytic hydrogen production using Fe2O3-based core shell nano particles with ZnS and CdS. Int. J. Hydrogen Energy 2014, 39, 1613–1622. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.-H.; Deep, A. Photocatalytic mineralization of hydrogen sulfide as a dual-phase technique for hydrogen production and environmental remediation. Appl. Catal. B 2019, 259, 118025. [Google Scholar] [CrossRef]
- Kida, T.; Guan, G.; Yamada, N.; Ma, T.; Kimura, K.; Yoshida, A. Hydrogen production from sewage sludge solubilized in hot-compressed water using photocatalyst under light irradiation. Int. J. Hydrogen Energy 2004, 29, 269–274. [Google Scholar] [CrossRef]
- Moon, S.Y.; Gwag, E.H.; Park, J.Y. Hydrogen Generation on Metal/Mesoporous Oxides: The Effects of Hierarchical Structure, Doping, and Co-catalysts. Energy Technol. 2018, 6, 459–469. [Google Scholar] [CrossRef]
- Concina, I.; Ibupoto, Z.H.; Vomiero, A. Semiconducting Metal Oxide Nanostructures for Water Splitting and Photovoltaics. Adv. Energy Mater. 2017, 7, 1700706. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Li, X.; Jin, X.; Wang, L.; Deng, Y.; Su, F.; Wong, P.K.; Ye, L. High-efficiency and stable photocatalytic hydrogen evolution of rhenium sulfide co-catalyst on Zn0.3Cd0.7S. Mater. Adv. 2020, 1, 363–370. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Ching Ng, A.M. Strategies for improving the efficiency of semiconductor metal oxide photocatalysis. Mater. Horiz. 2014, 1, 400–410. [Google Scholar] [CrossRef]
- Liu, J.; Ge, X.; Ye, X.; Wang, G.; Zhang, H.; Zhou, H.; Zhang, Y.; Zhao, H. 3D graphene/δ-MnO2 aerogels for highly efficient and reversible removal of heavy metal ions. J. Mater. Chem. A 2016, 4, 1970–1979. [Google Scholar] [CrossRef]
- Crespo, Y.; Seriani, N. A lithium peroxide precursor on the α-MnO2 (100) surface. J. Mater. Chem. A 2014, 2, 16538–16546. [Google Scholar] [CrossRef]
- Zhang, K.; Han, X.; Hu, Z.; Zhang, X.; Tao, Z.; Chen, J. Nanostructured Mn-based oxides for electrochemical energy storage and conversion. Chem. Soc. Rev. 2015, 44, 699–728. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.T.; Liu, Y.; Ren, Y.; Trahey, L.; Sun, Y. Morphological and Crystalline Evolution of Nanostructured MnO2 and Its Application in Lithium–Air Batteries. ACS Nano 2012, 6, 8067–8077. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Roy, A.S.; Kapri, S.; Bhattacharyya, S. Efficient Dye Degradation Catalyzed by Manganese Oxide Nanoparticles and the Role of Cation Valence. ChemistrySelect 2016, 1, 4265–4273. [Google Scholar] [CrossRef]
- Rahmat, M.; Rehman, A.; Rahmat, S.; Bhatti, H.N.; Iqbal, M.; Khan, W.S.; Bajwa, S.Z.; Rahmat, R.; Nazir, A. Highly efficient removal of crystal violet dye from water by MnO2 based nanofibrous mesh/photocatalytic process. J. Mater. Res. Technol. 2019, 8, 5149–5159. [Google Scholar] [CrossRef]
- Vidya Lekshmi, K.P.; Yesodharan, S.; Yesodharan, E.P. MnO2 efficiently removes indigo carmine dyes from polluted water. Heliyon 2018, 4, e00897. [Google Scholar] [CrossRef] [Green Version]
- Husnain, S.M.; Asim, U.; Yaqub, A.; Shahzad, F.; Abbas, N. Recent trends of MnO2-derived adsorbents for water treatment: A review. New J. Chem. 2020, 44, 6096–6120. [Google Scholar] [CrossRef]
