Effect of Polyethylene Glycol Incorporation in Electron Transport Layer on Photovoltaic Properties of Perovskite Solar Cells
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Methylammonium Iodide (CH3NH3I, MAI)
2.3. Synthesis of Zinc Oxide
2.4. Device Fabrication
2.5. Measurements and Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ke, W.; Fang, G.; Liu, Q.; Xiong, L.; Qin, P.; Tao, H.; Wang, J.; Lei, H.; Li, B.; Wan, J.; et al. Low-temperature solution-processed tin oxide as an alternative electron transporting layer for efficient perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 6730–6733. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron- and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.-J.; Shi, T.; Yan, Y. Unique properties of halide perovskites as possible origins of the superior solar cell performance. Adv. Mater. 2014, 26, 4653–4658. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Siempelkamp, B.D.; Mosconi, E.; De Angelis, F.; Kelly, T.L. Origin of the thermal instability in CH3NH3PbI3 thin films deposited on ZnO. Chem. Mater. 2015, 27, 4229–4236. [Google Scholar] [CrossRef]
- Edri, E.; Kirmayer, S.; Mukhopadhyay, S.; Gartsman, K.; Hodes, G.; Cahen, D. Elucidating the charge carrier separation and working mechanism of CH3NH3PbI3−xClx perovskite solar cells. Nat. Commun. 2014, 5, 3461. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photon. 2014, 8, 506. [Google Scholar] [CrossRef]
- Sacramento, A.S.; Moreira, F.T.C.; Guerreiro, J.L.; Tavares, A.P.; Sales, M.G.F. Novel biomimetic composite material for potentiometric screening of acetylcholine, a neurotransmitter in Alzheimer’s disease. Mater. Sci. Eng. C 2017, 79, 541–549. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)2PbI3-based perovskite solar cells. Nat. Energy 2016, 2, 16177. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Wan, J.; Tao, H.; Liu, Q.; Xiong, L.; Qin, P.; Wang, J.; Lei, H.; Yang, G.; et al. Efficient hole-blocking layer-free planar halide perovskite thin-film solar cells. Nat. Commun. 2015, 6, 6700. [Google Scholar] [CrossRef]
