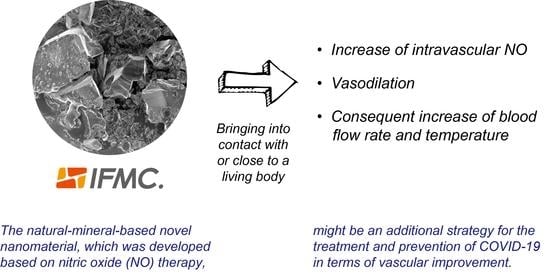
The Natural-Mineral-Based Novel Nanomaterial IFMC Increases Intravascular Nitric Oxide without Its Intake: Implications for COVID-19 and beyond
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Zeta Potential and Particle Size Measurement
2.3. Field Emission Scanning Electron Microscopy and Energy-Dispersive X-Ray Spectroscopy
2.4. The In Vivo Measurement of Intravascular NO Concentration Using a NO Sensor in the Hepatic Portal Vein of Rats
2.5. Measurements of Blood Flow Rate, Velocity and Vessel Diameter of the Human Brachial Artery Using an Ultrasonic Flow Meter
2.6. Measurement of Human Hand Surface Temperature Using Thermography
3. Results
3.1. SEM Image of IFMC
3.2. Changes in Intravascular NO Concentration before and after IFMC and Placebo Applications
3.3. Differences in Blood Flow Rate, Velocity and Vessel Diameter in the Brachial Artery between IFMC and Placebo Applications
3.4. Changes in Hand Surface Temperature after IFMC Application
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | angiotensin converting enzyme 2 |
| ACh | acetylcholine |
| aAPCs | artificial antigen presenting cells |
| ARDS | acute respiratory distress syndrome |
| CaM | calmodulin |
| Cav-1 | caveolin-1 |
| Cl | chlorine |
| COVID-19 | novel coronavirus disease 2019 |
| EDHF | endothelium-derived hyperpolarising factor |
| EDRF | endothelium-derived relaxing factor |
| EDS | energy-dispersive X-ray spectrometer |
| eNOS | endothelial nitric oxide synthase |
| Fe | iron |
| FE-SEM | field emission scanning electron microscopy |
| Hb | haemoglobin |
| HSP90 | heat shock protein 90 |
| IFMC | integrated functional mineral crystal |
| K | potassium |
| Mn | manganese |
| mRNA | messenger ribonucleic acid |
| Na | sodium |
| Nd | neodymium |
| NO | nitric oxide |
| O | oxygen |
| PGI2 | prostacyclin |
| S | sulphur |
| SARS-CoV-2 | severe acute respiratory syndrome-coronavirus 2 |
| SEM | scanning electron microscopy |
| SNO-Hb | S-nitrosohemoglobin |
| VEGFR2 | vascular endothelial growth factor receptor 2 |
| Zn | zinc |
References
- World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report-131. 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200530-covid-19-sitrep-131.pdf?sfvrsn=d31ba4b3_2 (accessed on 31 May 2020).
- Harari, Y.N. Yuval Noah Harari: The World after Coronavirus. Available online: https://www.ft.com/content/19d90308-6858-11ea-a3c9-1fe6fedcca75 (accessed on 1 April 2020).
- Venter, Z.S.; Aunan, K.; Chowdhury, S.; Lelieveld, J. COVID-19 Lockdowns Cause Global Air Pollution Declines with Implications for Public Health Risk. medRxiv 2020. Available online: http://xxx.lanl.gov/abs/https://www.medrxiv.org/content/early/2020/04/14/2020.04.10.20060673.full.pdf (accessed on 3 May 2020).