- Wang, R.; Hao, Q.; Feng, J.; Wang, G.-C.; Ding, H.; Chen, D.; Ni, B. Enhanced separation of photogenerated charge carriers and catalytic properties of ZnO-MnO2 composites by microwave and photothermal effect. J. Alloys Compd. 2019, 786, 418–427. [Google Scholar] [CrossRef]
- Chhabra, T.; Kumar, A.; Bahuguna, A.; Krishnan, V. Reduced graphene oxide supported MnO2 nanorods as recyclable and efficient adsorptive photocatalysts for pollutants removal. Vacuum 2019, 160, 333–346. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Z.; Li, N.; Nan, J.; Yu, R.; Du, J. Visible-light-driven photocatalytic degradation of ciprofloxacin by a ternary Mn2O3/Mn3O4/MnO2 valence state heterojunction. Chem. Eng. J. 2018, 353, 805–813. [Google Scholar] [CrossRef]
- Rajrana, K.; Gupta, A.; Mir, R.A.; Pandey, O.P. Facile sono-chemical synthesis of nanocrystalline MnO2 for catalytic and capacitive applications. Phys. B 2019, 564, 179–185. [Google Scholar] [CrossRef]
- Chiam, S.-L.; Pung, S.-Y.; Yeoh, F.-Y. Recent developments in MnO2-based photocatalysts for organic dye removal: A review. Environ. Sci. Pollut. Res. 2020, 27, 5759–5778. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Sharma, S.K.; An, N.; Lee, H.; Kim, D.Y.; Im, Y.B.; Cho, Y.D.; Ganesh, R.S.; Ponnusamy, S.; Raji, P.; et al. Photocatalytic properties of Mn-doped NiO spherical nanoparticles synthesized from sol-gel method. Optik 2016, 127, 10727–10734. [Google Scholar] [CrossRef]
- Ding, Y.; Wei, D.; He, R.; Yuan, R.; Xie, T.; Li, Z. Rational design of Z-scheme PtS-ZnIn2S4/WO3-MnO2 for overall photo-catalytic water splitting under visible light. Appl. Catal. B 2019, 258, 117948. [Google Scholar] [CrossRef]
- Zhen, W.; Ning, X.; Wang, M.; Wu, Y.; Lu, G. Enhancing hydrogen generation via fabricating peroxide decomposition layer over NiSe/MnO2-CdS catalyst. J. Catal. 2018, 367, 269–282. [Google Scholar] [CrossRef]
- Abuzeid, H.M.; Elsherif, S.A.; Abdel Ghany, N.A.; Hashem, A.M. Facile, cost-effective and eco-friendly green synthesis method of MnO2 as storage electrode materials for supercapacitors. J. Energy Storage 2019, 21, 156–162. [Google Scholar] [CrossRef]
- Hashem, A.M.; Abuzeid, H.M.; Winter, M.; Li, J.; Julien, C.M. Synthesis of High Surface Area α-KyMnO2 Nanoneedles Using Extract of Broccoli as Bioactive Reducing Agent and Application in Lithium Battery. Materials 2020, 13, 1269. [Google Scholar] [CrossRef] [Green Version]
- Zia, J.; Aazam, E.S.; Riaz, U. Synthesis of nanohybrids of polycarbazole with α-MnO2 derived from Brassica oleracea: A comparison of photocatalytic degradation of an antibiotic drug under microwave and UV irradiation. Environ. Sci. Pollut. Res. 2020, 27, 24137–24189. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [Green Version]
- Sunkar, S.; Nachiyar, C.V. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pac. J. Trop Biomed. 2012, 2, 953–959. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Botero, L.; Herrera, A.P.; Hinestroza, J.P. Oriented Growth of α-MnO2 Nanorods Using Natural Extracts from Grape Stems and Apple Peels. Nanomaterials 2017, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Yao, M.-S.; Lin, Y.-H.; Nan, C.-W. A comprehensive review on synthesis methods for transition-metal oxide nanostructures. CrystEngComm 2015, 17, 3551–3585. [Google Scholar] [CrossRef]