- Shi, J.; Dong, J.; Lv, S.; Xu, Y.; Zhu, L.; Xiao, J.; Xu, X.; Wu, H.; Li, D.; Luo, Y.; et al. Hole-conductor-free perovskite organic lead iodide heterojunction thin-film solar cells: High efficiency and junction property. Appl. Phys. Lett. 2014, 104, 063901. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Liang, J.; Li, T.; Fan, L.; Shi, B.; Wei, C.; Ding, Y.; Li, Y.; Zhao, Y.; Zhang, X. Electron transport layer driven to improve the open-circuit voltage of CH3NH3PbI3 planar perovskite solar cells. Sci. China Mater. 2018, 61, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Bera, A.; Sheikh, A.D.; Haque, M.A.; Bose, R.; Alarousu, E.; Mohammed, O.F.; Wu, T. Fast crystallization and improved stability of perovskite solar cells with Zn2SnO4 electron transporting layer: Interface matters. ACS Appl. Mater. Interfaces 2015, 7, 28404–28411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, F.; Feurer, T.; Jäger, T.; Avancini, E.; Bissig, B.; Yoon, S.; Buecheler, S.; Tiwari, A.N. Low-temperature-processed efficient semi-transparent planar perovskite solar cells for bifacial and tandem applications. Nat. Commun. 2015, 6, 8932. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, J.; Wu, T.; Bao, Q.; He, X.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L. Tuning the Fermi level of TiO2 electron transport layer through europium doping for highly efficient perovskite solar cells. Energy Tech. 2017, 5, 1820–1826. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, K.; Swain, B.S.; Kirmani, A.R.; Amassian, A. Highly efficient perovskite solar cells based on a nanostructured WO3–TiO2 core–shell electron transporting material. J. Mater. Chem. A 2015, 3, 9051–9057. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-b.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef]
