Adsorption of Cellulase on Wrinkled Silica Nanoparticles with Enhanced Inter-Wrinkle Distance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoparticle Synthesis
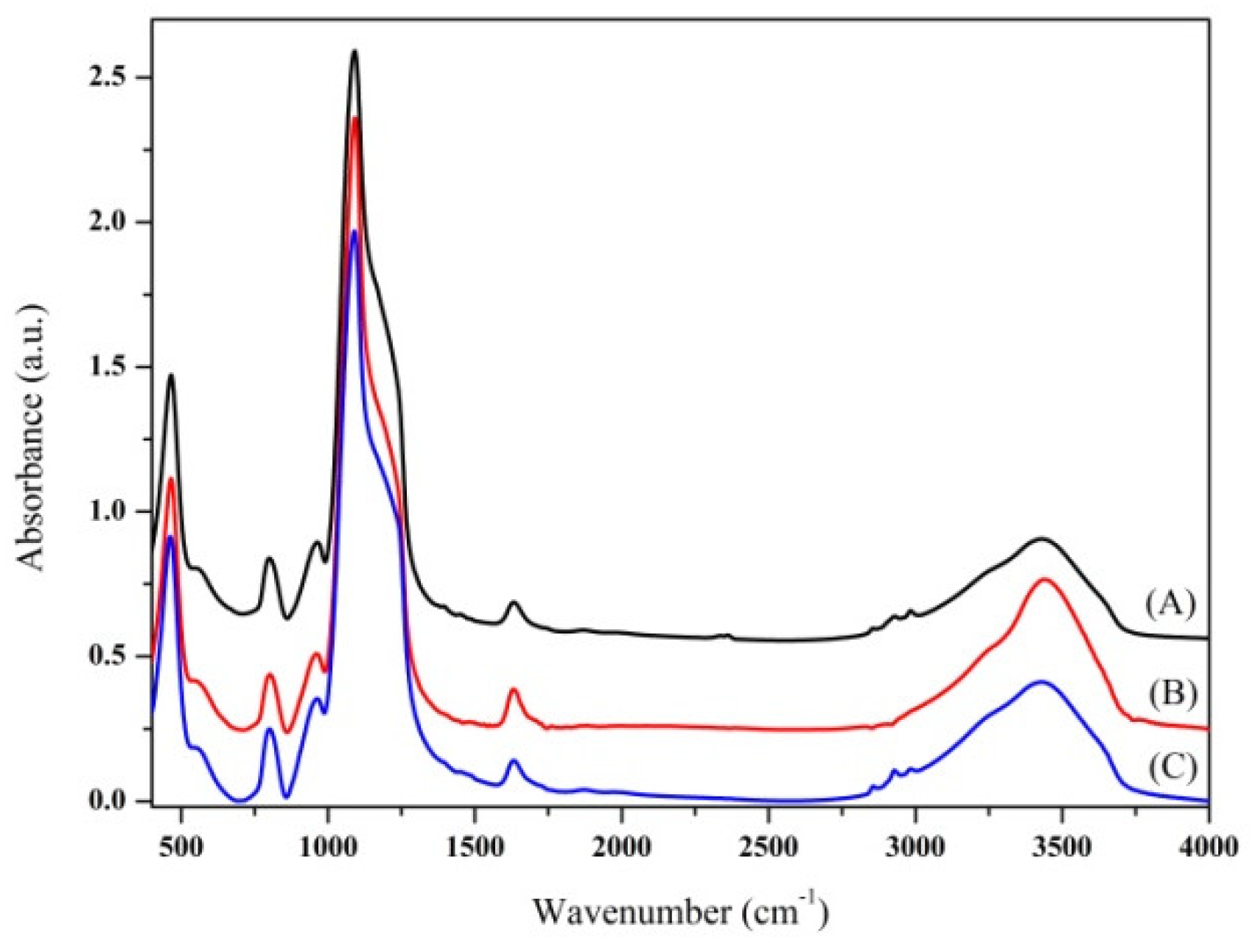
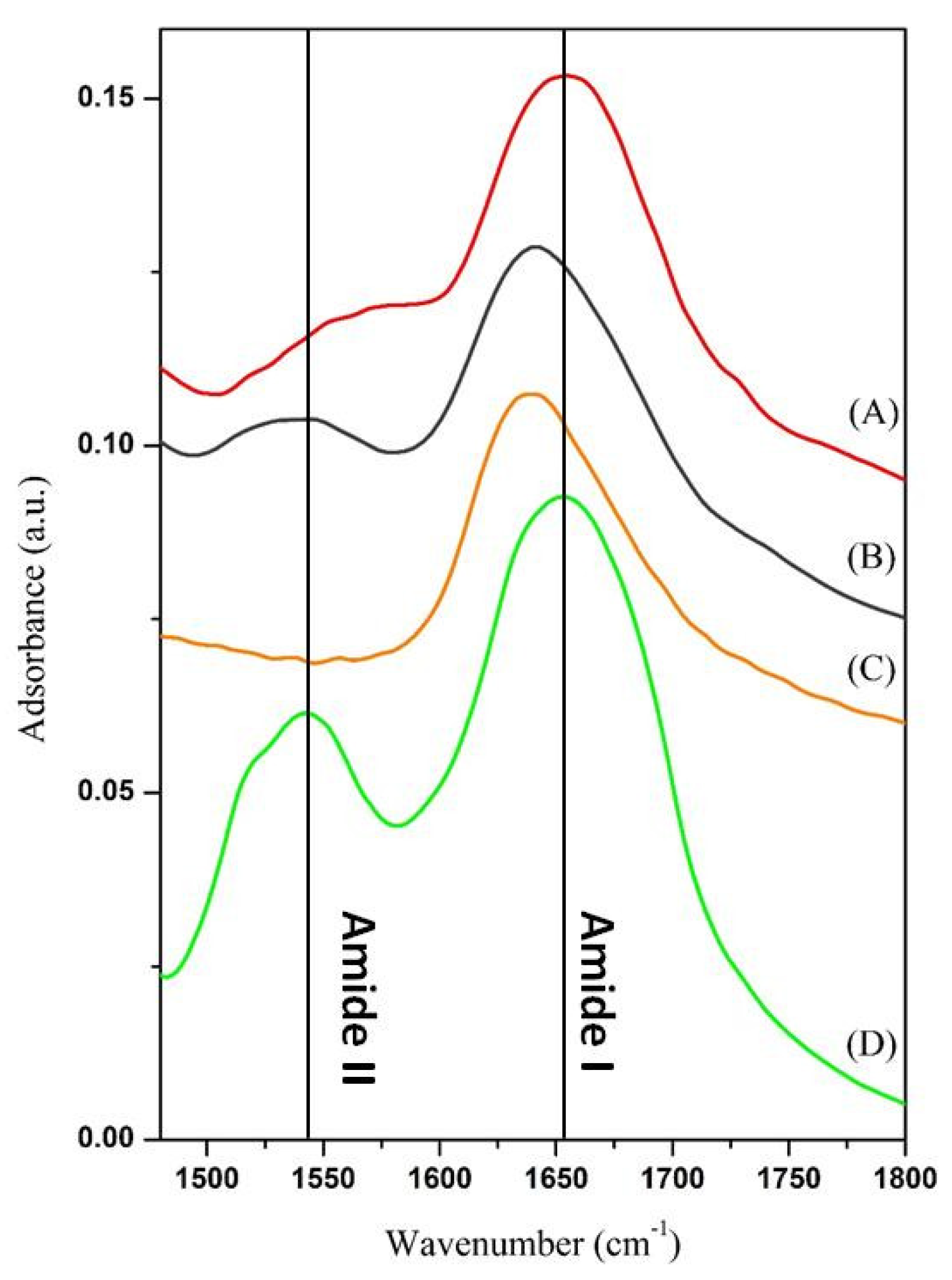
2.3. Chemical and Physical Characterization
2.4. Cellulase Immobilization
2.5. Catalytic Assays
2.6. Operational Stability
3. Results and Discussion
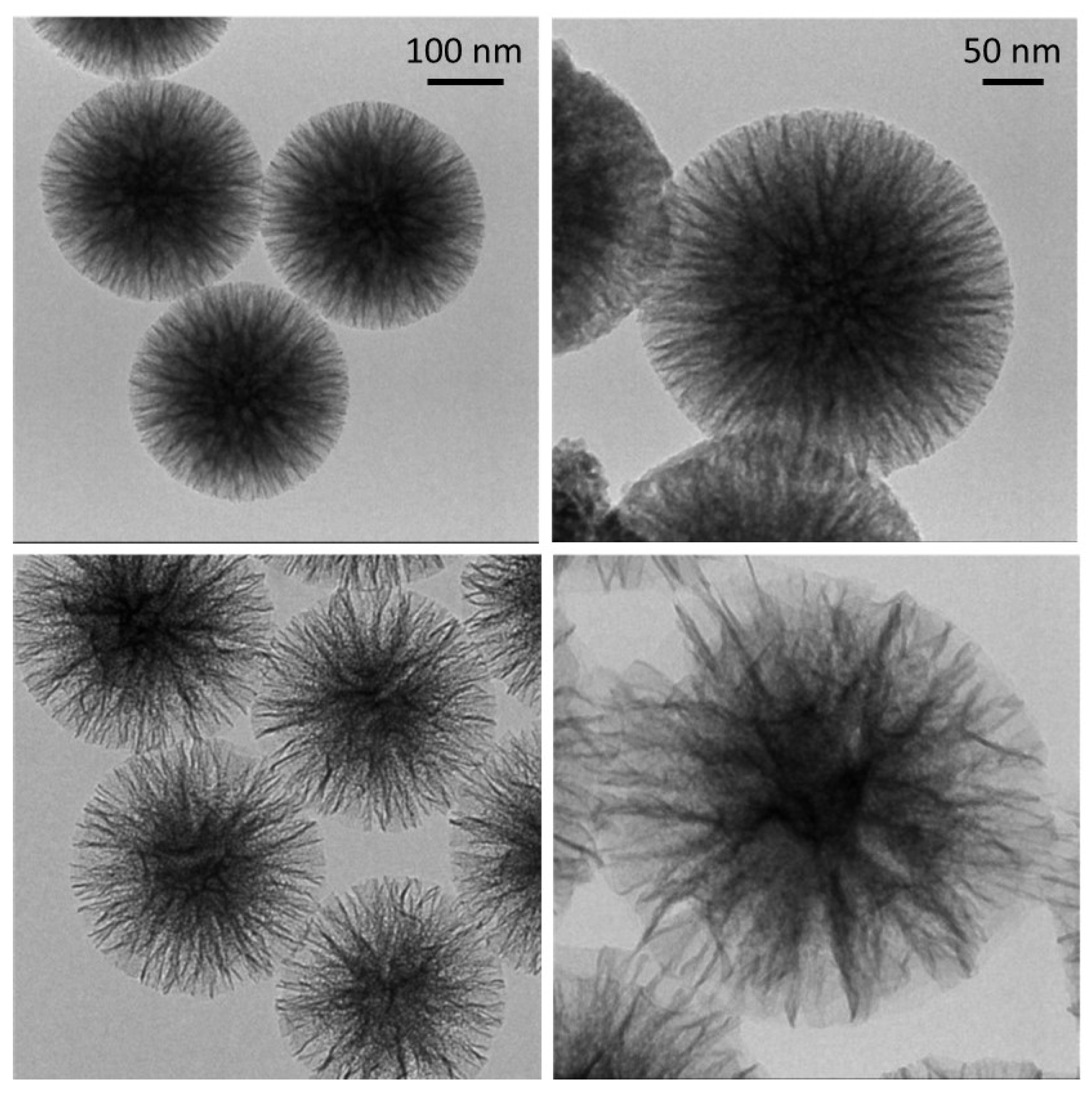
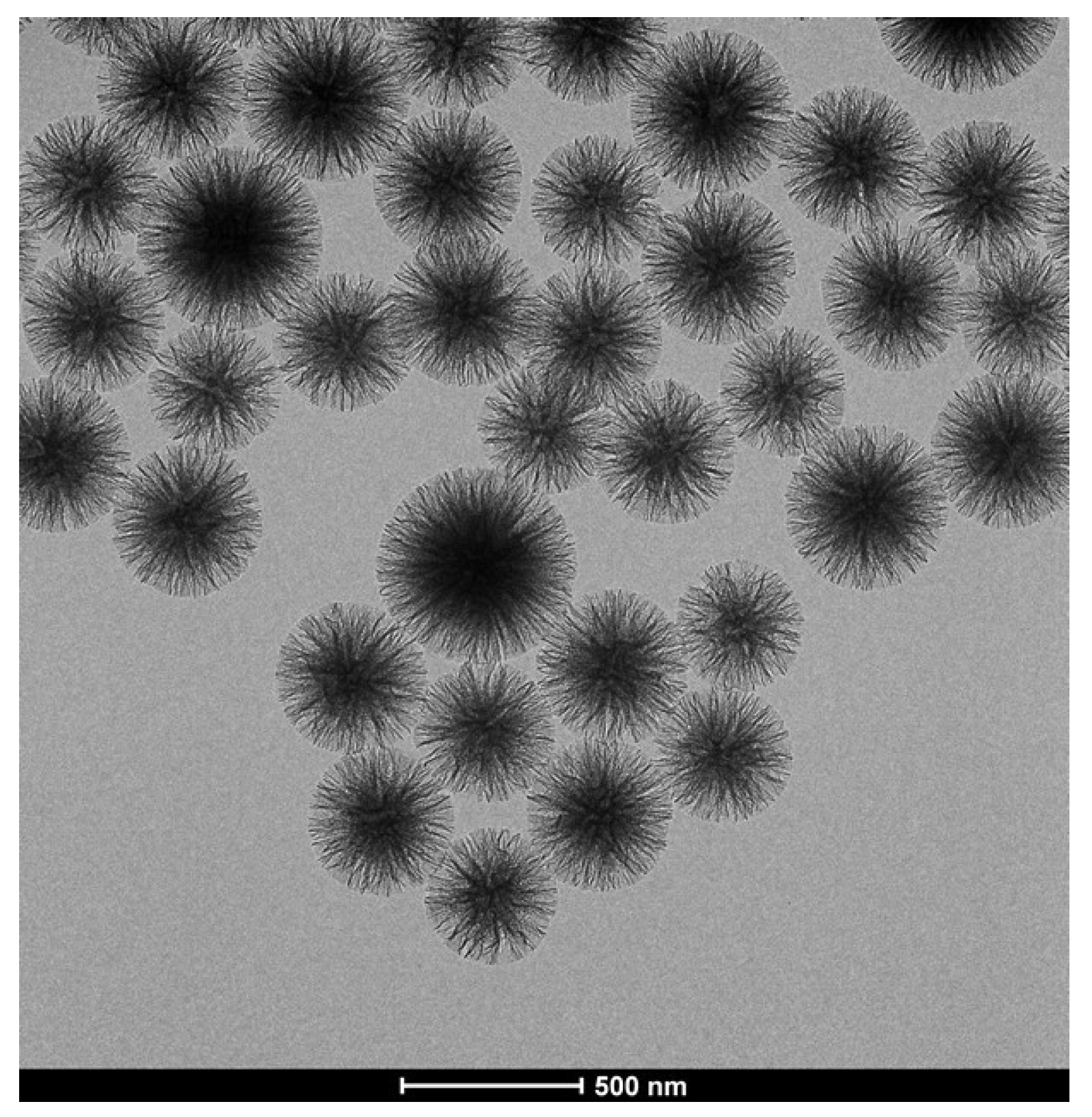
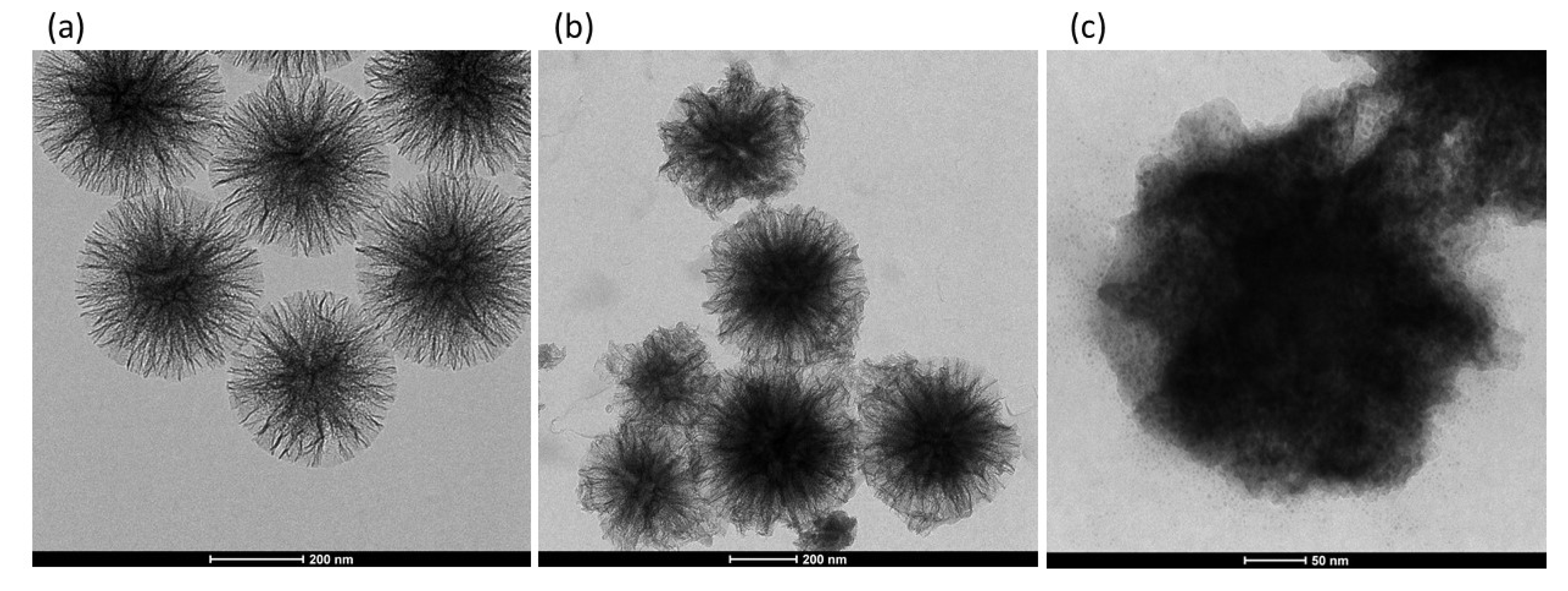
3.1. Choice of Supports
3.2. Optimization of the Adsorption Conditions
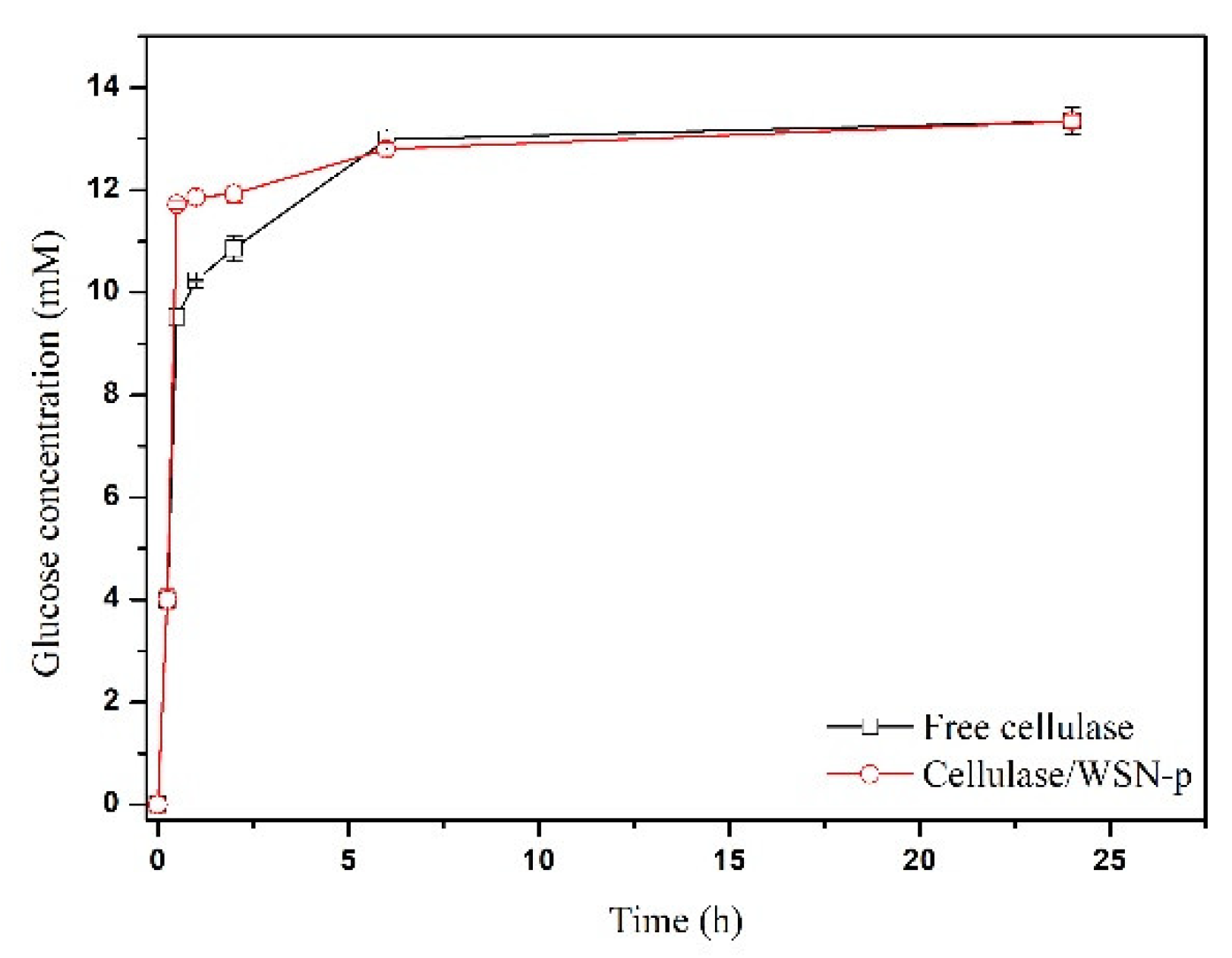
3.3. Catalytic Assays
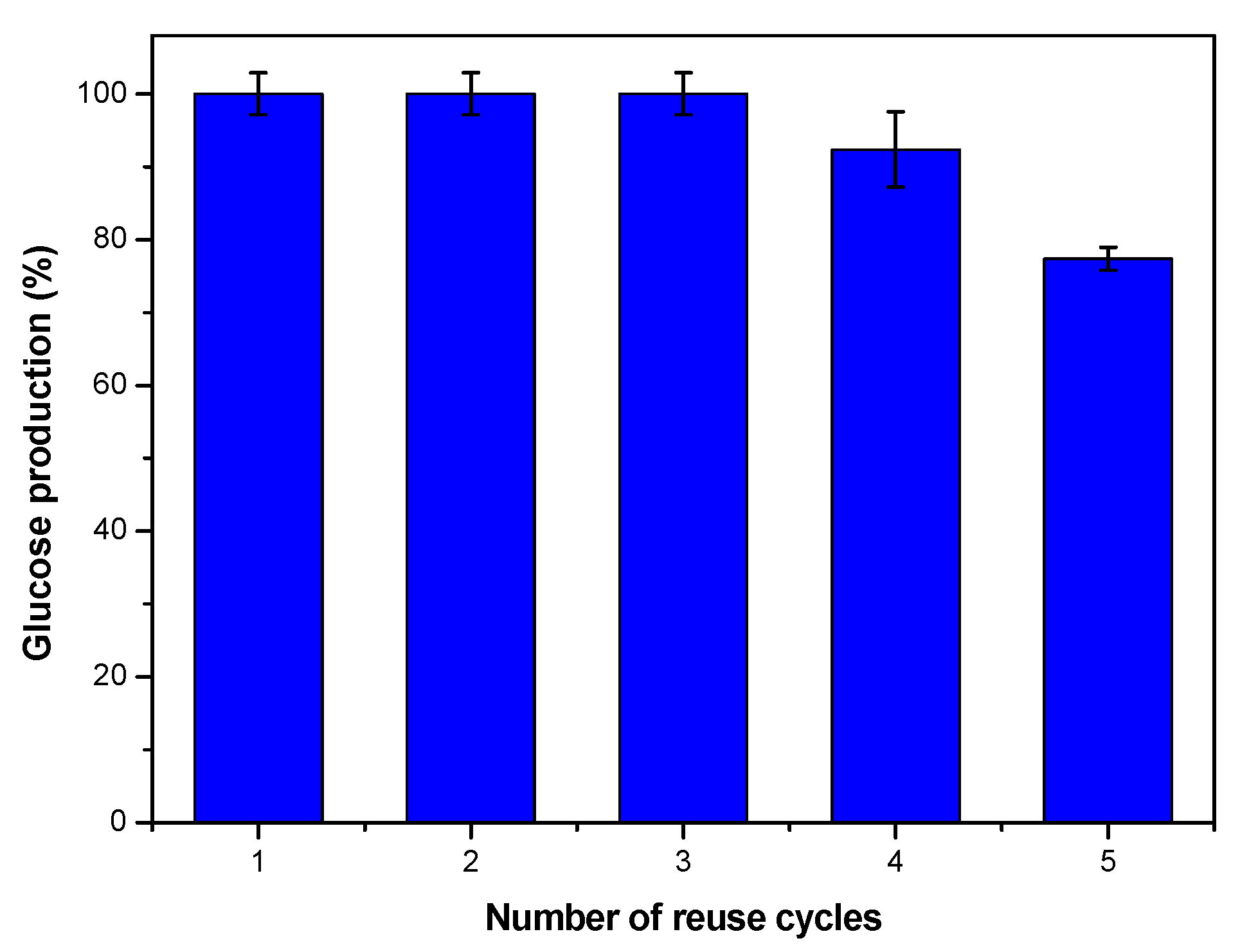
3.4. Reusability Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chang, R.H.Y.; Jang, J.; Wu, K.C.W. Cellulase immobilized mesoporous silica nanocatalysts for efficient cellulose-to-glucose conversion. Green Chem. 2011, 13, 2844–2850. [Google Scholar] [CrossRef]
- Yin, H.; Su, Z.L.; Shao, H.; Cai, J.; Wang, X.; Yin, H. Immobilization of cellulase on modified mesoporous silica shows improved thermal stability and reusability. Afr. J. Microbiol. Res. 2013, 7, 3248–3253. [Google Scholar]