- Chan, Y.L.; Pung, S.Y.; Hussain, N.S.; Sreekantan, S.; Yeoh, F.Y. Photocatalytic Degradation of Rhodamine B Using MnO2 and ZnO Nanoparticles. Mater. Sci. Forum 2013, 756, 167–174. [Google Scholar] [CrossRef]
- Zhang, G.; Qu, J.; Du, Y.; Guo, F.; Zhao, H.; Zhang, Y.; Xu, Y. Hydrogen production from CO2 reforming of methane over high pressure H2O2 modified different semi-cokes. J. Ind. Eng. Chem. 2014, 20, 2948–2957. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Dong, Y.; Feng, M.; Zhang, Y.; Zhao, W.; Cao, H. CO2 reforming of CH4 in coke oven gas to syngas over coal char catalyst. Chem. Eng. J. 2010, 156, 519–523. [Google Scholar] [CrossRef]
- Sekar, S.; Aqueel Ahmed, A.T.; Pawar, S.M.; Lee, Y.; Im, H.; Kim, D.Y.; Lee, S. Enhanced water splitting performance of biomass activated carbon-anchored WO3 nanoflakes. Appl. Surf. Sci. 2020, 508, 145127. [Google Scholar] [CrossRef]
- Sankar, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Im, Y.B.; Lee, Y.; Kim, D.Y.; Lee, S. Biomass-derived ultrathin mesoporous graphitic carbon nanoflakes as stable electrode material for high-performance supercapacitors. Mater. Des. 2019, 169, 107688. [Google Scholar] [CrossRef]
- Sekar, S.; Lee, Y.; Kim, D.Y.; Lee, S. Substantial LIB anode performance of graphitic carbon nanoflakes derived from biomass green-tea waste. Nanomaterials 2019, 9, 871. [Google Scholar] [CrossRef] [Green Version]
- Sankar, S.; Inamdar, A.I.; Im, H.; Lee, S.; Kim, D.Y. Template-free rapid sonochemical synthesis of spherical α-MnO2 nanoparticles for high-energy supercapacitor electrode. Ceram. Int. 2018, 44, 17514–17521. [Google Scholar] [CrossRef]
- Hashem, A.M.; Abuzeid, H.; Kaus, M.; Indris, S.; Ehrenberg, H.; Mauger, A.; Julien, C.M. Green synthesis of nanosized manganese dioxide as positive electrode for lithium-ion batteries using lemon juice and citrus peel. Electrochim. Acta 2018, 262, 74–81. [Google Scholar] [CrossRef]
- Fang, S.; Lin, F.; Qu, D.; Liang, X.; Wang, L. Characterization of Purified Red Cabbage Anthocyanins: Improvement in HPLC Separation and Protective Effect against H2O2-Induced Oxidative Stress in HepG2 Cells. Molecules 2019, 24, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Gong, X.; Zhang, B.; Liu, W.; Xu, M. Potassium catalytic hydrogen production in sorption enhanced gasification of biomass with steam. Int. J. Hydrogen Energy 2014, 39, 4234–4243. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Jia, L.; Beloshapkin, S.; Ross, J.R.H. Improved hydrogen production from formic acid on a Pd/C catalyst doped by potassium. Chem. Commun. 2012, 48, 4184–4186. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, V.; Ryu, J.W.; Kim, H.; Manivannan, R.; Son, Y.-A. Ultrasonic synthesis of α-MnO2 nanorods: An efficient catalytic conversion of refractory pollutant, methylene blue. Ultrason. Sonochem. 2020, 62, 104870. [Google Scholar] [CrossRef]
- Sekar, S.; Kim, D.Y.; Lee, S. Excellent Oxygen Evolution Reaction of Activated Carbon-Anchored NiO Nanotablets Prepared by Green Routes. Nanomaterials 2020, 10, 1382. [Google Scholar] [CrossRef]
- Sankar, S.; Saravanan, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Lee, S.; Kim, D.Y. Spherical activated-carbon nanoparticles derived from biomass green tea wastes for anode material of lithium-ion battery. Mater. Lett. 2019, 240, 189–192. [Google Scholar] [CrossRef]