- Liu, Z.; Deng, Z.; Ciais, P.; Lei, R.; Davis, S.J.; Feng, S.; Zheng, B.; Cui, D.; Dou, X.; He, P.; et al. COVID-19 causes record decline in global CO2 emissions. arXiv 2020, arXiv:econ.GN/2004.13614. [Google Scholar]
- Martial, C.; Cassol, H.; Laureys, S.; Gosseries, O. Near-Death Experience as a Probe to Explore (Disconnected) Consciousness. Trends Cogn. Sci. (Regul. Ed.) 2020, 24, 173–183. [Google Scholar] [CrossRef]
- van Lommel, P.; van Wees, R.; Meyers, V.; Elfferich, I. Near-death experience in survivors of cardiac arrest: A prospective study in the Netherlands. Lancet 2001, 358, 2039–2045. [Google Scholar] [CrossRef]
- Bianco, S.; Testoni, I.; Palmieri, A.; Solomon, S.; Hart, J. The Psychological Correlates of Decreased Death Anxiety After a Near-Death Experience: The Role of Self-Esteem, Mindfulness, and Death Representations. J. Humanist. Psychol. 2019. [Google Scholar] [CrossRef]
- Rouda, U. Prospects for democracy in Belarus. In Democratisation in the European Neighbourhood; Emerson, M., Ed.; Centre for European Policy Studies: Brussels, Belgium, 2005; pp. 71–92. [Google Scholar]
- Guo, Y.R.; Cao, Q.D.; Hong, Z.S.; Tan, Y.Y.; Chen, S.D.; Jin, H.J.; Tan, K.S.; Wang, D.Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lake, M.A. What we know so far: COVID-19 current clinical knowledge and research. Clin. Med. (Lond.) 2020, 20, 124–127. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses 2020, 12, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakodkar, P.; Kaka, N.; Baig, M.N. A Comprehensive Literature Review on the Clinical Presentation, and Management of the Pandemic Coronavirus Disease 2019 (COVID-19). Cureus 2020, 12, e7560. [Google Scholar] [PubMed] [Green Version]
- Lythgoe, M.P.; Middleton, P. Ongoing Clinical Trials for the Management of the COVID-19 Pandemic. Trends Pharmacol. Sci. 2020, 41, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.F.; Chien, C.S.; Yarmishyn, A.A.; Lin, Y.Y.; Luo, Y.H.; Lin, Y.T.; Lai, W.Y.; Yang, D.M.; Chou, S.J.; Yang, Y.P.; et al. A Review of SARS-CoV-2 and the Ongoing Clinical Trials. Int. J. Mol. Sci. 2020, 21, 2657. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Sokhansanj, B.A.; Rosen, G.L. Characterizing geographical and temporal dynamics of novel coronavirus SARS-CoV-2 using informative subtype markers. bioRxiv 2020. Available online: https://www.biorxiv.org/content/early/2020/04/20/2020.04.07.030759.full.pdf (accessed on 2 May 2020).
- van Dorp, L.; Acman, M.; Richard, D.; Shaw, L.P.; Ford, C.E.; Ormond, L.; Owen, C.J.; Pang, J.; Tan, C.C.S.; Boshier, F.A.T.; et al. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect. Genet. Evol. 2020, 83, 104351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Penninger, J.M. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ. J. 2013, 77, 301–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, V.B.; Zhong, J.C.; Grant, M.B.; Oudit, G.Y. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ. Res. 2016, 118, 1313–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, A.J.; Hiscox, J.A.; Hooper, N.M. ACE2: From vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004, 25, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef] [Green Version]
- Angeli, F.; Reboldi, G.; Verdecchia, P. Hypertensive urgencies and emergencies: Misconceptions and pitfalls. Eur. J. Intern. Med. 2020, 71, 15–17. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Wadman, M.; Couzin-Frankel, J.; Kaiser, J.; Matacic, C. A rampage through the body. Science 2020, 368, 356–360. [Google Scholar]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Calina, D.; Docea, A.O.; Petrakis, D.; Egorov, A.M.; Ishmukhametov, A.A.; Gabibov, A.G.; Shtilman, M.I.; Kostoff, R.; Carvalho, F.; Vinceti, M.; et al. Towards effective COVID-19 vaccines: Updates, perspectives and challenges (Review). Int. J. Mol. Med. 2020, 46, 3–16. [Google Scholar] [CrossRef]
