Crown Ether Grafted Graphene Oxide/Chitosan/Polyvinyl Alcohol Nanofiber Membrane for Highly Selective Adsorption and Separation of Lithium Ion
Abstract
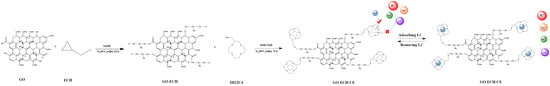
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Sample Preparation
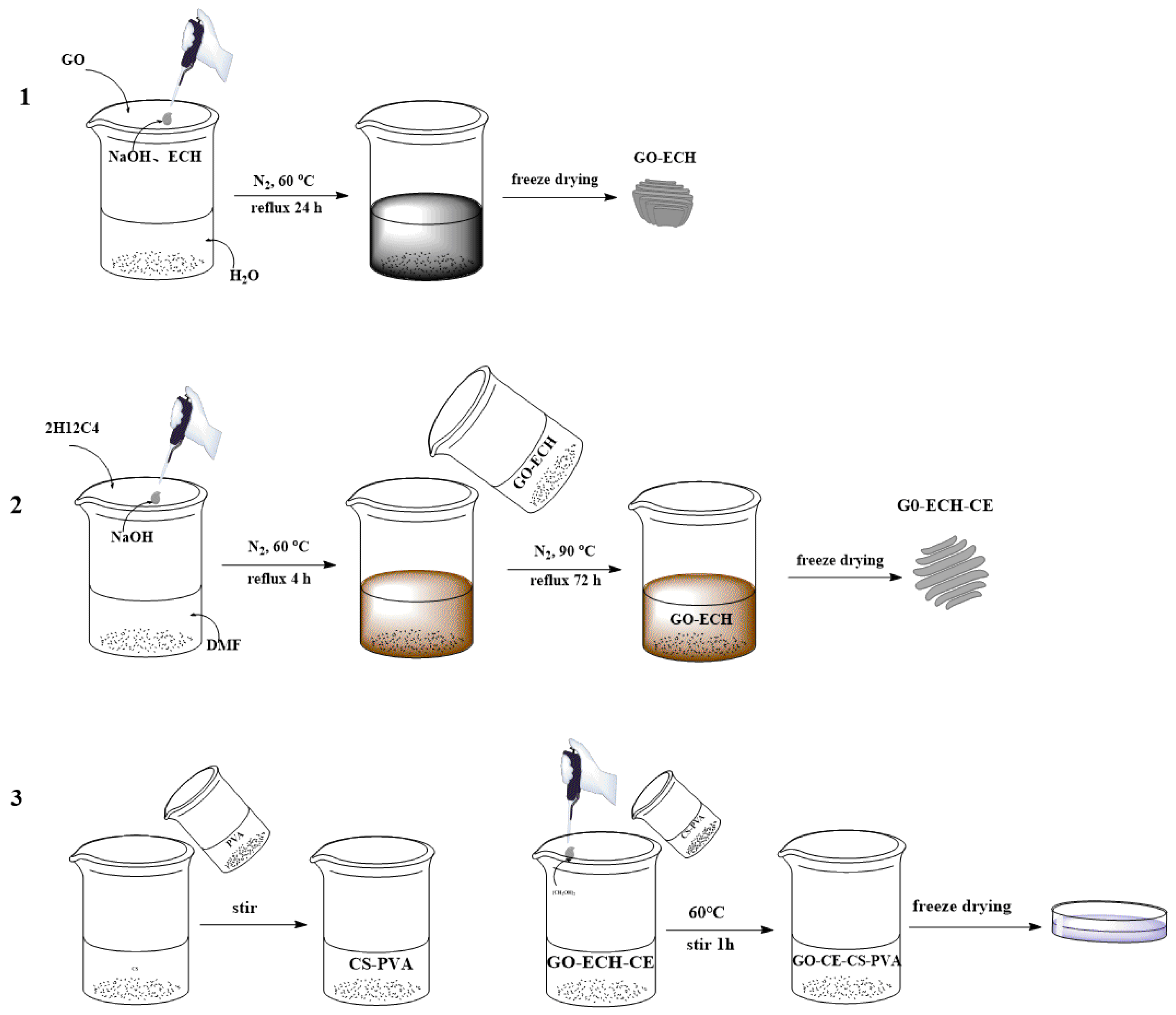
2.2.1. Preparation of Graphene Oxide (GO−ECH)
2.2.2. Preparation of 2-(Hydroxymethyl)-12-Crown 4-Ether Graft Modified Graphene Oxide (GO−ECH−CE)
2.2.3. Preparation of Crown Ether Grafted Graphene Oxide/Chitosan/Polyvinyl Alcohol Nanofiber Composite Film (GO–CE–CS–PVA)
2.2.4. Preparation of Graphene Oxide/Chitosan/Polyvinyl Alcohol Nanofiber Composite Membrane (GO–CS–PVA) Membrane
2.2.5. Repeat the Elution Procedure
2.3. Adsorption Experiment
2.4. Characterization Instruments
3. Results and Discussion
3.1. Characterization Results
3.1.1. Scanning Electron Microscope (SEM)
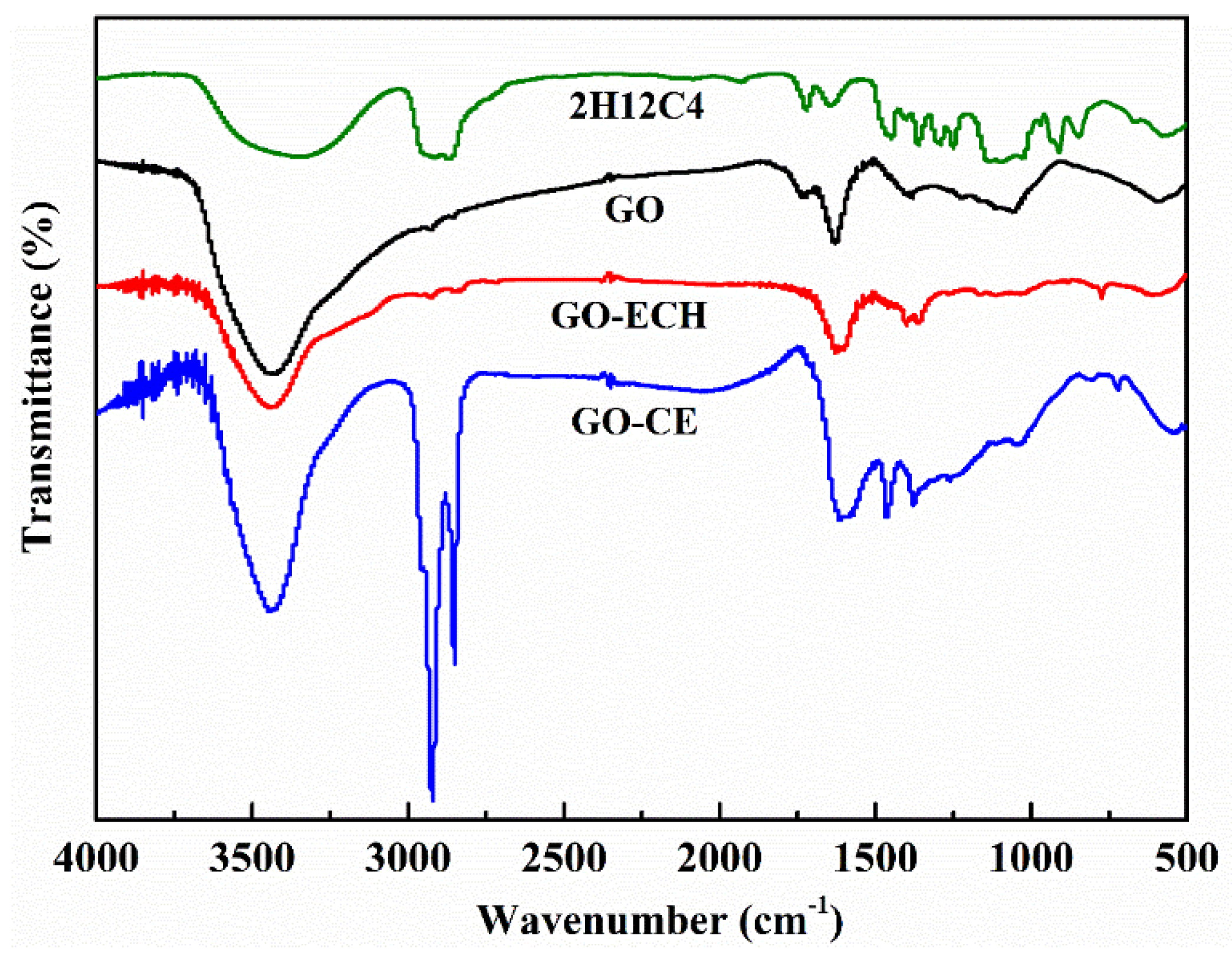
3.1.2. Fourier Infrared Spectroscopy (FTIR)
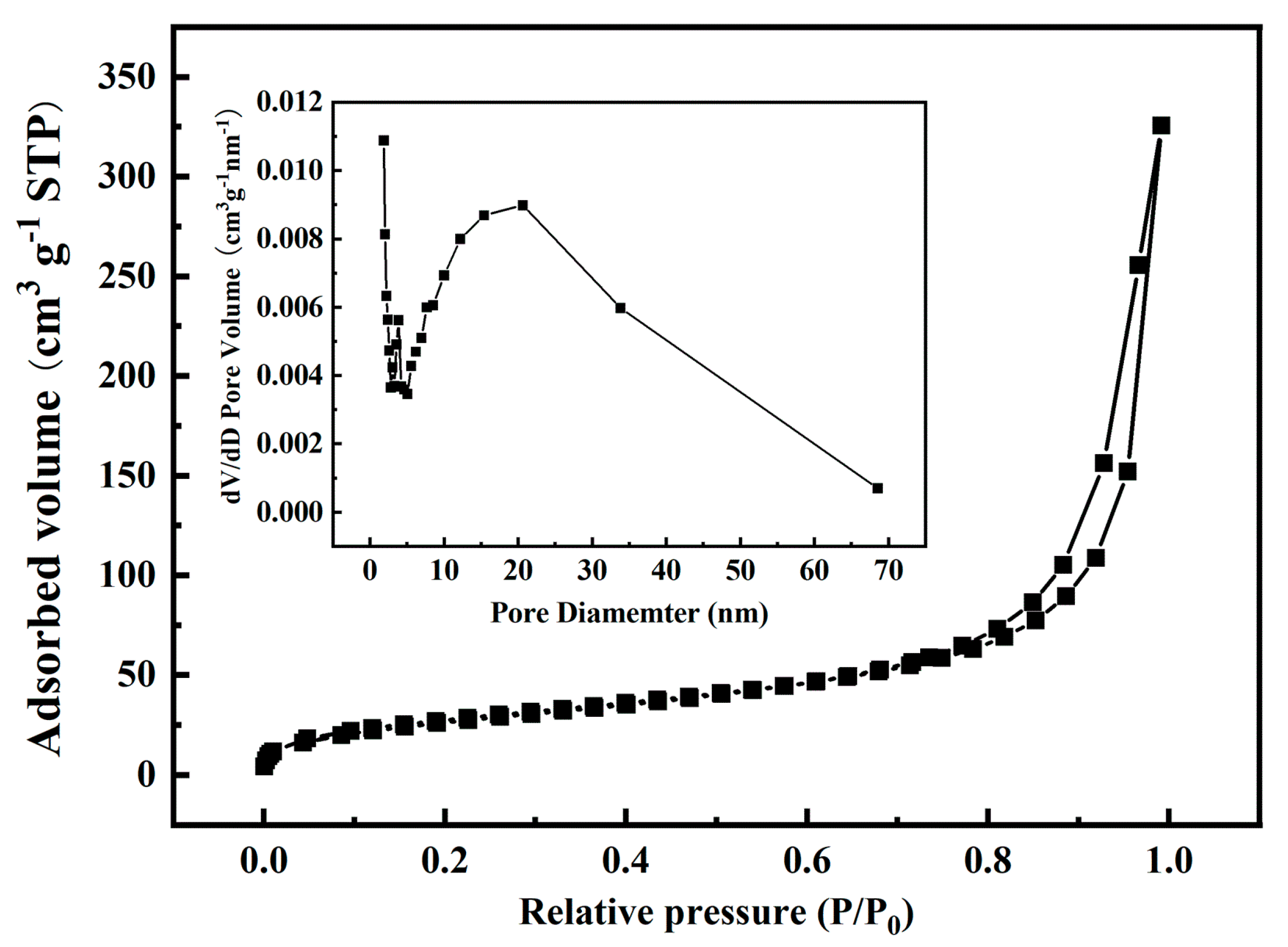
3.1.3. N2 Adsorption-Desorption (BET)
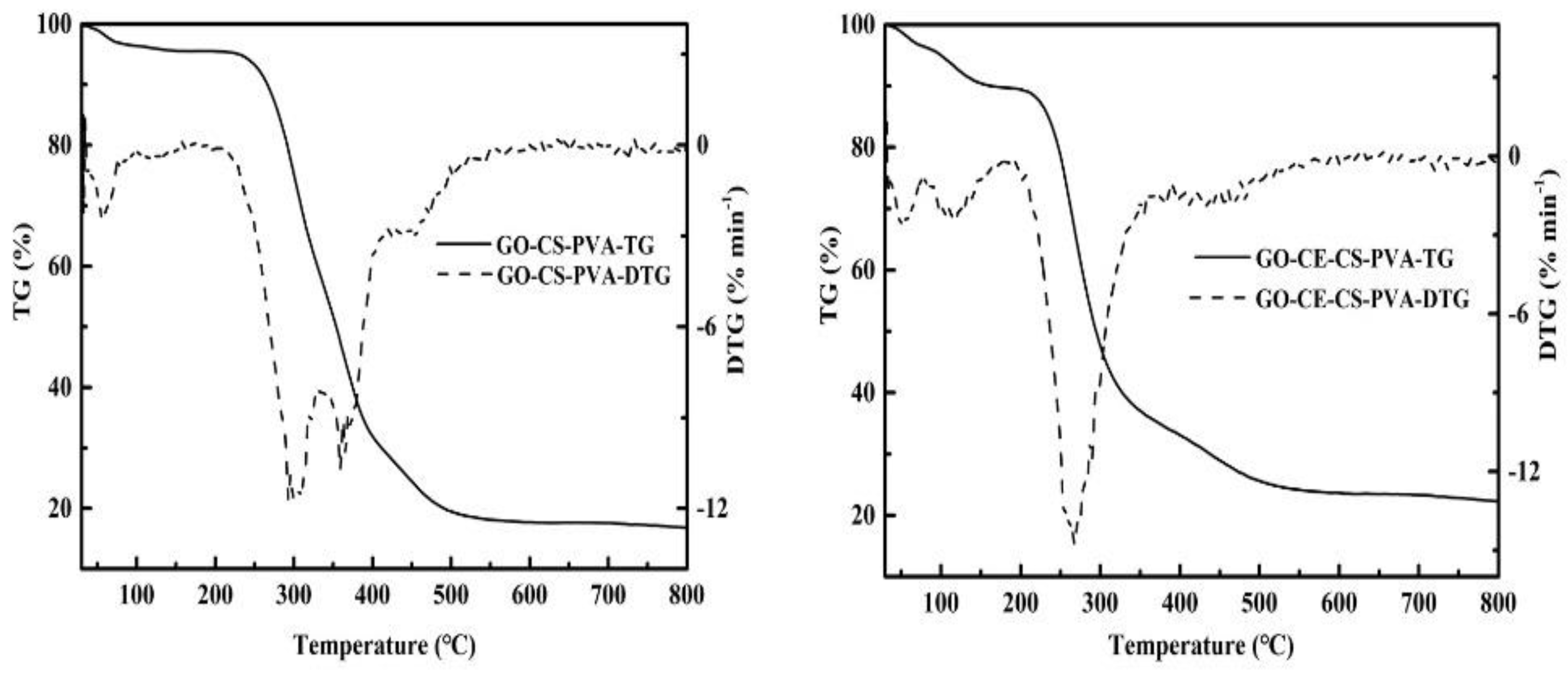
3.1.4. Thermogravimetric Analysis (TG/DTG)
3.2. Adsorption Experimental Analysis
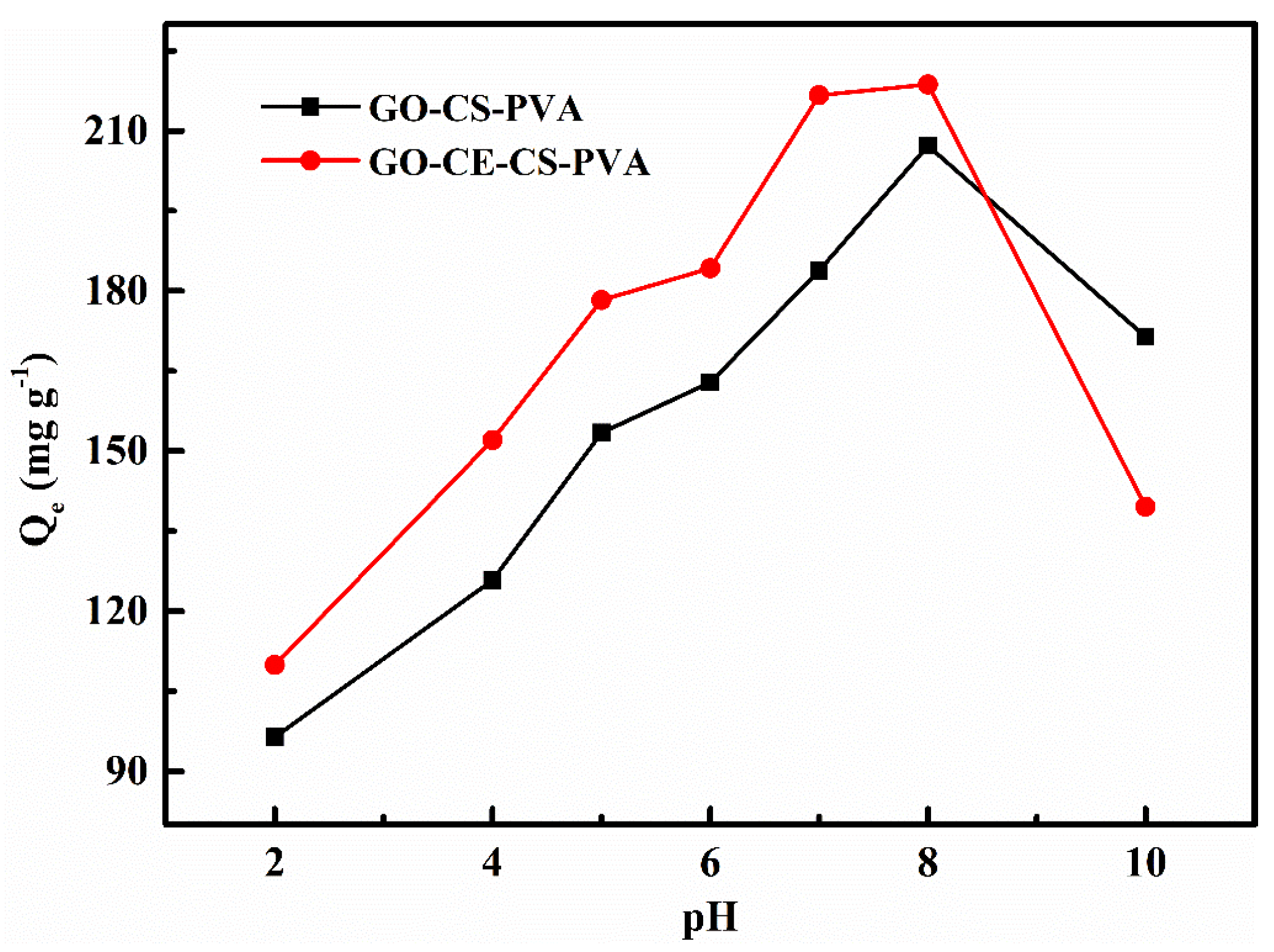
3.2.1. pH Experiment
3.2.2. Adsorption Kinetics Experiment
