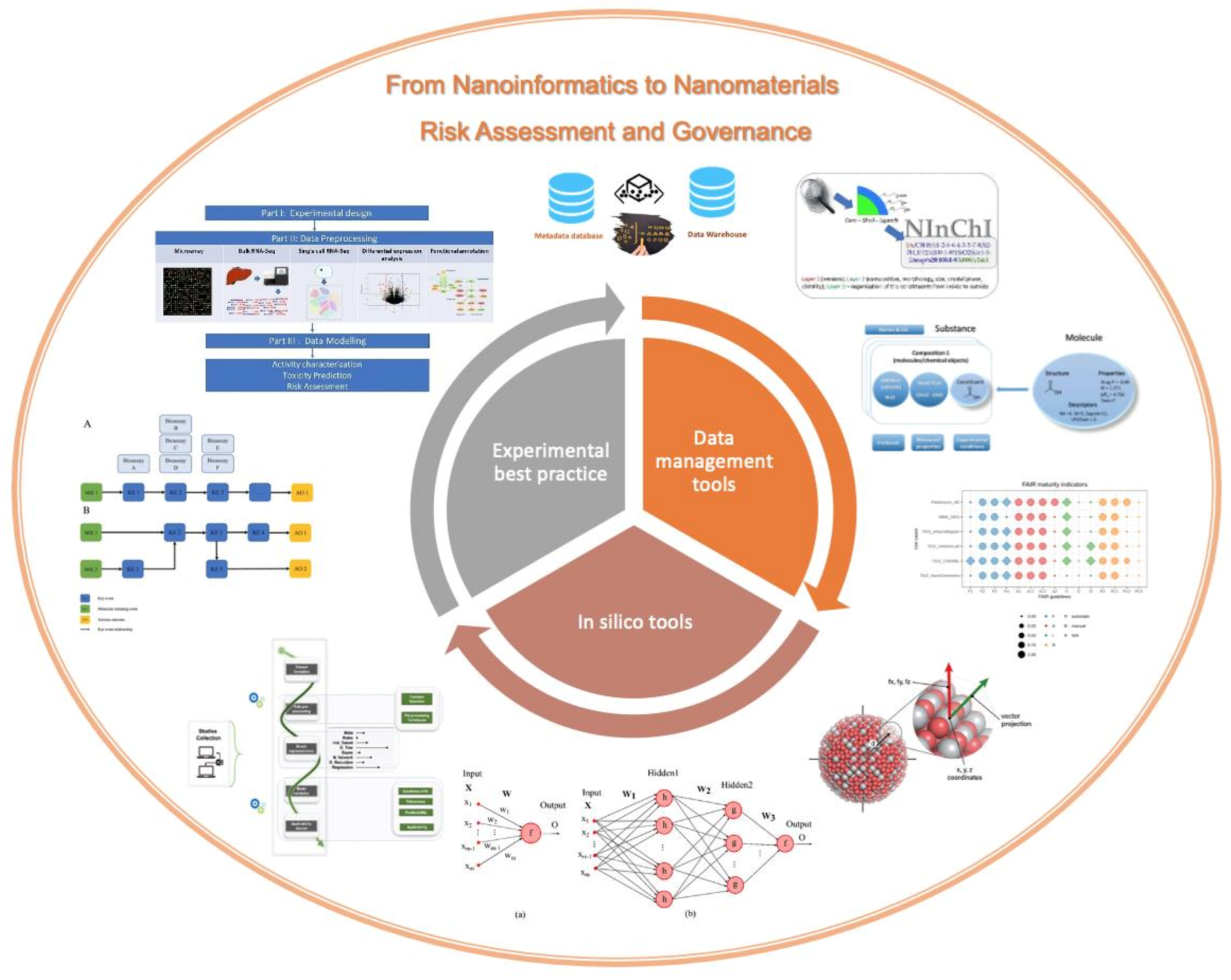
Editorial for the Special Issue From Nanoinformatics to Nanomaterials Risk Assessment and Governance
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ECHA. Appendix for Nanoforms Applicable to the Guidance on Registrationand Substance Identification. Available online: https://echa.europa.eu/documents/10162/13655/how_to_register_nano_en.pdf/f8c046ec-f60b-4349-492b-e915fd9e3ca0 (accessed on 21 December 2020).