- Walker, L.P.; Wilson, D.B. Enzymatic hydrolysis of cellulose: An overview. Bioresour. Technol. 1991, 36, 3–14. [Google Scholar] [CrossRef]
- Bhat, M. Cellulases and related enzymes in biotechnology. Biotechnol. Adv. 2000, 18, 355–383. [Google Scholar] [CrossRef]
- Bhat, M.K.; Bhat, S. Cellulose degrading enzymes and their potential industrial applications. Biotechnol. Adv. 1997, 15, 583–620. [Google Scholar] [CrossRef]
- Branda, F.; Silvestri, B.; Costantini, A.; Luciani, G. Effect of exposure to growth media on size and surface charge of silica based Stöber nanoparticles: A DLS and ζ-potential study. J. Sol–Gel Sci. Technol. 2015, 73, 54–61. [Google Scholar] [CrossRef]
- Silvestri, B.; Vitiello, G.; Luciani, G.; Calcagno, V.; Costantini, A.; Gallo, M.; Parisi, S.; Paladino, S.; Iacomino, M.; D’Errico, G.; et al. Probing the eumelanin–silica interface in chemically engineered bulk hybrid nanoparticles for targeted subcellular antioxidant protection. ACS Appl. Mater. Interfaces 2017, 9, 37615–37622. [Google Scholar] [CrossRef]
- Hartono, S.B.; Qiao, S.Z.; Liu, J.; Jack, K.; Ladewig, B.P.; Hao, Z.; Lu, G.Q.M. Functionalized mesoporous silica with very large pores for cellulase immobilization. J. Phys. Chem. C 2010, 114, 8353–8362. [Google Scholar] [CrossRef]