3.2.3. Adsorption Isotherm Experiment
3.2.4. Adsorption Thermodynamics Experiment
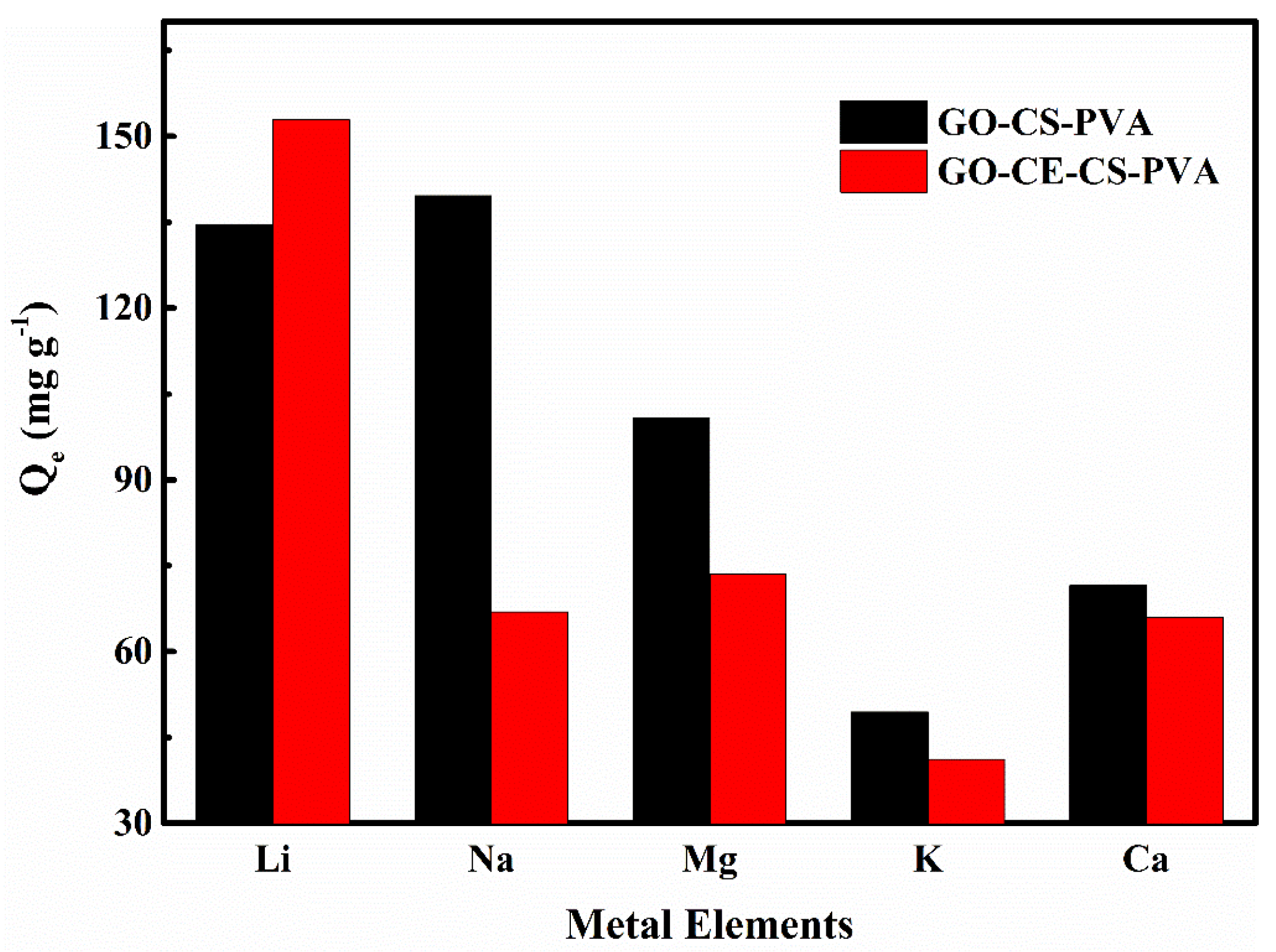
3.2.5. Selective Adsorption Experiment
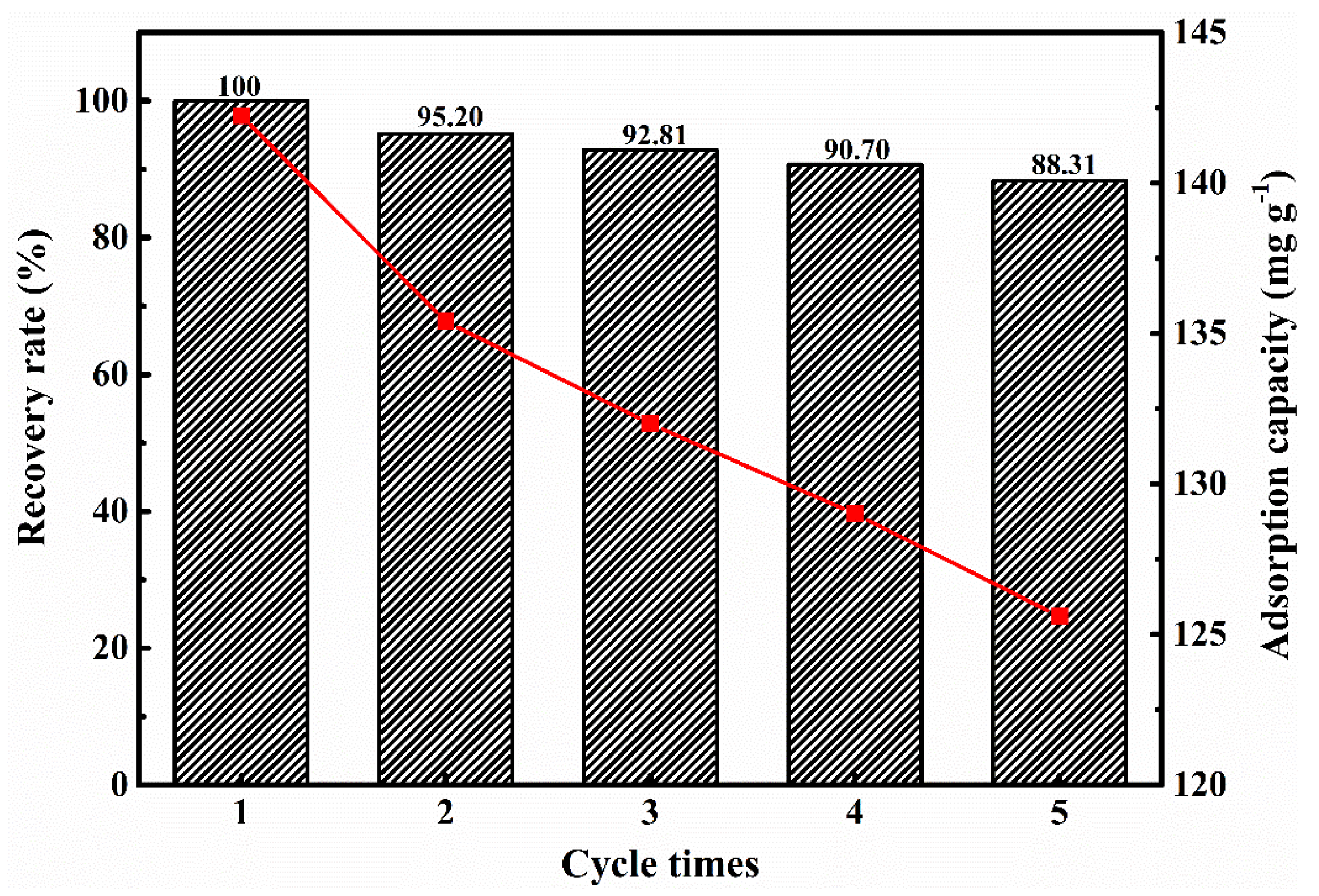
3.2.6. Repeatable Adsorption Experiment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Song, X.; Chen, Q.; Dong, M.; Yuan, G.; Li, D.; Ouyang, B.; Wang, Y.; Sun, W. Effects of chlorine adatoms on small lithium nanoclusters adsorbed on grapheme. Chem. Phys. Lett. 2020, 738, 136896. [Google Scholar] [CrossRef]
- Lan, Q.; Liu, Y.; Qin, J.; Zhao, Y.; Zhan, H.J.A.A.M. Interfaces. Electrochemically Regulated Li Deposition by Crown Ether. ACS Appl. Mater. Interfaces 2021, 13, 13. [Google Scholar] [CrossRef]
- Zhu, X.; Yue, H.; Sun, W.; Zhang, L.; Wang, H. Study on adsorption extraction process of lithium ion from West Taijinar brine by shaped titanium-based lithium ion sieves. Sep. Purif. Technol. 2021, 274, 119099. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Y.; Qiu, F.; Li, Z.; Yan, Y. Dual-Functional Mesoporous Films Templated by Cellulose Nanocrystals for the Selective Adsorption of Lithium and Rubidium. J. Chem. Eng. Data 2019, 64, 926–933. [Google Scholar] [CrossRef]