- Mohammad, F.S.; Karmakar, V.; Irfan, Z. Hydroxychloroquine and azithromycin combination could be lethal to COVID-19 patients. Farmacia 2020, 68, 384–389. [Google Scholar] [CrossRef]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef] [Green Version]
- Arsene, A.L.; Dumitrescu, I.; Drăgoi, C.M.; Udeanu, D.I.; Lupuliasa, D.; Jinga, V.; Drăgănescu, D.; Dinu-pîrvu, C.E.; Dragomiroiu, G.T.A.B.; Blejan, I.E.; et al. A new era for the therapeutic management of the ongoing COVID-19 pandemic. Farmacia 2020, 68, 185–196. [Google Scholar] [CrossRef]
- Zhang, D.H.; Wu, K.L.; Zhang, X.; Deng, S.Q.; Peng, B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J. Integr. Med. 2020, 18, 152–158. [Google Scholar] [CrossRef]
- Torequl Islam, M.; Nasiruddin, M.; Khan, I.N.; Mishra, S.K.; Kudrat-E-Zahan, M.; Alam Riaz, T.; Ali, E.S.; Rahman, M.S.; Mubarak, M.S.; Martorell, M.; et al. A Perspective on Emerging Therapeutic Interventions for COVID-19. Front. Public Health 2020, 8, 281. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y. Potential interventions for novel coronavirus in China: A systematic review. J. Med. Virol. 2020, 92, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Hou, Y.; Shen, J.; Huang, Y.; Martin, W.; Cheng, F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignarro, L.J. Inhaled NO and COVID-19. Br. J. Pharmacol. 2020, 177, 3848–3849. [Google Scholar] [CrossRef] [PubMed]
- Murad, F. Discovery of some of the biological effects of nitric oxide and its role in cell signaling. Biosci. Rep. 1999, 19, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F. Endothelium-derived relaxing factor: Discovery, early studies, and identification as nitric oxide. Biosci. Rep. 1999, 19, 235–251. [Google Scholar] [CrossRef]
- Ignarro, L.J. Nitric oxide: A unique endogenous signaling molecule in vascular biology. Biosci. Rep. 1999, 19, 51–71. [Google Scholar] [CrossRef]
- Keyaerts, E.; Vijgen, L.; Chen, L.; Maes, P.; Hedenstierna, G.; Van Ranst, M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int. J. Infect. Dis. 2004, 8, 223–226. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, P.; Gao, H.; Sun, B.; Chao, D.; Wang, F.; Zhu, Y.; Hedenstierna, G.; Wang, C.G. Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: A rescue trial in Beijing. Clin. Infect. Dis. 2004, 39, 1531–1535. [Google Scholar] [CrossRef] [Green Version]
- Akerström, S.; Mousavi-Jazi, M.; Klingström, J.; Leijon, M.; Lundkvist, A.; Mirazimi, A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005, 79, 1966–1969. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, J.; Murata, I. Nitric oxide inhalation as an interventional rescue therapy for COVID-19-induced acute respiratory distress syndrome. Ann. Intensive Care 2020, 10, 61. [Google Scholar] [CrossRef]
- Berra, L.; Lei, C.; Su, B.; Dong, H.; Safaee Fakhr, B.; Grassi, L.G.; Di Fenza, R.; Gianni, S.; Pinciroli, R.; Vassena, E.; et al. Protocol for a randomized controlled trial testing inhaled nitric oxide therapy in spontaneously breathing patients with COVID-19. medRxiv 2020. Available online: https://www.medrxiv.org/content/early/2020/03/13/2020.03.10.20033522.full.pdf (accessed on 1 May 2020).
- Lei, C.; Su, B.; Dong, H.; Bellavia, A.; Di Fenza, R.; Safaee Fakhr, B.; Gianni, S.; Grassi, L.G.; Kacmarek, R.; Araujo Morais, C.C.; et al. Protocol of a randomized controlled trial testing inhaled Nitric Oxide in mechanically ventilated patients with severe acute respiratory syndrome in COVID-19 (SARS-CoV-2). medRxiv 2020. Available online: https://www.medrxiv.org/content/early/2020/03/13/2020.03.09.20033530.full.pdf (accessed on 1 May 2020).
