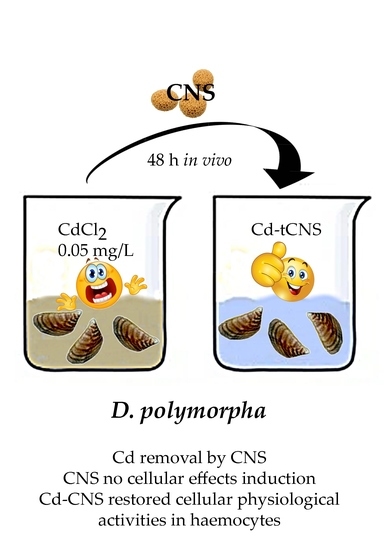
Suitability of a Cellulose-Based Nanomaterial for the Remediation of Heavy Metal Contaminated Freshwaters: A Case-Study Showing the Recovery of Cadmium Induced DNA Integrity Loss, Cell Proliferation Increase, Nuclear Morphology and Chromosomal Alterations on Dreissena polymorpha
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Preparation and Characterization of the Cellulose Nanosponges (CNS)
2.3. Sampling and Maintenance Condition
2.4. In Vivo Exposure
2.5. Cadmium Concentration in Water
2.6. Viability Assessment
2.7. Comet Assay
2.8. Cytome Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. CNS Synthesis and Characterization
3.2. Cadmium Concentration in Water
3.3. Cellular Responses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vincent-Hubert, F.; Arini, A.; Gourlay-Francé, C. Early genotoxic effects in gill cells and haemocytes of Dreissena polymorpha exposed to cadmium, B(a)P and a combination of B(a)P and Cd. Mutat. Res. 2011, 723, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Evariste, L.; Rioult, D.; Brousseau, P.; Geffard, A.; David, E.; Auffret, M.; Fournier, M.; Betoulle, S. Differential sensitivity to cadmium of immunomarkers measured in hemocyte subpopulations of zebra mussel Dreissena polymorpha. Ecotox. Envir. Saf. 2017, 137, 78–85. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme Unep. Available online: http://wedocs.unep.org/bitstream/handle/20.500.11822/27636/Cadmium_Review.pdf (accessed on 17 July 2020).
- Ravera, O.; Mislin, H. Cadmium in freshwater ecosystems. In Cadmium in the Environment; Birkhäuser: Basel, Switzerland, 1986; pp. 75–79. [Google Scholar]
- World Health Organization. Chapter 6.3. In Cadmium Air Quality Guidelines, 2nd ed.; Regional Office for Europe: Copenhagen, Denmark, 2000; pp. 136–139. [Google Scholar]
- Morin, S.; Duong, T.T.; Dabrin, A.; Coynel, A.; Herlory, O.; Baudrimont, M.; Delmas, F.; Durrieu, G.; Schäfer, J.; Winterton, F.; et al. Long-term survey of heavy-metal pollution, biofilm contamination and diatom community structure in the Riou Mort watershed, South-West France. Environ. Pollut. 2008, 15, 532–542. [Google Scholar] [CrossRef] [PubMed]
- CE. CEE/CEEA/CE n.83. 1998. Available online: https://eur-lex.europa.eu/eli/dir/1998/83/oj (accessed on 17 July 2020).