- Shang, X.; Hu, B.; Nie, P.; Shi, W.; Hussain, T.; Liu, J. LiNi0.5Mn1.5O4-based hybrid capacitive deionization for highly selective adsorption of lithium from brine. Sep. Purif. Technol. 2021, 258, 118009. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, D.; Lu, Y.; Yan, Y.; Hu, B. Zwitterion imprinted composite membranes with obvious antifouling character for selective separation of Li ions. Korean J. Chem. Eng. 2020, 37, 707–715. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, X.; Hu, S.; Xiang, X. Highly efficient extraction of lithium from salt lake brine by LiAl-layered double hydroxides as lithium-ion-selective capturing material. J. Energy Chem. 2019, 34, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Zhao, Q. A Nano-Heterogeneous Membrane for Efficient Separation of Lithium from High Magnesium/Lithium Ratio Brine. Adv. Funct. Mater. 2021, 31, 2009430. [Google Scholar] [CrossRef]
- Xu, P.; Qian, X.; Guo, C.; Xu, Z.; Zhao, L.; Mai, W.; Li, J.; Tian, X.; Duo, Y. Nanofiltration Technology Used for Separation of Magnesium and Lithium from Salt Lake Brine: A Survey. Mater. Rep. 2019, 33, 410–417. [Google Scholar]
- Su, H.; Zhu, Z.; Wang, L.; Qi, T. Advances and Prospects of Extracting and Recovering Lithium from Salt Lake Brines. Mater. Rep. 2019, 33, 2119–2126. [Google Scholar]