- Wang, J.; Qin, M.; Tao, H.; Ke, W.; Chen, Z.; Wan, J.; Qin, P.; Xiong, L.; Lei, H.; Yu, H.; et al. Performance enhancement of perovskite solar cells with Mg-doped TiO2 compact film as the hole-blocking layer. Appl. Phys. Lett. 2015, 106, 121104. [Google Scholar] [CrossRef]
- Dymshits, A.; Iagher, L.; Etgar, L. Parameters influencing the growth of ZnO nanowires as efficient low temperature flexible perovskite-based solar cells. Materials 2016, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Zhao, Y.; Shi, J.; Wei, H.; Xiao, J.; Xu, X.; Luo, J.; Xu, J.; Li, D.; Luo, Y.; et al. Impressive enhancement in the cell performance of ZnO nanorod-based perovskite solar cells with Al-doped ZnO interfacial modification. Chem. Commun. 2014, 50, 13381–13384. [Google Scholar] [CrossRef]
- Kim, K.-D.; Lim, D.C.; Hu, J.; Kwon, J.-D.; Jeong, M.-G.; Seo, H.O.; Lee, J.Y.; Jang, K.-Y.; Lim, J.-H.; Lee, K.H.; et al. Surface modification of a ZnO electron-collecting layer using atomic layer deposition to fabricate high-performing inverted organic photovoltaics. ACS Appl. Mater. Interfaces 2013, 5, 8718–8723. [Google Scholar] [CrossRef]
- Lee, K.-M.; Lin, C.-J.; Chang, Y.-H.; Lin, T.-H.; Suryanarayanan, V.; Wu, M.-C. The effect of post-baking temperature and thickness of ZnO electron transport layers for efficient planar heterojunction organometal-trihalide perovskite solar cells. Coatings 2017, 7, 215. [Google Scholar] [CrossRef] [Green Version]
- Matas Adams, A.; Marin-Beloqui, J.M.; Stoica, G.; Palomares, E. The influence of the mesoporous TiO2 scaffold on the performance of methyl ammonium lead iodide (MAPI) perovskite solar cells: Charge injection, charge recombination and solar cell efficiency relationship. J. Mater. Chem. A 2015, 3, 22154–22161. [Google Scholar] [CrossRef]