- Li, X.; He, Q.; Shi, J. Global gene expressionaAnalysis of cellular death mechanisms induced by mesoporous silica nanoparticle-based drug delivery system. ACS Nano 2014, 8, 1309–1320. [Google Scholar] [CrossRef]
- Wei, Y.; Jin, D.; Ding, T.; Shih, W.H.; Liu, X.; Cheng, S.Z.D.; Fu, Q. A non-surfactant templating route to mesoporous silica materials. Adv. Mater. 1998, 10, 313–316. [Google Scholar] [CrossRef]
- Takimoto, A.; Shiomi, T.; Ino, K.; Tsunoda, T.; Kawai, A.; Mizukami, F.; Sakaguchi, K. Encapsulation of cellulase with mesoporous silica (SBA-15). Micropor. Mesopor. Mater. 2008, 116, 601–606. [Google Scholar] [CrossRef]
- Chen, B.; Qiu, J.; Mo, H.; Yu, Y.; Ito, K.; Sakai, E.; Feng, H. Synthesis of mesoporous silica with different pore sizes for cellulase immobilization: Pure physical adsorption. New J. Chem. 2017, 41, 9338–9345. [Google Scholar] [CrossRef]
- Chong, A.S.M.; Zhao, X.S. Design of large-pore mesoporous materials for immobilization of penicillin G acylase biocatalyst. Catal. Today 2004, 93–95, 293–299. [Google Scholar] [CrossRef]
- Yiu, H.H.P.; Botting, C.H.; Botting, N.P.; Wright, P.A. Size selective protein adsorption on thiol-functionalised SBA-15 mesoporous molecular sieve. Phys. Chem. Chem. Phys. 2001, 3, 2983–2985. [Google Scholar] [CrossRef]