- Xu, S.; Song, J.; Bi, Q.; Chen, Q.; Zhang, W.-M.; Qian, Z.; Zhang, L.; Xu, S.; Tang, N.; He, T. Extraction of lithium from Chinese salt-lake brines by membranes: Design and practice. J. Membr. Sci. 2021, 635, 119441. [Google Scholar] [CrossRef]
- Cao, Z.J.; Gao, M.; Ning, Z.Y.; Wang, W. Lithium Resources and Lithium Extraction Technology in Qinghai Salt Lake. Chem. Eng. Des. Commun. 2019, 45, 190–207. [Google Scholar]
- Zhang, Y.; Zheng, X.; Bian, T.; Zhang, Y.; Mei, J.; Li, Z. Phosphorylated-CNC/MWCNT thin films-toward efficient adsorption of rare earth La (III). Cellulose 2020, 27, 3379–3390. [Google Scholar] [CrossRef]
- Huang, X.; Zhuang, D.; Chen, Z.; Gong, H.; Wang, T.; He, J.; Zhang, X. The Investigation for Electrodeposition Behavior of Lithium Metal in a Crown Ether/Propylene Carbonate Electrolyte. J. Electroanal. Chem. 2021, 887, 115156. [Google Scholar] [CrossRef]
- Salinas, G.; Marquez, A.V.; Idir, M.; Shinde, S.; Frontana-Uribe, B.; Raoux, M.; Lang, J.; Cloutet, E.; Kuhn, A. Sodium-Ion Selectivity Study of a Crown-Ether-Functionalized PEDOT Analog. ChemElectroChem 2020, 7, 2826–2830. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, B.; Xin, Y.; Li, L.; Ye, G.; Wang, J.; Shen, J.; Cui, X.; Yang, Q. Preparation, Mechanical Properties, and Biocompatibility of Graphene Oxide-Reinforced Chitin Monofilament Absorbable Surgical Sutures. Mar. Drugs 2019, 17, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Bian, T.; Zhang, Y.; Zhang, Y.; Li, Z. Construction of ion-imprinted nanofiber chitosan films using low-temperature thermal phase separation for selective and efficiency adsorption of Gd (III). Cellulose 2020, 27, 455–467. [Google Scholar] [CrossRef]
- Hu, D.; Lian, Z.; Xian, H.; Jiang, R.; Wang, N.; Weng, Y.; Peng, X.; Wang, S.; Ouyang, X.-K. Adsorption of Pb (II) from aqueous solution by polyacrylic acid grafted magnetic chitosan nanocomposite. Int. J. Biol. Macromol. 2019, 154, 1537–1547. [Google Scholar] [CrossRef]
- Taghizadeh, M.T.; Sabouri, N.; Ghanbarzadeh, B.J.S. Polyvinyl alcohol:starch:carboxymethyl cellulose containing sodium montmorillonite clay blends; mechanical properties and biodegradation behavior. SpringerPlus 2013, 2, 376. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liang, J. Insight: High intensity and activity carrier granular sludge cultured using polyvinyl alcohol/chitosan and polyvinyl alcohol/chitosan/iron gel beads. Bioresour. Technol. 2021, 326, 124778. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Yue, J.; Cheng, Y.; Xu, K.; Wang, Q.; Fan, F.; Wang, Z.; Cui, Z. Fabrication of poly(vinylidene fluoride) membrane via thermally induced phase separation using ionic liquid as green diluent. Chin. J. Chem. Eng. 2020, 28, 1415–1423. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhang, Y.; Zheng, X.; Sun, W.; Li, B.T.; Wang, D.; Li, Z. High specific surface crown ether modified chitosan nanofiber membrane by low-temperature phase separation for efficient selective adsorption of lithium. Sep. Purif. Technol. 2021, 262, 118312. [Google Scholar] [CrossRef]
- Abbas, T.; Lavadiya, D.N.; Kiran, R. Exploring the Use of Polyols, Corn, and Beet Juice for Decreasing the Freezing Point of Brine Solution for Deicing of Pavements. Sustainability 2021, 13, 5765. [Google Scholar] [CrossRef]
- Zhang, Y.; Bian, T.; Xia, D.; Wang, D.; Zhang, Y.; Zheng, X.; Li, Z. Optimum selective separation of Cu(ii) using 3D ordered macroporous chitosan films with different pore sizes. RSC Adv. 2019, 9, 13065–13076. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.; Bian, T.; Zheng, X.; Cui, Y.; Zhang, Y.; Li, M.; Li, Z. Corrosion-resistant porous hydrophobic PVDF-CBC foam for the treatment of oil-water separation. Mater. Chem. Phys. 2021, 273, 125080. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y.; Zhang, F.; Li, Z.; Yan, Y. Dual-template docking oriented ionic imprinted bilayer mesoporous films with efficient recovery of neodymium and dysprosium. J. Hazard. Mater. 2018, 353, 496–504. [Google Scholar] [CrossRef] [PubMed]