- Pereira, L.S.; Ribas, J.L.C.; Vicari, T.; Silva, S.B.; Stival, J.; Baldan, A.P.; Valdez Domingos, F.X.; Grassi, M.T.; Cestari, M.M.; Silvade Assis, H.C. Effects Eofecologically relevant concentrations of cadmium I na freshwater fish. Ecotoxicol. Environ. Saf. 2016, 13, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Urbano, M.R.; Almeida Lopes, A.C.B.; Carvalho, M.F.H.; Buzzo, M.L.; Docea, A.O.; Mesas, A.E.; Aschner, M.; Silva, A.M.R.; Silbergeld, E.K.; et al. Blood cadmium levels and sources of exposure in an adult urban populationin southern Brazil. Environ. Res. 2020, 187, 109618. [Google Scholar] [CrossRef]
- Aitio, A.; Alessio, L.; Axelson, O.; Coenen, J.; de Flora, S.; Grandjean, P.; Heinrich, U.; Huff, J.E.; Ikeda, M.; Kavlock, R.; et al. IARC monographs on the evaluation of carcinogenic risks to humans: Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. IARC Monogr. Eval. Carcinog. Risks Hum. 1993, 58, 1–415. [Google Scholar]
- Xue, W.; Peng, Z.; Huang, D.; Zeng, G.; Wan, J.; Wan, J.; Xui, R.; Cheng, M.; Zhang, C.; Jiang, D.; et al. Nanoremediation of Cadmium Contaminated River Sediments: Microbial Response and Organic Carbon Changes. J. Hazard. Mater. 2019, 359, 290–299. [Google Scholar] [CrossRef]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.K.; Thévenod, F. Cell organelles as targets of mammalian cadmium toxicity. Arch. Toxicol. 2020, 94, 1017–1049. [Google Scholar] [CrossRef] [Green Version]
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in situ remediation: A review of the benefits and potential risks. Environ. Health Perspect. 2009, 117, 1813–1831. [Google Scholar] [CrossRef] [Green Version]
- Oughton, D.; Koin, P.; Bleyl, S.; Filip, J.; Skácelová, P.; Klaas, N.; von der Kammer, F.; Gondikas, A. Development and Application of Analytical Methods for Monitoring Nanoparticles in Remediation: NanoRem 5; CL:AIRE: London, UK, 2017; p. 6. [Google Scholar]
- Fu, F.L.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–220. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Leitch, M.; Naknakom, B.; Tilton, R.D.; Lowry, G.V. Effect of emplaced nZVI mass and groundwater velocity on PCE dechlorination and hydrogen evolution in water-saturated sand. J. Hazard. Mater. 2017, 322, 136–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, C.; Sun, Y.; Tsang, D.C.W.; Lin, D. Environmental transformations and ecological effects of iron-based nanoparticles. Environ. Pollut. 2018, 232, 10–30. [Google Scholar] [CrossRef]
- Hanna, S.K.; Miller, R.J.; Lenihan, H.S. Deposition of carbon nanotubes by a marine suspension feeder revealed by chemical and isotopic tracers. J. Hazard. Mater. 2014, 279, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, N.I.; MacCormack, T.J. Ecophysiological perspectives on engineered nanomaterial toxicity in fish and crustaceans. Comp. Biochem. Physiol. Part C. Toxicol. Pharmacol. 2017, 193, 30–41. [Google Scholar] [CrossRef]
- Ogunkunle, O.C.; Odulaja, D.A.; Akande, F.O.; Varun, M.; Vishwakarma, V.; Fatoba, O.P. Cadmium toxicity in cowpea plant: Effect of foliar intervention of nano-TiO2 on tissue Cd bioaccumulation, stress enzymes and potential dietary health risk. J. Biotechnol. 2020, 310, 54–61. [Google Scholar] [CrossRef]
- Corsi, I.; Fiorati, A.; Grassi, G.; Bartolozzi, I.; Daddi, T.; Melone, L.; Punta, C. Environmentally Sustainable and Ecosafe Polysaccharide-Based Materials for Water Nano-Treatment: An Eco-Design Study. Materials 2018, 11, 1228. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, A.W.; de Lannoy, C.F.; Wiesner, M.R. Cellulose nanomaterials in water treatment technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A.P. Nanocellulose-Based Materials for Water Purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Pierre, G.; Punta, C.; Delattre, C.; Melone, L.; Dubessay, P.; Fiorati, A.; Pastori, N.; Galante, Y.M.; Michaud, P. TEMPO-mediated oxidation of polysaccharides: An ongoing story. Carbohyd. Polym. 