- Kao, H.M.; Chang, P.C.; Wu, J.D.; Chiang, A.S.; Lee, C.H. Direct synthesis, characterization and solid-state NMR spectroscopy of large-pore vinyl-functionalized cubic mesoporous silica FDU-12. Micropor. Mesopor. Mater. 2006, 97, 9–20. [Google Scholar] [CrossRef]
- Harmoko, C.; Sucipto, K.I.; Retnoningtyas, E.S.; Hartono, S.B. Vinyl functionalized cubic mesoporous silica nanoparticles as supporting materials to enhance cellulase enzyme stability. ARPN J. Eng. Appl. Sci. 2016, 11, 2981–2992. [Google Scholar]
- Kannan, K.; Jasra, R.V. Improved catalytic hydrolysis of carboxy methyl cellulose using cellulase immobilized on functionalized meso cellular foam. J. Porous Mater. 2011, 18, 409–416. [Google Scholar] [CrossRef]
- Califano, V.; Costantini, A. Immobilization of Cellulolytic Enzymes in Mesostructured Silica Materials. Catalysts 2020, 10, 706. [Google Scholar] [CrossRef]
- Hirsh, S.L.; Bilek, M.M.M.; Nosworthy, N.J.; Kondyurin, A.; dos Remedios, C.G.; McKenzie, D.R. A Comparison of Covalent Immobilization and Physical Adsorption of a Cellulase Enzyme Mixture. Langmuir 2010, 26, 14380–14388. [Google Scholar] [CrossRef]
- Talukder, M.M.R.; Goh, H.Y.; Puah, S.M. Interaction of silica with cellulase and minimization of its inhibitory effect on cellulose hydrolysis. Biochem. Eng. J. 2017, 118, 91–96. [Google Scholar] [CrossRef]