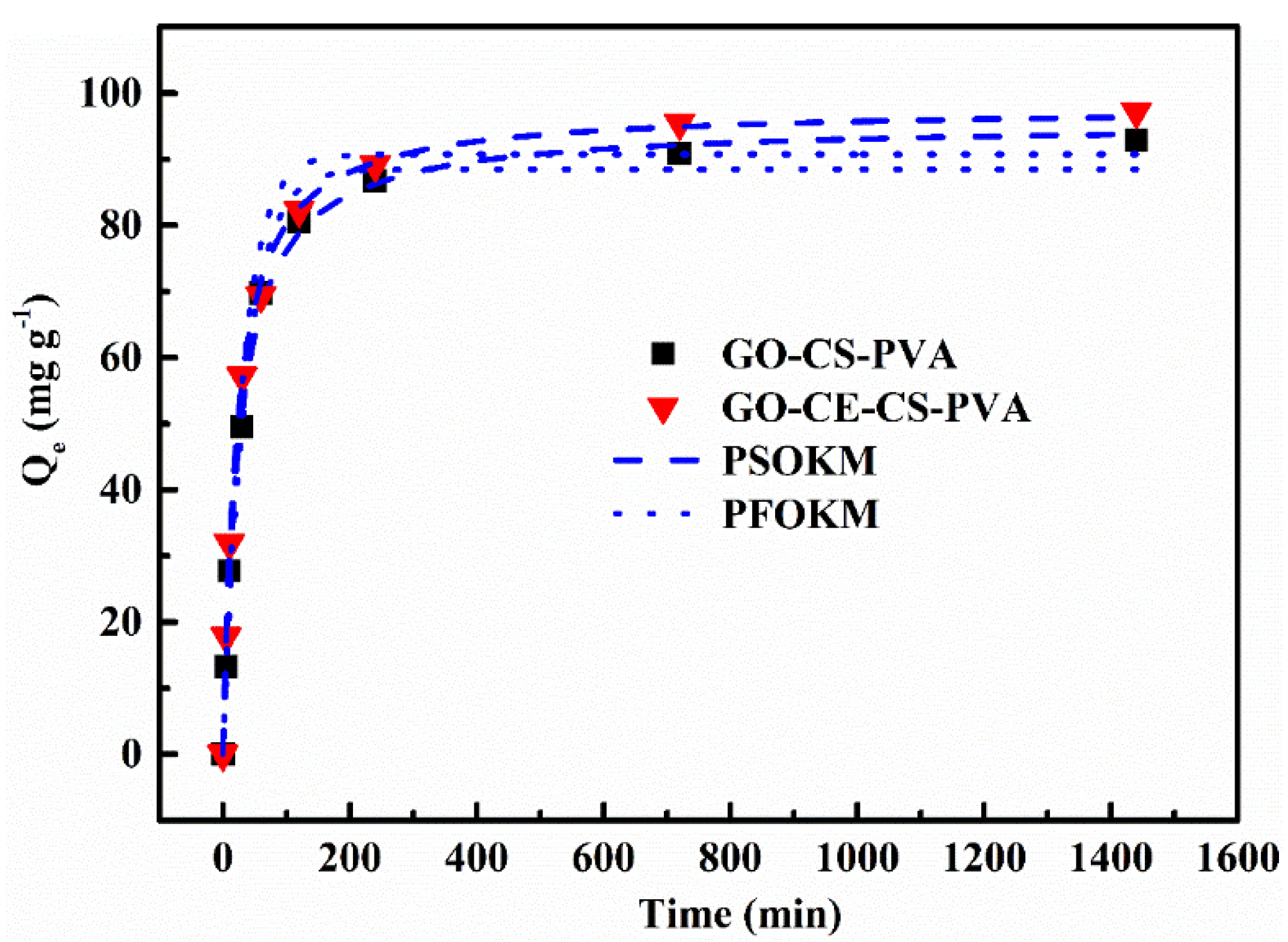


| Adsorbents | Qe,exp (mg g−1) | PFOKM | PSOKM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qe,c (mg g−1) | k1 (min−1) | R2 | Qe,c (mg g−1) | K2 × 10−2 (g mg−1 min−1) | h (mg g−1 min−1) | t1/2 (min) | R2 | ||
| GO-CS-PVA | 92.82 | 88.49 | 0.028 | 0.983 | 95.42 | 0.041 | 3.77 | 25.28 | 0.996 |
| GO-CE-CS-PVA | 97.23 | 90.76 | 0.032 | 0.954 | 97.83 | 0.046 | 4.43 | 22.07 | 0.998 |
| Adsorbents | Langmuir Isotherm Equation | Freundlich Isotherm Equation | |||||
|---|---|---|---|---|---|---|---|
| R2 | KL (L mg−1) | Qm (mg g−1) | RL | R2 | KF (mg g−1) | 1/n | |
| GO-CS-PVA | 0.992 | 0.007 | 148.75 | 0.069 | 0.900 | 9.69 | 0.366 |
| GO-CE-CS-PVA | 0.994 | 0.004 | 168.50 | 0.117 | 0.933 | 6.46 | 0.427 |
| Adsorbents | ∆H (kJ mol−1) | ∆S (J mol−1) | T (K) | Ko | ∆G (kJ mol−1) |
|---|---|---|---|---|---|
| GO-CS-PVA | −1.28 | 10.49 | 308.15 | 5.83 | −4.516 |
| 318.15 | 5.74 | −4.624 | |||
| 328.15 | 5.65 | −4.725 | |||
| GO-CE-CS-PVA | −3.44 | 3.52 | 308.15 | 5.91 | −4.551 |
| 318.15 | 5.50 | −4.511 | |||
| 328.15 | 5.45 | −4.625 |