- Wang, H.-H.; Chen, Q.; Zhou, H.; Song, L.; Louis, Z.S.; Marco, N.D.; Fang, Y.; Sun, P.; Song, T.-B.; Chen, H.; et al. Improving the TiO2 electron transport layer in perovskite solar cells using acetylacetonate-based additives. J. Mater. Chem. A 2015, 3, 9108–9115. [Google Scholar] [CrossRef]
- Carcia, P.F.; McLean, R.S.; Reilly, M.H. High-performance ZnO thin-film transistors on gate dielectrics grown by atomic layer deposition. Appl. Phys. Lett. 2006, 88, 123509. [Google Scholar] [CrossRef]
- Hadouchi, W.; Rousset, J.; Tondelier, D.; Geffroy, B.; Bonnassieux, Y. Zinc oxide as a hole blocking layer for perovskite solar cells deposited in atmospheric conditions. RSC Adv. 2016, 6, 67715–67723. [Google Scholar] [CrossRef]
- Ahmed, M.I.; Hussain, Z.; Mujahid, M.; Khan, A.N.; Javaid, S.S.; Habib, A. Low resistivity ZnO-GO electron transport layer based CH3NH3PbI3 solar cells. AIP Adv. 2016, 6, 065303. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zang, Z.; Han, C.; Hu, Z.; Tang, X.; Du, J.; Leng, Y.; Sun, K. Highly compact CsPbBr3 perovskite thin films decorated by ZnO nanoparticles for enhanced random lasing. Nano Energy 2017, 40, 195–202. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, Q.; Wu, H.; Li, H.; Lai, J.; Ji, G.; Yan, L.; Wang, X.; Zhang, D.; Lin, J.; et al. Roll-to-roll micro-gravure printed large-area zinc oxide thin film as the electron transport layer for solution-processed polymer solar cells. Org. Electron. 2017, 45, 190–197. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Jiang, Q.; Wang, P.; Yin, Z.; Zhang, X.; Tan, H.; Yang, Y.; Wei, M.; Sutherland, B.R.; et al. Ultra-bright and highly efficient inorganic based perovskite light-emitting diodes. Nat. Commun. 2017, 8, 15640. [Google Scholar] [CrossRef]