- Cao, L. Carrier-Bound Immobilized Enzymes; Wiley-VCH: Weinhiem, Germany, 2005. [Google Scholar]
- Hisamatsu, K.; Shiomi, T.; Matsuura, S.; Nara, T.Y.; Tsunoda, T.; Mizukami, F.; Sakaguchi, K.J. α-Amylase immobilization capacities of mesoporous silicas with different morphologies and surface properties. Porous Mater. 2012, 19, 95–102. [Google Scholar] [CrossRef]
- Lei, J.; Fan, J.; Yu, C.; Zhang, L.; Jiang, S.; Tu, B.; Zhao, D. Immobilization of enzymes in mesoporous materials: Controlling the entrance to nanospace. Micropor. Mesopor. Mater. 2004, 73, 121–128. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, Y.; Yang, S. Comparative studies on catalytic properties of immobilized Candida rugosa lipase in ordered mesoporous rod-like silica and vesicle-like silica. Micropor. Mesopor. Mater. 2009, 119, 223–229. [Google Scholar] [CrossRef]
- Moon, D.; Lee, J. Tunable synthesis of hierarchical mesoporous silica nanoparticles with radial wrinkle structure. Langmuir 2012, 28, 12341–12347. [Google Scholar] [CrossRef]
- Avossa, J.; Bifulco, A.; Amendola, E.; Gesuele, F.; Oscurato, S.L.; Gizaw, Y.; Mensitieri, G.; Branda, F. Forming nanostructured surfaces through Janus colloidal silica particles with nanowrinkles: A new strategy to superhydrophobicity. Appl. Surf. Sci. 2019, 465, 73–81. [Google Scholar] [CrossRef]
- Pang, J.; Zhou, G.; Liu, R.; Li, T. Esterification of oleic acid with methanol by immobilized lipase on wrinkled silica nanoparticles with highly ordered, radially oriented mesochannels. Mater. Sci. Eng. C 2016, 59, 35–42. [Google Scholar] [CrossRef]
- Califano, V.; Costantini, A.; Silvestri, B.; Venezia, V.; Cimino, S.; Sannino, F. The effect of pore morphology on the catalytic performance of β-glucosidase immobilized into mesoporous silica. Pure Appl. Chem. 2019, 91, 1583–1592. [Google Scholar] [CrossRef]
- Califano, V.; Sannino, F.; Costantini, A.; Avossa, J.; Cimino, S.; Aronne, A. Wrinkled silica nanoparticles: Efficient matrix for β-glucosidase immobilization. J. Phys. Chem. C 2018, 122, 8373–8379. [Google Scholar] [CrossRef]
- Sannino, F.; Costantini, A.; Ruffo, F.; Aronne, A.; Venezia, V.; Califano, V. Covalent immobilization of β-glucosidase into mesoporous silica nanoparticles from anhydrous acetone enhances its catalytic performance. Nanomaterials 2020, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Bergmeyer, H.U.; Bernt, E. Methods of Enzymatic