| Metal Ions | GO-CS-PVA | GO-CE-CS-PVA | ||||
|---|---|---|---|---|---|---|
| Cf (mg·L−1) | Kd (mL·g−1) | k | Cf (mg·L−1) | Kd (mL·g−1) | k | |
| Li+ | 865.38 | 0.156 | / | 847.13 | 0.180 | / |
| Na+ | 860.35 | 0.162 | 0.96 | 933.08 | 0.072 | 2.51 |
| Mg2+ | 899.08 | 0.112 | 1.39 | 926.49 | 0.079 | 2.27 |
| K+ | 950.56 | 0.052 | 3.00 | 958.81 | 0.043 | 4.19 |
| Ca2+ | 928.48 | 0.077 | 2.03 | 933.96 | 0.071 | 2.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Li, A.; Hua, J.; Zhang, Y.; Li, Z. Crown Ether Grafted Graphene Oxide/Chitosan/Polyvinyl Alcohol Nanofiber Membrane for Highly Selective Adsorption and Separation of Lithium Ion. Nanomaterials 2021, 11, 2668. https://doi.org/10.3390/nano11102668
Zheng X, Li A, Hua J, Zhang Y, Li Z. Crown Ether Grafted Graphene Oxide/Chitosan/Polyvinyl Alcohol Nanofiber Membrane for Highly Selective Adsorption and Separation of Lithium Ion. Nanomaterials. 2021; 11(10):2668. https://doi.org/10.3390/nano11102668
Chicago/Turabian StyleZheng, Xudong, Ang Li, Jie Hua, Yuzhe Zhang, and Zhongyu Li. 2021. "Crown Ether Grafted Graphene Oxide/Chitosan/Polyvinyl Alcohol Nanofiber Membrane for Highly Selective Adsorption and Separation of Lithium Ion" Nanomaterials 11, no. 10: 2668. https://doi.org/10.3390/nano11102668