- Ikeda, Y.; Biro, S.; Kamogawa, Y.; Yoshifuku, S.; Eto, H.; Orihara, K.; Yu, B.; Kihara, T.; Miyata, M.; Hamasaki, S.; et al. Repeated sauna therapy increases arterial endothelial nitric oxide synthase expression and nitric oxide production in cardiomyopathic hamsters. Circ. J. 2005, 69, 722–729. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.N.; Thipse, M.; Mahdi, M.B.; Azad, S.; Davies, R.; Ruel, M.; Silver, M.A.; Hakami, L.; Mesana, T.; Leenen, F.; et al. Can heat therapy help patients with heart failure? Artif Organs 2020, 44, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Brunt, V.E.; Eymann, T.M.; Francisco, M.A.; Howard, M.J.; Minson, C.T. Passive heat therapy improves cutaneous microvascular function in sedentary humans via improved nitric oxide-dependent dilation. J. Appl. Physiol. 2016, 121, 716–723. [Google Scholar] [CrossRef] [Green Version]
- Oyama, J.i.; Kudo, Y.; Maeda, T.; Node, K.; Makino, N. Hyperthermia by bathing in a hot spring improves cardiovascular functions and reduces the production of inflammatory cytokines in patients with chronic heart failure. Heart Vessel. 2013, 28, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Karaarslan, F.; Ozkuk, K.; Seringec Karabulut, S.; Bekpinar, S.; Karagulle, M.Z.; Erdogan, N. How does spa treatment affect cardiovascular function and vascular endothelium in patients with generalized osteoarthritis? A pilot study through plasma asymmetric di-methyl arginine (ADMA) and L-arginine/ADMA ratio. Int. J. Biometeorol. 2018, 62, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Sobajima, M.; Nozawa, T.; Shida, T.; Ohori, T.; Suzuki, T.; Matsuki, A.; Inoue, H. Repeated sauna therapy attenuates ventricular remodeling after myocardial infarction in rats by increasing coronary vascularity of noninfarcted myocardium. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H548–H554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, M.; Hori, S. Experimental investigation in rats to identify the cause of sudden death during bathing in Japan. Acute Med. Surg. 2014, 1, 101–104. [Google Scholar] [CrossRef]
- Pieretti, J.C.; Pelegrino, M.T.; Nascimento, M.H.M.; Tortella, G.R.; Rubilar, O.; Seabra, A.B. Small molecules for great solutions: Can nitric oxide-releasing nanomaterials overcome drug resistance in chemotherapy? Biochem. Pharmacol. 2019, 176, 113740. [Google Scholar] [CrossRef]
- Su, H.; Wang, Y.; Gu, Y.; Bowman, L.; Zhao, J.; Ding, M. Potential applications and human biosafety of nanomaterials used in nanomedicine. J. Appl. Toxicol. 2018, 38, 3–24. [Google Scholar] [CrossRef]
- Teikoku Pharmaceutical Co. 2018. Available online: https://IFMC.jp (accessed on 31 May 2020).