Analysis, 2nd ed.; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974. [Google Scholar]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Ausanio, G.; Califano, V.; Costantini, A.; Perretta, G.; Aronne, A.; Pepe, G.P.; Sannino, F.; Vicari, L.R. Matrix-assisted pulsed laser evaporation of β-glucosidase from a dopa/quinone target. Enzym. Microb. Technol. 2020, 132, 109414. [Google Scholar] [CrossRef] [PubMed]
- Tebeka, I.R.; Silva, A.G.; Petri, D.F. Hydrolytic activity of free and immobilized cellulase. Langmuir 2009, 25, 1582–1587. [Google Scholar] [CrossRef]
- Zhang, D.; Hegab, H.E.; Lvov, Y.; Snow, L.D.; Palmer, J. Immobilization of cellulase on a silica gel substrate modified using a 3-APTES self-assembled monolayer. SpringerPlus 2016, 5, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungurean, M.; Paul, C.; Peter, F. Cellulase immobilized by sol–gel entrapment for efficient hydrolysis of cellulose. Bioproc. Biosys. Eng. 2013, 36, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Parashar, A.; Bressler, D.C. Highly retained enzymatic activities of two different cellulases immobilized on non-porous and porous silica particles. Biotechnol. Bioproc. Eng. 2014, 19, 621–628. [Google Scholar] [CrossRef]