2017, 165, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Melone, L.; Rossi, B.; Pastori, N.; Panzeri, W.; Mele, A.; Punta, C. TEMPO-Oxidized Cellulose Cross-Linked with Branched Polyethyleneimine: Nanostructured Adsorbent Sponges for Water Remediation. Chem.Plus.Chem. 2015, 80, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Fiorati, A.; Turco, G.; Travan, A.; Caneva, E.; Pastori, N.; Cametti, M.; Punta, C.; Melone, L. Mechanical and Drug Release Properties of Sponges from Cross-linked Cellulose Nanofibers. ChemPlusChem 2017, 82, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Paladini, G.; Venuti, V.; Almásy, L.; Melone, L.; Crupi, V.; Majolino, D.; Pastori, N.; Fiorati, A.; Punta, C. Cross-linked cellulose nano-sponges: A small angle neutron scattering (SANS) study. Cellulose 2019, 26, 9005–9019. [Google Scholar] [CrossRef]
- Riva, L.; Fiorati, A.; Sganappa, A.; Melone, L.; Punta, C.; Cametti, M. Naked-eye heterogeneous sensing of fluoride ions by co-polymeric nanosponge systems comprising aromatic-imide-functionalized nanocellulose and branched polyethyleneimine. ChemPlusChem 2019, 84, 1512–1518. [Google Scholar] [CrossRef]
- Bartolozzi, I.; Daddi, T.; Punta, C.; Fiorati, A.; Iraldo, F. Life cycle assessment of emerging environmental technologies in the early stage of development: A case study on nanostructured materials. J. Ind. Ecol. 2020, 24, 101–115. [Google Scholar] [CrossRef]
- Fiorati, A.; Grassi, G.; Pastori, N.; Graziano, A.; Liberatori, G.; Melone, L.; Punta, C.; Corsi, I. Eco-design of nanostructured cellulose sponges for sea-water decontamination from heavy metal ions. J. Clean. Prod. 2020, 246, 119009. [Google Scholar] [CrossRef]
- Liberatori, G.; Grassi, G.; Guidi, P.; Bernardeschi, M.; Fiorati, A.; Scarcelli, V.; Genovese, M.; Faleri, C.; Protano, G.; Frenzilli, G.; et al. Effect-Based Approach to Assess Nanostructured Cellulose Sponge Removal Efficacy of Zinc Ions from Seawater to Prevent Ecological Risks. Nanomaterials 2020, 10, 1283. [Google Scholar] [CrossRef]
- Binelli, A.; Della Torre, C.; Magni, S.; Parolini, M. Does zebra mussel (Dreissena polymorpha) represent the freshwater counterpart of Mytilus in ecotoxicological studies? A critical review. Environ. Pollut. 2015, 196, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, C.; Fenech, M. Mussel micronucleus cytome assay. Nat. Protoc. 2012, 7, 1125–1137. [Google Scholar] [CrossRef]
- Binelli, A.; Cogni, D.; Parolini, M.; Riva, C.; Provini, A. In vivo experiments for the evaluation of genotoxic and cytotoxic effects of Triclosan in Zebra mussel hemocytes. Aquat. Toxicol. 2009, 91, 238–244. [Google Scholar] [CrossRef] [PubMed]
- De Lafontaine, Y.; Gagné, F.; Blaise, C.; Costan, G.; Gagnon, P.; Chan, H. Biomarkers in zebra mussels (Dreissena polymorpha) for the assessment and monitoring of water quality of the St Lawrence River (Canada). Aquat. Toxicol. 2000, 50, 51–71. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Available online: https://www.epa.gov (accessed on 17 July 2020).
- Naimo, T.J.; Atchison, G.J.; Holland-Bartels, L.E. Sublethal effects of cadmium on physiological responses in the pocketbook mussel, Lampsilis ventricosa. Environ. Toxicol. Chem. 1992, 11, 1013–1021. [Google Scholar] [CrossRef]
- Ivankovic, D.; Pavicic, J.; Beatovic, V.; Sauerborn Klobucar, R.; Klobucar, G.I.V. Inducibility of Metallothionein Biosynthesis in the Whole Soft Tissue of Zebra Mussels Dreissena polymorpha Exposed to Cadmium, Copper, and Pentachlorophenol. Environ. Toxicol. 2010, 25, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Guidi, P.; Bernardeschi, M.; Scarcelli, V.; Cantafora, E.; Benedetti, M.; Falleni, A.; Frenzilli, G. Lysosomal, genetic and chromosomal damage in haemocytes of the freshwater bivalve (Unio pictorum) exposed to polluted sediments from the River Cecina (Italy). Chem. Ecol. 2017, 33, 516–527. [Google Scholar] [CrossRef]
- Chiba, K.; Kawakami, K.; Tohyama, K. Simultaneous evaluation of cell viability by neutral red, MTT and crystal violet staining assays of the same cells. Toxicol. In Vitro 1998, 12, 251–258. [Google Scholar] [CrossRef]