- Kochev, N.; Jeliazkova, N.; Paskaleva, V.; Tancheva, G.; Iliev, L.; Ritchie, P.; Jeliazkov, V. Your Spreadsheets Can Be FAIR: A Tool and FAIRification Workflow for the eNanoMapper Database. Nanomaterials 2020, 10, 1908. [Google Scholar] [CrossRef] [PubMed]
- Papadiamantis, A.G.; Klaessig, F.C.; Exner, T.E.; Hofer, S.; Hofstaetter, N.; Himly, M.; Williams, M.A.; Doganis, P.; Hoover, M.D.; Afantitis, A.; et al. Metadata Stewardship in Nanosafety Research: Community-Driven Organisation of Metadata Schemas to Support FAIR Nanoscience Data. Nanomaterials 2020, 10, 2033. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Bonaretti, S.; Winckers, L.; Quik, J.; Bakker, M.; Maier, D.; Lynch, I.; van Rijn, J.; Willighagen, E. A Semi-Automated Workflow for FAIR Maturity Indicators in the Life Sciences. Nanomaterials 2020, 10, 2068. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.S.T.; Da Silva, G.H.; de Medeiros, A.M.Z.; Khan, L.U.; Papadiamantis, A.G.; Lynch, I. Effect of the Albumin Corona on the Toxicity of Combined Graphene Oxide and Cadmium to Daphnia magna and Integration of the Datasets into the NanoCommons Knowledge Base. Nanomaterials 2020, 10, 1936. [Google Scholar] [CrossRef] [PubMed]
- Heller, S.R.; McNaught, A.; Pletnev, I.; Stein, S.; Tchekhovskoi, D. InChI, the IUPAC International Chemical Identifier. J. Cheminform. 2015, 7, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, I.; Afantitis, A.; Exner, T.; Himly, M.; Lobaskin, V.; Doganis, P.; Maier, D.; Sanabria, N.; Papadiamantis, A.G.; Rybinska-Fryca, A.; et al. Can an InChI for Nano Address the Need for a Simplified Representation of Complex Nanomaterials across Experimental and Nanoinformatics Studies? Nanomaterials 2020, 10, 2493. [Google Scholar] [CrossRef] [PubMed]
- Shateri, M.; Sobhanigavgani, Z.; Alinasab, A.; Varamesh, A.; Hemmati-Sarapardeh, A.; Mosavi, A.; Shamshirband, S.S. Comparative Analysis of Machine Learning Models for Nanofluids Viscosity Assessment. Nanomaterials 2020, 10, 1767. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, S.A.; Power, D.; Rouse, I.; Lobaskin, V. In Silico Prediction of Protein Adsorption Energy on Titanium Dioxide and Gold Nanoparticles. Nanomaterials 2020, 10, 1967. [Google Scholar] [CrossRef] [PubMed]
- Papadiamantis, A.G.; Jänes, J.; Voyiatzis, E.; Sikk, L.; Burk, J.; Burk, P.; Tsoumanis, A.; Ha, M.K.; Yoon, T.H.; Valsami-Jones, E.; et al. Predicting Cytotoxicity of Metal Oxide Nanoparticles Using Isalos Analytics Platform. Nanomaterials 2020, 10, 2017. [Google Scholar] [CrossRef] [PubMed]
- Utembe, W.; Clewell, H.; Sanabria, N.; Doganis, P.; Gulumian, M. Current Approaches and Techniques in Physiologically Based Pharmacokinetic (PBPK) Modelling of Nanomaterials. Nanomaterials 2020, 10, 1267. [Google Scholar] [CrossRef] [PubMed]
- Furxhi, I.; Murphy, F.; Mullins, M.; Arvanitis, A.; Poland, C.A. Practices and Trends of Machine Learning Application in Nanotoxicology. Nanomaterials 2020, 10, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinaret, P.A.S.; Serra, A.; Federico, A.; Kohonen, P.; Nymark, P.; Liampa, I.; Ha, M.K.; Choi, J.-S.; Jagiello, K.; Sanabria, N.; et al. Transcriptomics in Toxicogenomics, Part I: Experimental Design, Technologies, Publicly Available Data, and Regulatory Aspects. Nanomaterials 2020, 10, 750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federico, A.; Serra, A.; Ha, M.K.; Kohonen, P.; Choi, J.-S.; Liampa, I.; Nymark, P.; Sanabria, N.; Cattelani, L.; Fratello, M.; et al. Transcriptomics in Toxicogenomics, Part II: Preprocessing and Differential Expression Analysis for High Quality Data. Nanomaterials 2020, 10, 903. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Fratello, M.; Cattelani, L.; Liampa, I.; Melagraki, G.; Kohonen, P.; Nymark, P.; Federico, A.; Kinaret, P.A.S.; Jagiello, K.; et al. Transcriptomics in Toxicogenomics, Part III: Data Modelling for Risk Assessment. Nanomaterials 2020, 10, 708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ede, J.D.; Lobaskin, V.; Vogel, U.; Lynch, I.; Halappanavar, S.; Doak, S.H.; Roberts, M.G.; Shatkin, J.A. Translating Scientific Advances in the AOP Framework to Decision Making for Nanomaterials. Nanomaterials 2020, 10, 1229. [Google Scholar] [CrossRef] [PubMed]
- Kohl, Y.; Rundén-Pran, E.; Mariussen, E.; Hesler, M.; El Yamani, N.; Longhin, E.M.; Dusinska, M. Genotoxicity of Nanomaterials: Advanced In Vitro Models and High Throughput Methods for Human Hazard Assessment—A Review. Nanomaterials 2020, 10, 1911. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Tchetchik, A.; Furxhi, I. Reduction of Health Care-Associated Infections (HAIs) with Antimicrobial Inorganic Nanoparticles Incorporated in Medical Textiles: An Economic Assessment. Nanomaterials 2020, 10, 999. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lynch, I.; Afantitis, A.; Greco, D.; Dusinska, M.; Banares, M.A.; Melagraki, G. Editorial for the Special Issue From Nanoinformatics to Nanomaterials Risk Assessment and Governance. Nanomaterials 2021, 11, 121. https://doi.org/10.3390/nano11010121
Lynch I, Afantitis A, Greco D, Dusinska M, Banares MA, Melagraki G. Editorial for the Special Issue From Nanoinformatics to Nanomaterials Risk Assessment and Governance. Nanomaterials. 2021; 11(1):121. https://doi.org/10.3390/nano11010121
Chicago/Turabian StyleLynch, Iseult, Antreas Afantitis, Dario Greco, Maria Dusinska, Miguel A. Banares, and Georgia Melagraki. 2021. "Editorial for the Special Issue From Nanoinformatics to Nanomaterials Risk Assessment and Governance" Nanomaterials 11, no. 1: 121. https://doi.org/10.3390/nano11010121