- Park, H.-Y.; Lim, D.; Kim, K.-D.; Jang, S.-Y. Performance optimization of low-temperature-annealed solution-processable ZnO buffer layers for inverted polymer solar cells. J. Mater. Chem. A 2013, 1, 6327–6334. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Elumalai, N.K.; Upama, M.B.; Wang, D.; Chan, K.H.; Wright, M.; Xu, C.; Haque, F.; Uddin, A. Low temperature processed ZnO thin film as electron transport layer for efficient perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 159, 251–264. [Google Scholar] [CrossRef]
- Xu, L.; Guo, Y.; Liao, Q.; Zhang, J.; Xu, D. Morphological control of ZnO nanostructures by electrodeposition. J. Phys. Chem. B 2005, 109, 13519–13522. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-M.; Chang, S.H.; Wang, K.-H.; Chang, C.-M.; Cheng, H.-M.; Kei, C.-C.; Tseng, Z.-L.; Wu, C.-G. Thickness effects of ZnO thin film on the performance of tri-iodide perovskite absorber based photovoltaics. Sol. Energy 2015, 120, 117–122. [Google Scholar] [CrossRef]
- Han, G.S.; Shim, H.-W.; Lee, S.; Duff, M.L.; Lee, J.-K. Low-temperature modification of ZnO nanoparticles film for electron-transport layers in perovskite solar cells. ChemSusChem 2017, 10, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Seo, J.-Y.; Park, N.-G. Material and device stability in perovskite solar cells. ChemSusChem 2016, 9, 2528–2540. [Google Scholar] [CrossRef]
- Dong, Q.; Liu, F.; Wong, M.K.; Tam, H.W.; Djurišić, A.B.; Ng, A.; Surya, C.; Chan, W.K.; Ng, A.M.C. Encapsulation of perovskite solar cells for high humidity conditions. ChemSusChem 2016, 9, 2597–2603. [Google Scholar] [CrossRef]
- Dkhissi, Y.; Meyer, S.; Chen, D.; Weerasinghe, H.C.; Spiccia, L.; Cheng, Y.-B.; Caruso, R.A. Stability comparison of perovskite solar cells based on zinc oxide and titania on polymer substrates. ChemSusChem 2016, 9, 687–695. [Google Scholar] [CrossRef]
- Cao, J.; Wu, B.; Chen, R.; Wu, Y.; Hui, Y.; Mao, B.-W.; Zheng, N. Efficient, Hysteresis-Free, and Stable Perovskite Solar Cells with ZnO as Electron-Transport Layer: Effect of Surface Passivation. Adv. Mater. 2018, 30, 1705596. [Google Scholar] [CrossRef]