- Guidi, P.; Frenzilli, G.; Benedetti, M.; Bernardeschi, M.; Falleni, A.; Fattorini, D.; Regoli, F.; Scarcelli, V.; Nigro, M. Antioxidant, genotoxic and lysosomal biomarkers in the freshwater bivalve (Unio pictorum) transplanted in a metal polluted river basin. Aquat. Toxicol. 2010, 100, 75–83. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Kumaravel, T.S.; Jha, A.N. Reliable Comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2006, 605, 7–16. [Google Scholar] [CrossRef]
- Asker, N.; Carney Almroth, B.; Albertsson, E.; Coltellaro, M.; Bignell, J.P.; Hanson, N.; Scarcelli, V.; Fagerholm, B.; Parkkonen, J.; Wijkmark, E.; et al. A gene to organism approach—assessing the impact of environmental pollution in eelpout (Zoarces viviparus) females and larvae. Environ. Toxicol. Chem. 2015, 34, 1511–1523. [Google Scholar] [CrossRef] [Green Version]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panzella, L.; Melone, L.; Pezzella, A.; Rossi, B.; Pastori, N.; Perfetti, M.; D’Errico, G.; Punta, C.; d’Ischia, M. Surface-functionalization of nanostructured cellulose aerogels by solid state eumelanin coating. Biomacromolecules 2016, 17, 564–571. [Google Scholar] [CrossRef]
- Huff, J.; Lunn, R.M.; Waalkes, M.P.; Tomatis, L.; Infante, P.F. Cadmium-induced cancers in animals and in humans. Int. J. Occup. Environ. Health. 2007, 13, 202–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J. Cadmium stress: An oxidative challenge. Biometals 2010, 23, 927–940. [Google Scholar] [CrossRef]
- Guo, S.N.; Zheng, J.L.; Yuan, S.S.; Zhu, Q.L.; Wu, C.W. Immunosuppressive effects and associated compensatory responses in zebrafish after full life-cycle exposure to environmentally relevant concentrations of cadmium. Aquat. Toxicol. 2017, 88, 64–71. [Google Scholar] [CrossRef]
- Thévenod, F.; Lee, W.K. Cadmium and cellular signaling cascades: Interactions between cell death and survival pathways. Arch. Toxicol. 2013, 87, 1743–1786. [Google Scholar] [CrossRef]
- McMurray, C.T.; Tainer, J.A. Cancer, cadmium and genome integrity. Nat. Genet. 2003, 34, 239–241. [Google Scholar] [CrossRef]
- Apykhtina, O.L.; Dybkova, S.M.; Sokurenko, L.M.; Yu, B.; Chaikovsky, C. Cytotoxic and genotoxic effects of cadmium sulfide nanoparticles. Exp. Oncol. 2018, 40, 194–199. [Google Scholar] [CrossRef]
- Oporto, C.; Vandecasteele, C.; Smolders, E. Elevated Cadmium Concentrations in Potato Tubers Due to Irrigation with River Water Contaminated by Mining in Potosí, Bolivia. J. Environ. Qual. 2007, 36, 1181–1186. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Regoli, F.; Winston, G.W.; Gorbi, S.; Frenzilli, G.; Nigro, M.; Corsi, I.; Focardi, S. Integrating enzymatic responses to organic chemical exposure with total oxyradical absorbing capacity and DNA damage in the European eel Anguilla anguilla. Environ. Toxicol. Chem. 2003, 22, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Nacci, D.E.; Cayula, S.; Jackim, E. Detection of DNA damage in individual cells from marine organisms using the single cell gel assay. Aquat. Toxicol. 1996, 35, 197–210. [Google Scholar] [CrossRef]
- Buschini, A.; Carboni, P.; Martino, A.; Poli, P.; Ross, C. Effects of temperature on baseline and genotoxicant-induced DNA damage in haemocytes of Dreissena polymorpha. Mutat. Res. 2003, 537, 81–92. [Google Scholar] [CrossRef]
- Thevenod, F. Cadmium and cellular signaling cascades: To be or not to be? Toxicol. Appl. Pharmacol. 2009, 238, 221–239. [Google Scholar] [CrossRef]
- Templeton, D.M.; Liu, Y. Multiple roles of cadmium in cell death and survival. Chem. Biol. Interact. 2010, 188, 267–275. [Google Scholar] [CrossRef]
- Ho, J.L.; Ju, H.L.; Seon, M.L.; Na, H.K.; Yeon, G.M.; Tae, K.T. Cadmium induces cytotoxicity in normal mouse renal MM55.K cells. Int. J. Environ. Health Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Kant, U.; Govind, M.; Gomal, G.; Salvatore, A.; Pizzo, V. Cadmium-induced DNA synthesis and cell proliferation in macrophages: The role of intracellular calcium and signal transduction mechanisms. Cell. Signal. 2002, 14, 327–340. [Google Scholar] [CrossRef]