- Lee, S.; Kang, T.W.; Kim, D.Y. Correlation of Magnetic Properties with Microstructural Properties for Columnar-Structured (Zn1−xMnx)O/Al2O3 (0001) Thin Films. J. Cryst. Growth 2005, 284, 6–14. [Google Scholar] [CrossRef]
- Kaur, N.; Lee, Y.; Kim, D.Y.; Lee, S. Optical bandgap tuning in nanocrystalline ZnO:Y films via forming defect-induced localized bands. Mater. Des. 2018, 148, 30–38. [Google Scholar] [CrossRef]
- Lee, S.; Seong, J.; Kim, D. Effects of laser-annealing using a KrF excimer laser on the surface, structural, optical, and electrical properties of AlZnO thin films. J. Korean Phys. Soc. 2010, 56, 782–786. [Google Scholar]
- Ali, G.A.M.; Yusoff, M.M.; Shaaban, E.R.; Chong, K.F. High performance MnO2 nanoflower supercapacitor electrode by electrochemical recycling of spent batteries. Ceram. Int. 2017, 43, 8440–8448. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-W.; Chen, Y.; Chen, B.-Z. A Synthesis Method of MnO2/Activated Carbon Composite for Electrochemical Supercapacitors. J. Electrochem. Soc. 2015, 162, A1654–A1661. [Google Scholar] [CrossRef]
- Liu, R.; Liu, E.; Ding, R.; Liu, K.; Teng, Y.; Luo, Z.; Li, Z.; Hu, T.; Liu, T. Facile in-situ redox synthesis of hierarchical porous activated carbon@MnO2 core/shell nanocomposite for supercapacitors. Ceram. Int. 2015, 41, 12734–12741. [Google Scholar] [CrossRef]
- Kumar, A.; Aathira, M.S.; Pal, U.; Jain, S.L. Photochemical Oxidative Coupling of 2-Naphthols using a Hybrid Reduced Graphene Oxide/Manganese Dioxide Nanocomposite under Visible-Light Irradiation. ChemCatChem 2018, 10, 1844–1852. [Google Scholar] [CrossRef]
- Darari, A.; Ardiansah, H.R.; Arifin Rismaningsih, N.; Ningrum, A.N.; Subagio, A. Characterization of CNT-MnO2 nanocomposite by electrophoretic deposition as potential electrode for supercapacitor. AIP Conf. Proc. 2016, 1725, 020012. [Google Scholar]
- Jamesh, M.-I. Recent advances on flexible electrodes for Na-ion batteries and Li–S batteries. J. Energy Chem. 2019, 32, 15–44. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Ren, L.; Hu, D.; Gu, H.; Zhang, S. Sulfuric acid etching for fabrication of porous MnO2 for high-performance supercapacitor. J. Colloid Interface Sci. 2018, 518, 84–91. [Google Scholar] [CrossRef]
- Abdel-Aal, S.K.; Aly, A.E.; Chanduví, H.H.M.; Gil Rebaza, A.V.; Atteia, E.; Shankar, A. Magnetic and optical properties of perovskite-graphene nanocomposites LaFeO3-rGO: Experimental and DFT calculations. Chem. Phys. 2020, 538, 110874. [Google Scholar] [CrossRef]
- Ateia, E.E.; Mohamed, A.T. Nonstoichiometry and phase stability of Al and Cr substituted Mg ferrite nanoparticles synthesized by citrate method. J. Magn. Magn. Mater. 2017, 426, 217–224. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.; Kim, D.Y.; Panin, G.N. Multicolor Emission from Poly(p-Phenylene)/Nanoporous ZnMnO Organic–Inorganic Hybrid Light-Emitting Diode. ACS Appl. Mater. Interfaces 2016, 8, 35435–35439. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.; Panin, G.N. Novel Green Luminescent and Phosphorescent Material: Semiconductive Nanoporous ZnMnO with Photon Confinement. ACS Appl. Mater. Interfaces 2017, 9, 20630–20636. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.; Kim, D.Y.; Panin, G.N. Highly Efficient Low-Voltage Cathodoluminescence of Semiconductive Nanoporous ZnMnO Green Phosphor Films. Appl. Surf. Sci. 2019, 470, 234–240. [Google Scholar] [CrossRef]