- Si, H.; Liao, Q.; Zhang, Z.; Li, Y.; Yang, X.; Zhang, G.; Kang, Z.; Zhang, Y. An innovative design of perovskite solar cells with Al2O3 inserting at ZnO/perovskite interface for improving the performance and stability. Nano Energy 2016, 22, 223–231. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Zhang, Q. Progress in perovskite solar cells based on ZnO nanostructures. Sol. Energy 2018, 163, 289–306. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, Q.-D.; Xiao, J.; Xue, Q.; Li, H.-W.; Guan, Z.; Yip, H.-L.; Tsang, S.-W. Decomposition of organometal halide perovskite films on zinc oxide nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 19986–19993. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Gu, Z.; Ye, T.; Fu, W.; Wu, G.; Li, H.; Chen, H. Enhanced photovoltaic performance of CH3NH3PbI3 perovskite solar cells through interfacial engineering using self-assembling monolayer. J. Am. Chem. Soc. 2015, 137, 2674–2679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ling, H.; Lu, X.; Xia, J. The facile modification of PEDOT: PSS buffer layer by polyethyleneglycol and their effects on inverted perovskite solar cell. Sol. Energy 2019, 186, 398–403. [Google Scholar] [CrossRef]
- Qin, Q.; Zhang, Z.; Cai, Y.; Zhou, Y.; Liu, H.; Lu, X.; Gao, X.; Shui, L.; Wu, S.; Liu, J. Improving the performance of low-temperature planar perovskite solar cells by adding functional fullerene end-capped polyethylene glycol derivatives. J. Power Sources 2018, 396, 49–56. [Google Scholar] [CrossRef]
- Fu, Q.; Xiao, S.; Tang, X.; Chen, Y.; Hu, T. Amphiphilic fullerenes employed to improve the quality of perovskite films and the stability of perovskite solar cells. ACS Appl. Mater. Interfaces 2019, 11, 24782–24788. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Zhang, B.; Guo, Z.-A.; Bala, H.; Yao, S.; Zhang, J.; Chen, C.; Fu, W.; Cao, J. Perovskite solar cells based on the synergy between carbon electrodes and polyethylene glycol additive with excellent stability. Org. Electron. 2020, 83, 105734. [Google Scholar] [CrossRef]