- Uozumi, Y.; Nawashiro, H.; Sato, S.; Kawauchi, S.; Shima, K.; Kikuchi, M. Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg. Med. 2010, 42, 566–576. [Google Scholar] [CrossRef]
- Nakagawa, A.; Yokoyama, Y.; Suzuki, H.; Shoji, K.; Watanabe, Y.; Imamura, A.; Kokuryo, T.; Nagino, M. Real-time monitoring of liver damage during experimental ischaemia-reperfusion using a nitric oxide sensor. Br. J. Surg. 2012, 99, 1120–1128. [Google Scholar] [CrossRef]
- Yang, C.C.; Hsu, S.P.; Chen, K.H.; Chien, C.T. Effect of Adenoviral Catalase Gene Transfer on Renal Ischemia/Reperfusion Injury in Rats. Chin. J. Physiol. 2015, 58, 420–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.H.; Lin, C.L.; Wang, S.E.; Sheu, S.J.; Chien, C.T.; Wu, C.H. Oral treatment with herbal formula B401 alleviates penile toxicity in aging mice with manganism. Clin. Interv. Aging 2015, 10, 907–918. [Google Scholar] [PubMed] [Green Version]
- Tsutsui, C.; Lee, M.; Takahashi, G.; Murata, S.; Hirata, T.; Kanai, T.; Mori, A. Treatment of cardiac disease by inhalation of atmospheric pressure plasma. Jpn. J. Appl. Phys. 2014, 53, 060309. [Google Scholar] [CrossRef]
- Imanishi, T.; Kobayashi, K.; Kuroi, A.; Mochizuki, S.; Goto, M.; Yoshida, K.; Akasaka, T. Effects of angiotensin II on NO bioavailability evaluated using a catheter-type NO sensor. Hypertension 2006, 48, 1058–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kume, T.; Kawamoto, T.; Okura, H.; Neishi, Y.; Hashimoto, K.; Hayashida, A.; Watanabe, N.; Kanda, Y.; Mochizuki, S.; Goto, M.; et al. Evaluation of coronary endothelial function by catheter-type NO sensor in high-fat-diet-induced obese dogs. Circ. J. 2009, 73, 562–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mochizuki, S.; Miyasaka, T.; Goto, M.; Ogasawara, Y.; Yada, T.; Akiyama, M.; Neishi, Y.; Toyoda, T.; Tomita, J.; Koyama, Y.; et al. Measurement of acetylcholine-induced endothelium-derived nitric oxide in aorta using a newly developed catheter-type nitric oxide sensor. Biochem. Biophys. Res. Commun. 2003, 306, 505–508. [Google Scholar] [CrossRef]
- Neishi, Y.; Mochizuki, S.; Miyasaka, T.; Kawamoto, T.; Kume, T.; Sukmawan, R.; Tsukiji, M.; Ogasawara, Y.; Kajiya, F.; Akasaka, T.; et al. Evaluation of bioavailability of nitric oxide in coronary circulation by direct measurement of plasma nitric oxide concentration. Proc. Natl. Acad. Sci. USA 2005, 102, 11456–11461. [Google Scholar] [CrossRef] [Green Version]
- Scheel, P.; Ruge, C.; Schöning, M. Flow velocity and flow volume measurements in the extracranial carotid and vertebral arteries in healthy adults: Reference data and the effects of age. Ultrasound Med. Biol. 2000, 26, 1261–1266. [Google Scholar] [CrossRef]
- Turnbull, A.V.; Rivier, C.L. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: Actions and mechanisms of action. Physiol. Rev. 1999, 79, 1–71. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, G.; Zhao, J.; Nie, X.; Wan, C.; Liu, J.; Duan, Z.; Xu, G. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces microglial nitric oxide production and subsequent rat primary cortical neuron apoptosis through p38/JNK MAPK pathway. Toxicology 2013, 312, 132–141. [Google Scholar] [CrossRef]
- Roosterman, D.; Goerge, T.; Schneider, S.W.; Bunnett, N.W.; Steinhoff, M. Neuronal control of skin function: The skin as a neuroimmunoendocrine organ. Physiol. Rev. 2006, 86, 1309–1379. [Google Scholar] [PubMed]
- Ghimire, K.; Altmann, H.M.; Straub, A.C.; Isenberg, J.S. Nitric oxide: What’s new to NO? Am. J. Physiol. Cell Physiol. 2017, 312, C254–C262. [Google Scholar] [CrossRef] [PubMed]