- Kamran, M.A. The aggregation of Mn2+, its d-d transition in CdS:Mn(II) nanobelts and bound magnetic polaron formation at room temperature. Nanotechnology 2018, 29, 435702. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, Z.; Yan, W.; Zhong, W.; Pan, Z.; Hao, L.; Wei, S. Anomalous magnetic behavior of Mn-Mn dimers in the dilute magnetic semiconductor (Ga,Mn)N. Phys. Rev. B 2007, 76, 245210. [Google Scholar] [CrossRef]
- Li, T.; Wu, J.; Xiao, X.; Zhang, B.; Hu, Z.; Zhou, J.; Yang, P.; Chen, X.; Wang, B.; Huang, L. Band gap engineering of MnO2 through in situ Al-doping for applicable pseudocapacitors. RSC Adv. 2016, 6, 13914–13919. [Google Scholar] [CrossRef]
- Navakoteswara Rao, V.; Lakshmana Reddy, N.; Mamatha Kumari, M.; Ravi, P.; Sathish, M.; Kuruvilla, K.M.; Preethi, V.; Reddy, K.R.; Shetti, N.P.; Aminabhavi, T.M.; et al. Photocatalytic recovery of H2 from H2S containing wastewater: Surface and interface control of photo-excitons in Cu2S@TiO2 core-shell nanostructures. Appl. Catal. B 2019, 254, 174–185. [Google Scholar] [CrossRef]
- Jayabalan, T.; Matheswaran, M.; Preethi, V.; Naina Mohamed, S. Enhancing biohydrogen production from sugar industry wastewater using metal oxide/graphene nanocomposite catalysts in microbial electrolysis cell. Int. J. Hydrogen Energy 2020, 45, 7647–7655. [Google Scholar] [CrossRef]
- Anthony Raja, M.; Preethi, V. Photocatalytic hydrogen production using bench-scale trapezoidal photocatalytic reactor. Int. J. Hydrogen Energy 2020, 45, 7574–7583. [Google Scholar] [CrossRef]
- Anthony Raja, M.; Preethi, V. Performance of Square and Trapezoidal photoreactors for solar hydrogen recovery from various industrial sulphide wastewater using CNT/Ce3+ doped TiO2. Int. J. Hydrogen Energy 2020, 45, 7616–7626. [Google Scholar] [CrossRef]
- Li, P.; Chen, R.; Tian, S.; Xiong, Y. Efficient Oxygen Evolution Catalysis Triggered by Nickel Phosphide Nanoparticles Compositing with Reduced Graphene Oxide with Controlled Architecture. ACS Sustain. Chem. Eng. 2019, 7, 9566–9573. [Google Scholar] [CrossRef]
- Luo, Z.; Zhao, X.; Zhang, H.; Jiang, Y. Zn0.3Cd0.7S nanorods loaded with noble-metal-free Ni3C co-catalyst enhancing photocatalytic hydrogen evolution. Appl. Catal. A 2019, 582, 117115. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekar, S.; Lee, S.; Vijayarengan, P.; Kalirajan, K.M.; Santhakumar, T.; Sekar, S.; Sadhasivam, S. Upcycling of Wastewater via Effective Photocatalytic Hydrogen Production Using MnO2 Nanoparticles—Decorated Activated Carbon Nanoflakes. Nanomaterials 2020, 10, 1610. https://doi.org/10.3390/nano10081610
Sekar S, Lee S, Vijayarengan P, Kalirajan KM, Santhakumar T, Sekar S, Sadhasivam S. Upcycling of Wastewater via Effective Photocatalytic Hydrogen Production Using MnO2 Nanoparticles—Decorated Activated Carbon Nanoflakes. Nanomaterials. 2020; 10(8):1610. https://doi.org/10.3390/nano10081610
Chicago/Turabian StyleSekar, Sankar, Sejoon Lee, Preethi Vijayarengan, Kaliyappan Mohan Kalirajan, Thirumavalavan Santhakumar, Saravanan Sekar, and Sutha Sadhasivam. 2020. "Upcycling of Wastewater via Effective Photocatalytic Hydrogen Production Using MnO2 Nanoparticles—Decorated Activated Carbon Nanoflakes" Nanomaterials 10, no. 8: 1610. https://doi.org/10.3390/nano10081610