- Jo, S.B.; Lee, J.H.; Sim, M.; Kim, M.; Park, J.H.; Choi, Y.S.; Kim, Y.; Ihn, S.G.; Cho, K. High performance organic photovoltaic cells using polymer-hybridized ZnO nanocrystals as a cathode interlayer. Adv. Energy Mater. 2011, 1, 690–698. [Google Scholar] [CrossRef]
- Shao, S.; Zheng, K.; Pullerits, T.n.; Zhang, F. Enhanced performance of inverted polymer solar cells by using poly (ethylene oxide)-modified ZnO as an electron transport layer. ACS Appl. Mater. Interfaces 2013, 5, 380–385. [Google Scholar] [CrossRef]
- Cheng, C.-J.; Balamurugan, R.; Liu, B.-T. Enhanced efficiencies of perovskite solar cells by incorporating silver nanowires into the hole transport layer. Micromachines 2019, 10, 682. [Google Scholar] [CrossRef] [Green Version]
- Li, P.-S.; Balamurugan, R.; Liu, B.-T.; Lee, R.-H.; Chou, H.-T. MAPbI3 incorporated with carboxyl group chelated titania for planar perovskite solar cells in low-temperature process. Nanomaterials 2019, 9, 908. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Kelly, T.L. Perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques. Nat. Photon. 2013, 8, 133. [Google Scholar] [CrossRef]
- Mohammadian-Sarcheshmeh, H.; Mazloum-Ardakani, M. Recent advancements in compact layer development for perovskite solar cells. Heliyon 2018, 4, e00912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, X.; Shao, C.; Liu, Y. Photoluminescence of polyethylene oxide–ZnO composite electrospun fibers. Polymer 2007, 48, 1459–1463. [Google Scholar] [CrossRef]
- Tshabalala, M.A.; Dejene, F.; Swart, H. Synthesis and characterization of ZnO nanoparticles using polyethylene glycol (PEG). Physica B 2012, 407, 1668–1671. [Google Scholar] [CrossRef]
- Zhao, J.-H.; Liu, C.-J.; Lv, Z.-H. Photoluminescence of ZnO nanoparticles and nanorods. Optik 2016, 127, 1421–1423. [Google Scholar] [CrossRef]