- Vallet, B.; Curtis, S.E.; Winn, M.J.; King, C.E.; Chapler, C.K.; Cain, S.M. Hypoxic vasodilation does not require nitric oxide (EDRF/NO) synthesis. J. Appl. Physiol. 1994, 76, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.W.; Piantadosi, C.A. How do red blood cells cause hypoxic vasodilation? The SNO-hemoglobin paradigm. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1507–H1512. [Google Scholar] [PubMed] [Green Version]
- Straub, A.C.; Lohman, A.W.; Billaud, M.; Johnstone, S.R.; Dwyer, S.T.; Lee, M.Y.; Bortz, P.S.; Best, A.K.; Columbus, L.; Gaston, B.; et al. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature 2012, 491, 473–477. [Google Scholar] [CrossRef] [Green Version]
- Helms, C.; Kim-Shapiro, D.B. Hemoglobin-mediated nitric oxide signaling. Free Radic. Biol. Med. 2013, 61, 464–472. [Google Scholar]
- Lebrun, R.; Ross, A.; Bender, S.A.; Qaiumzadeh, A.; Baldrati, L.; Cramer, J.; Brataas, A.; Duine, R.A.; Kläui, M. Tunable long-distance spin transport in a crystalline antiferromagnetic iron oxide. Nature 2018, 561, 222–225. [Google Scholar] [CrossRef]
- Culotta, E.; Koshland, D. NO news is good news. Science 1992, 258, 1862–1865. [Google Scholar] [CrossRef]
- Chen, G.; Suzuki, H.; Weston, A.H. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br. J. Pharmacol. 1988, 95, 1165–1174. [Google Scholar]
- Takamura, Y.; Shimokawa, H.; Zhao, H.; Igarashi, H.; Egashira, K.; Takeshita, A. Important role of endothelium-derived hyperpolarizing factor in shear stress–induced endothelium-dependent relaxations in the rat mesenteric artery. J. Cardiovasc. Pharmacol. 1999, 34, 381–387. [Google Scholar]
- Lakshminrusimha, S.; Porta, N.F.; Farrow, K.N.; Chen, B.; Gugino, S.F.; Kumar, V.H.; Russell, J.A.; Steinhorn, R.H. Milrinone enhances relaxation to prostacyclin and iloprost in pulmonary arteries isolated from lambs with persistent pulmonary hypertension of the newborn. Pediatr. Crit. Care Med. 2009, 10, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Takaki, A.; Morikawa, K.; Tsutsui, M.; Murayama, Y.; Tekes, E.; Yamagishi, H.; Ohashi, J.; Yada, T.; Yanagihara, N.; Shimokawa, H. Crucial role of nitric oxide synthases system in endothelium-dependent hyperpolarization in mice. J. Exp. Med. 2008, 205, 2053–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkor, M.A.; Quyyumi, A.A. Endothelium-derived hyperpolarizing factor and vascular function. Cardiol. Res. Pract. 2011, 2011, 156146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, P.J. Nitric Oxide and Airway Disease. Ann. Med. 1995, 27, 389–393. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the IFMC may be available from the corresponding author. Original images and CSV files may also be obtained from the corresponding author upon request. |





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akiyama, T.; Hirata, T.; Fujimoto, T.; Hatakeyama, S.; Yamazaki, R.; Nomura, T. The Natural-Mineral-Based Novel Nanomaterial IFMC Increases Intravascular Nitric Oxide without Its Intake: Implications for COVID-19 and beyond. Nanomaterials 2020, 10, 1699. https://doi.org/10.3390/nano10091699
Akiyama T, Hirata T, Fujimoto T, Hatakeyama S, Yamazaki R, Nomura T. The Natural-Mineral-Based Novel Nanomaterial IFMC Increases Intravascular Nitric Oxide without Its Intake: Implications for COVID-19 and beyond. Nanomaterials. 2020; 10(9):1699. https://doi.org/10.3390/nano10091699
Chicago/Turabian StyleAkiyama, Tomohiro, Takamichi Hirata, Takahiro Fujimoto, Shinnosuke Hatakeyama, Ryuhei Yamazaki, and Tomohiro Nomura. 2020. "The Natural-Mineral-Based Novel Nanomaterial IFMC Increases Intravascular Nitric Oxide without Its Intake: Implications for COVID-19 and beyond" Nanomaterials 10, no. 9: 1699. https://doi.org/10.3390/nano10091699