- Yua, X.; Filardo, E.J.; Shaikha, Z.A. The membrane estrogen receptor GPR30 mediates cadmium-induced proliferation of breast cancer cells. Toxicol. Appl. Pharmacol. 2010, 245, 83–90. [Google Scholar] [CrossRef]
- Olabarrieta, I.; L’Azou, B.; Yuric, S.; Cambar, J.; Cajaraville, M.P. In vitro effects of cadmium on two different animal cell models. Toxicol. Vitro 2011, 15, 511–517. [Google Scholar] [CrossRef]
- Gómez-Mendikute, A.; Cajaraville, M.P. Comparative effects of cadmium, copper, paraquat and benzo[a]pyrene on the actin cytoskeleton and production of reactive oxygen species (ROS) in mussel haemocytes. Toxicol. Vitro 2003, 17, 539–546. [Google Scholar] [CrossRef]
- Hsu, T.; Huang, K.M.; Tsai, H.T.; Sung, S.T.; Ho, T.N. Cadmium (Cd) induced oxidative stress down-regulates the gene expression of DNA mismatch recognition proteins MutS homolog 2 (MSH2) 580 and MSH6 in zebrafish (Danio rerio) embryos. Aquat. Toxicol. 2013, 126, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Pierron, F.; Baillon, L.; Sow, M.; Gotreau, S.; Gonzalez, P. Effect of Low-Dose Cadmium Exposure on DNA Methylation in the Endangered European Eel Environ. Sci. Technol. 2014, 48, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Bourgeault, A.; Gourlay-Francé, C.; Vincent-Hubert, F.; Palais, F.; Geffard, A.; Biagiant-Risbourg, S.; Pain-Devin, S.; Tusseau-Vuillemin, M.H. Lessons from a transplantation of zebra mussels into a small urban river: An integrated ecotoxicological assessment. Environ. Toxicol. 2010, 25, 10. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, C.; Costa, G.; Nande, M.; Martín, C.; Martín, M.; Sánchez-Fortún, S. Heavy metals immobilization capability of two iron-based nanoparticles (nZVI and Fe3O4): Soil and freshwater bioassays to assess ecotoxicological impact. Sci. Total Environ. 2019, 656, 421–432. [Google Scholar] [CrossRef]
- Vicari, T.; Ferraro, M.V.M.; Ramsdorf, W.A.; Mela, M.; Ribeiro, C.A.O.; Cestari, M.M. Genotoxic evaluation of different dosages of methylmercury (CH3Hg+) in Hoplias malabaricus. Ecotoxicol. Environ. Saf. 2012, 82, 47–55. [Google Scholar] [CrossRef] [PubMed]






| Experimental Group | Cd(II) mg L−1 |
|---|---|
| AFW | <0.001 |
| CNS | <0.001 |
| Cd(II) | 0.0537 ± 0.005 |
| Cd-t CNS | 0.0060 ± 0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guidi, P.; Bernardeschi, M.; Palumbo, M.; Genovese, M.; Scarcelli, V.; Fiorati, A.; Riva, L.; Punta, C.; Corsi, I.; Frenzilli, G. Suitability of a Cellulose-Based Nanomaterial for the Remediation of Heavy Metal Contaminated Freshwaters: A Case-Study Showing the Recovery of Cadmium Induced DNA Integrity Loss, Cell Proliferation Increase, Nuclear Morphology and Chromosomal Alterations on Dreissena polymorpha. Nanomaterials 2020, 10, 1837. https://doi.org/10.3390/nano10091837
Guidi P, Bernardeschi M, Palumbo M, Genovese M, Scarcelli V, Fiorati A, Riva L, Punta C, Corsi I, Frenzilli G. Suitability of a Cellulose-Based Nanomaterial for the Remediation of Heavy Metal Contaminated Freshwaters: A Case-Study Showing the Recovery of Cadmium Induced DNA Integrity Loss, Cell Proliferation Increase, Nuclear Morphology and Chromosomal Alterations on Dreissena polymorpha. Nanomaterials. 2020; 10(9):1837. https://doi.org/10.3390/nano10091837
Chicago/Turabian StyleGuidi, Patrizia, Margherita Bernardeschi, Mara Palumbo, Massimo Genovese, Vittoria Scarcelli, Andrea Fiorati, Laura Riva, Carlo Punta, Ilaria Corsi, and Giada Frenzilli. 2020. "Suitability of a Cellulose-Based Nanomaterial for the Remediation of Heavy Metal Contaminated Freshwaters: A Case-Study Showing the Recovery of Cadmium Induced DNA Integrity Loss, Cell Proliferation Increase, Nuclear Morphology and Chromosomal Alterations on Dreissena polymorpha" Nanomaterials 10, no. 9: 1837. https://doi.org/10.3390/nano10091837