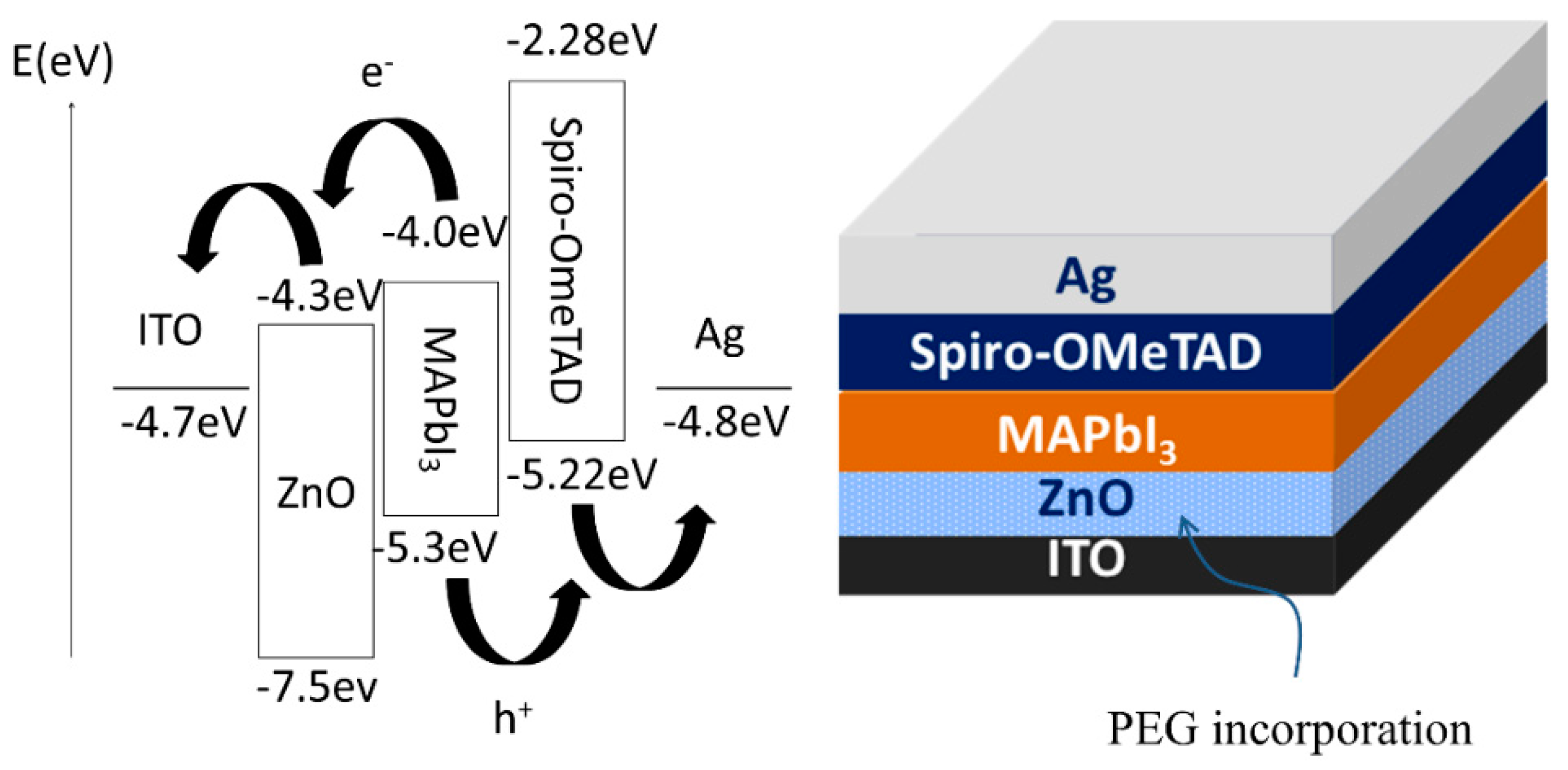
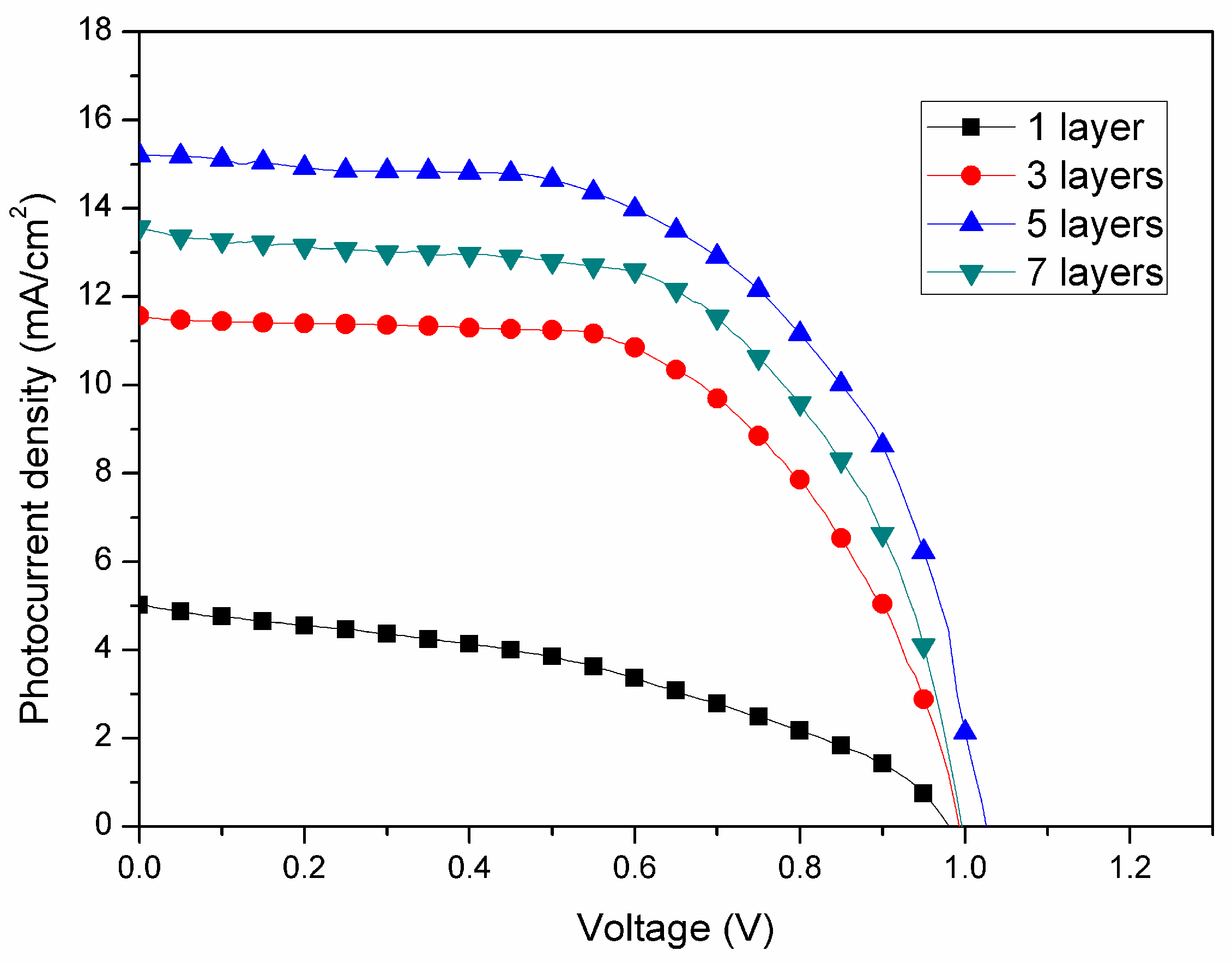
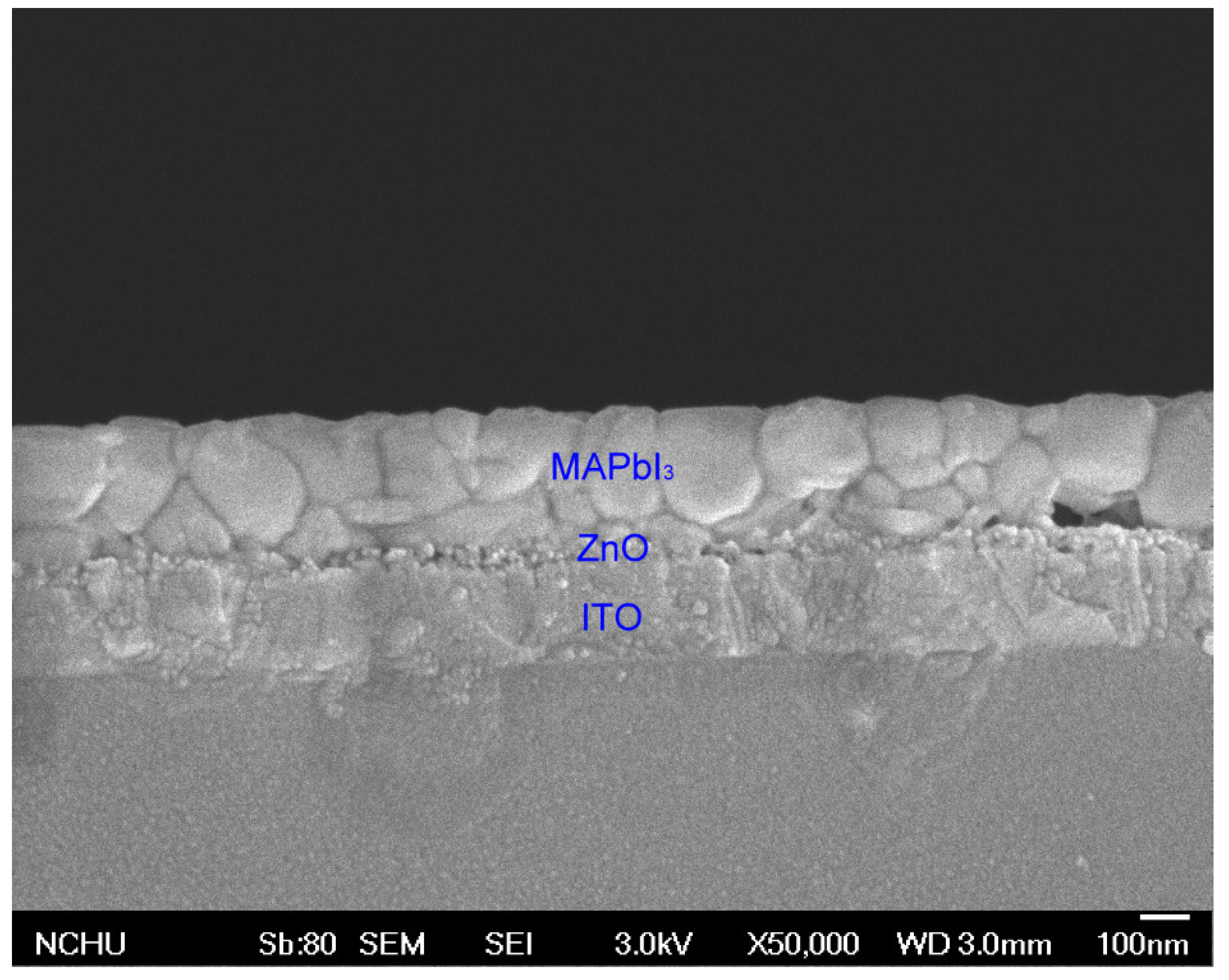
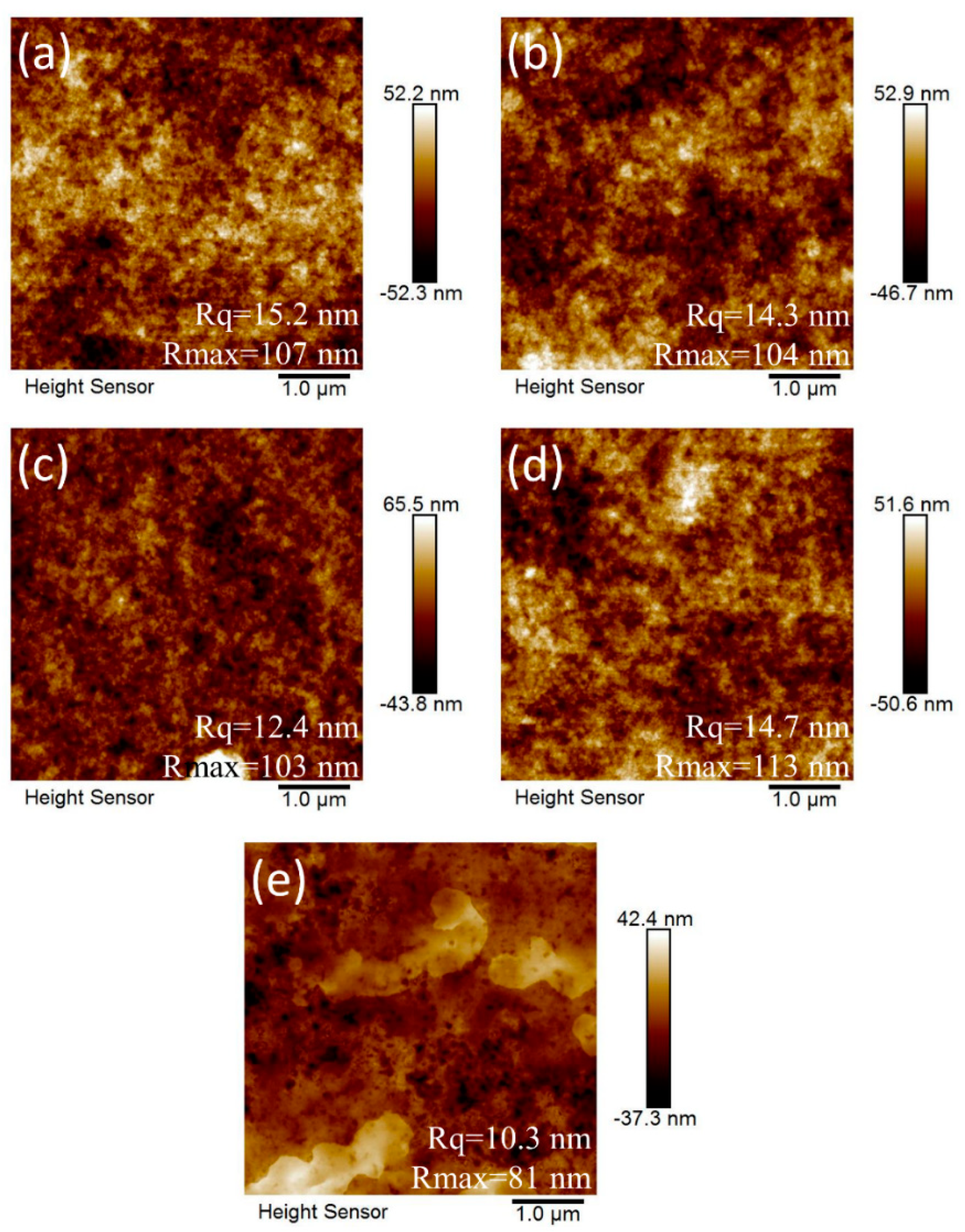

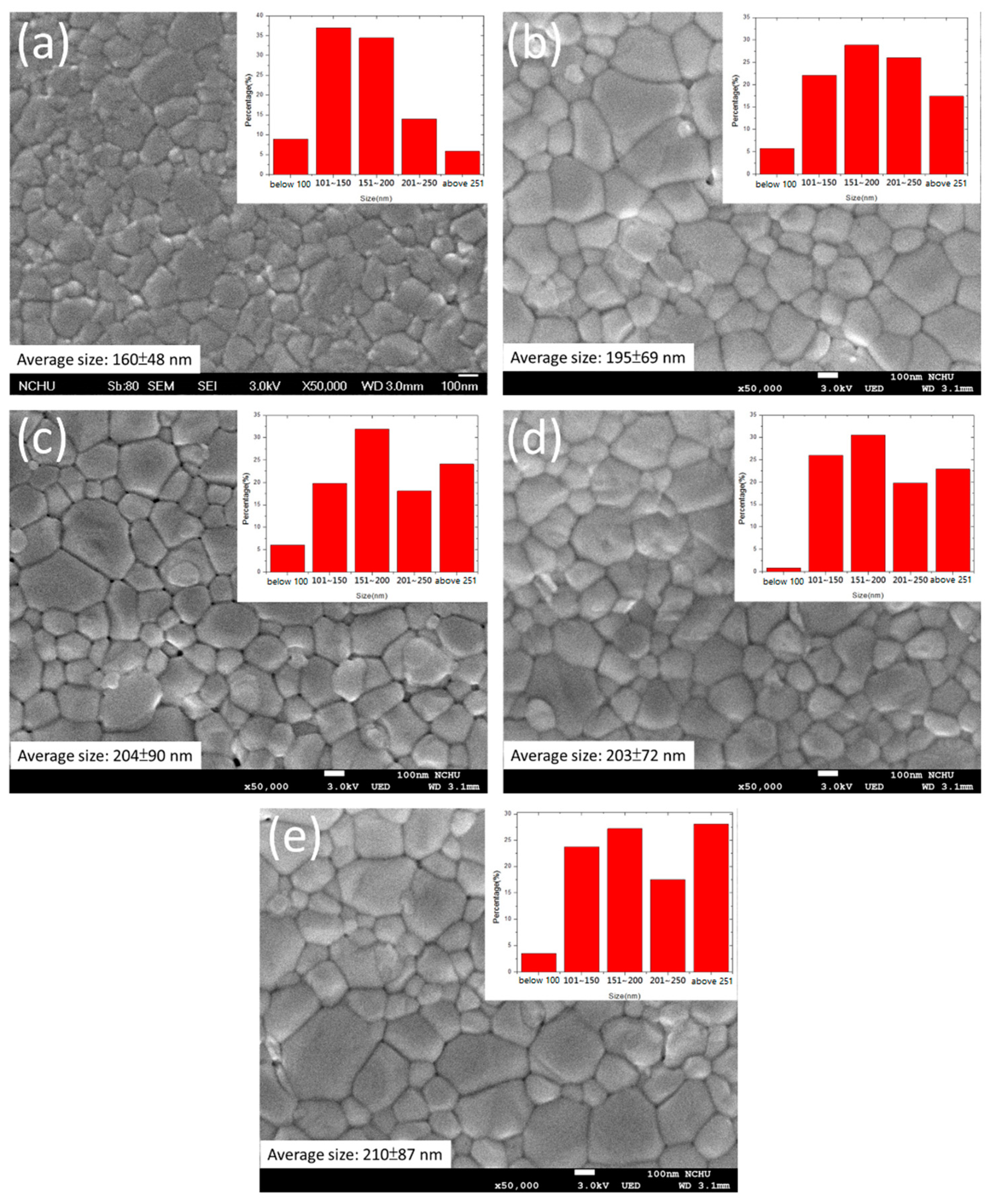

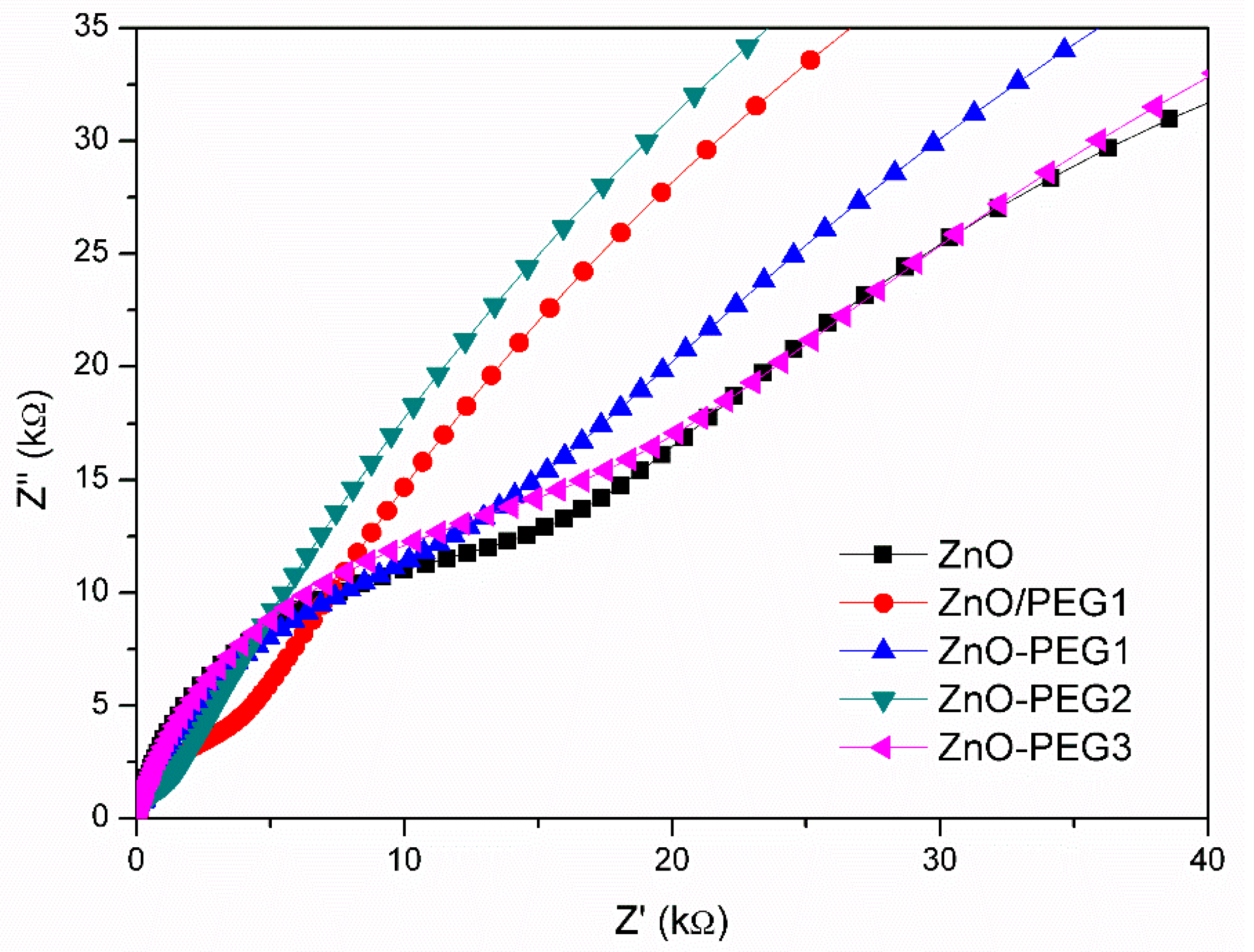
| No. of ZnO Layer | Voc (V) | Jsc (mA/cm2) | FF (%) | η (%) |
|---|---|---|---|---|
| 1 | 0.98 | 5.0 | 41.0 | 2.0 |
| 3 | 0.99 | 11.6 | 59.5 | 6.8 |
| 5 | 1.02 | 15.2 | 59.3 | 9.2 |
| 7 | 0.99 | 13.6 | 60.1 | 8.1 |
| Sample | PEG content (wt%) | Voc (V) | Jsc (mA/cm2) | FF (%) | η (STD c) (%) |
|---|---|---|---|---|---|
| ZnO | 0 a | 1.02 | 15.2 | 59.3 | 9.2 (0.21) |
| ZnO-PEG1 | 0.1 a | 1.02 | 16.6 | 61.5 | 10.0 (0.30) |
| ZnO-PEG2 | 0.2 a | 1.01 | 19.2 | 59.4 | 11.5 (0.12) |
| ZnO-PEG3 | 0.3 a | 1.02 | 16.5 | 59.4 | 10.4 (0.21) |
| ZnO/PEG1 | 1b | 1.02 | 17.6 | 59.6 | 10.7 (0.25) |
| Sample | R1 (Ω) | R2 (kΩ) | R3 (kΩ) |
|---|---|---|---|
| ZnO | 36.8 | 14.0 | 104 |
| ZnO-PEG1 | 35.1 | 8.5 | 177.9 |
| ZnO-PEG2 | 34.0 | 0.81 | 205.7 |
| ZnO-PEG3 | 35.1 | 15.1 | 131.8 |
| ZnO/PEG1 | 34.1 | 2.7 | 179.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.-T.; Guo, B.-W.; Balamurugan, R. Effect of Polyethylene Glycol Incorporation in Electron Transport Layer on Photovoltaic Properties of Perovskite Solar Cells. Nanomaterials 2020, 10, 1753. https://doi.org/10.3390/nano10091753
Liu B-T, Guo B-W, Balamurugan R. Effect of Polyethylene Glycol Incorporation in Electron Transport Layer on Photovoltaic Properties of Perovskite Solar Cells. Nanomaterials. 2020; 10(9):1753. https://doi.org/10.3390/nano10091753
Chicago/Turabian StyleLiu, Bo-Tau, Bo-Wei Guo, and Rathinam Balamurugan. 2020. "Effect of Polyethylene Glycol Incorporation in Electron Transport Layer on Photovoltaic Properties of Perovskite Solar Cells" Nanomaterials 10, no. 9: 1753. https://doi.org/10.3390/nano10091753