- Amaly, N.; Si, Y.; Chen, Y.; El-Moghazy, A.Y.; Zhao, C.; Zhang, R.; Sun, G. Reusable anionic sulfonate functionalized nanofibrous membranes for cellulase enzyme adsorption and separation. Colloid Surface B 2018, 170, 588–595. [Google Scholar] [CrossRef]
- Daoud, F.B.O.; Kaddour, S.; Sadoun, T. Adsorption of cellulase Aspergillus niger on a commercial activated carbon: Kinetics and equilibrium studies. Colloid Surface B 2010, 75, 93–99. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantini, A.; Venezia, V.; Pota, G.; Bifulco, A.; Califano, V.; Sannino, F. Adsorption of Cellulase on Wrinkled Silica Nanoparticles with Enhanced Inter-Wrinkle Distance. Nanomaterials 2020, 10, 1799. https://doi.org/10.3390/nano10091799
Costantini A, Venezia V, Pota G, Bifulco A, Califano V, Sannino F. Adsorption of Cellulase on Wrinkled Silica Nanoparticles with Enhanced Inter-Wrinkle Distance. Nanomaterials. 2020; 10(9):1799. https://doi.org/10.3390/nano10091799
Chicago/Turabian StyleCostantini, Aniello, Virginia Venezia, Giulio Pota, Aurelio Bifulco, Valeria Califano, and Filomena Sannino. 2020. "Adsorption of Cellulase on Wrinkled Silica Nanoparticles with Enhanced Inter-Wrinkle Distance" Nanomaterials 10, no. 9: 1799. https://doi.org/10.3390/nano10091799







