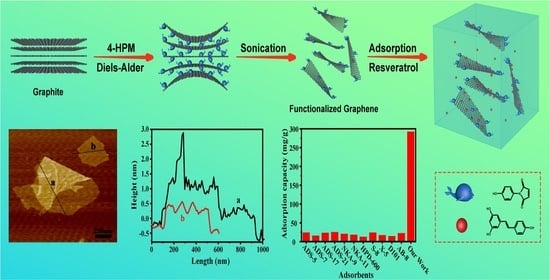
Ultrasonic-Assisted Diels–Alder Reaction Exfoliation of Graphite into Graphene with High Resveratrol Adsorption Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
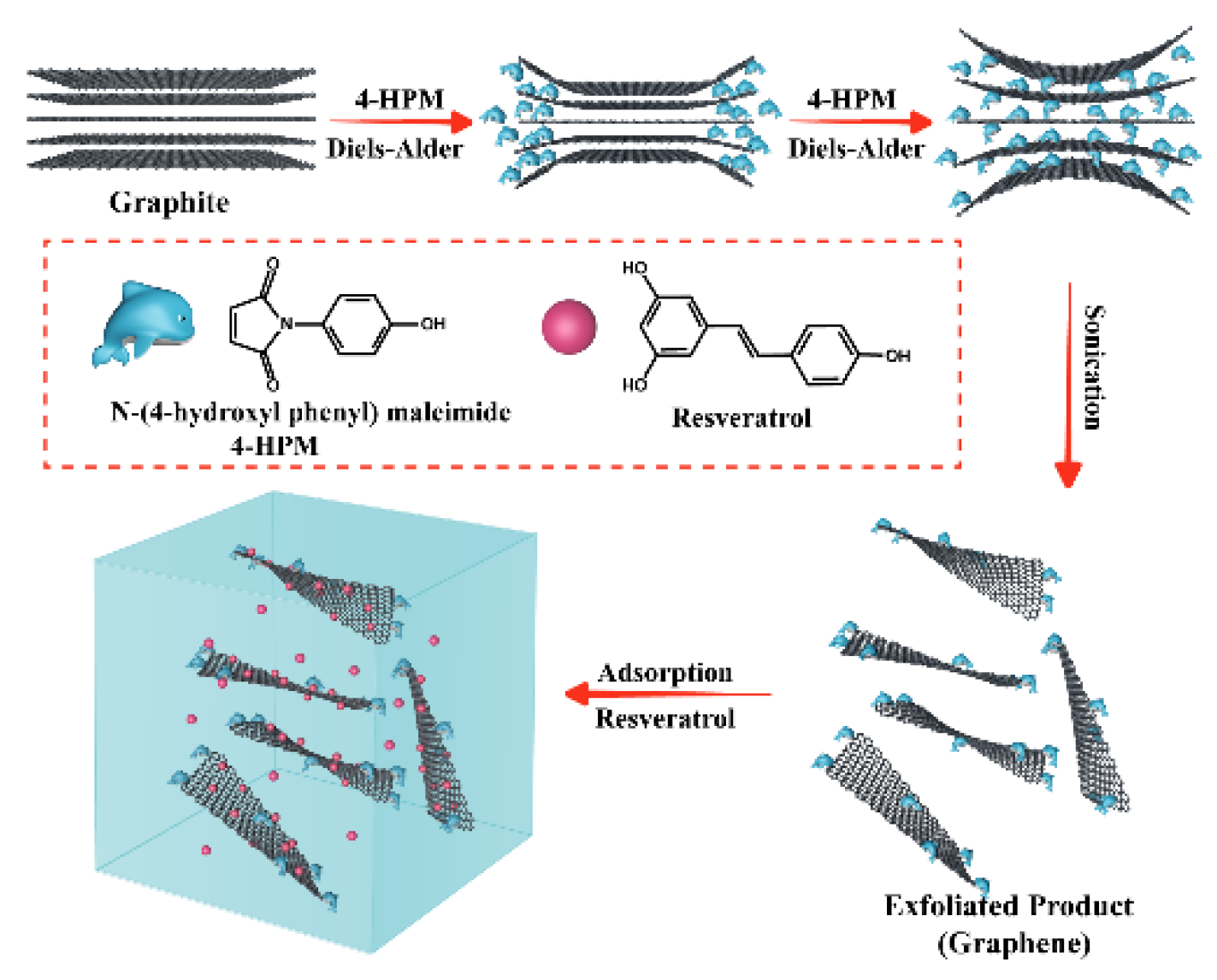
2.2. Exfoliation of Graphite via One-Step Diels–Alder Reaction
2.3. Characterization
3. Results and Discussion
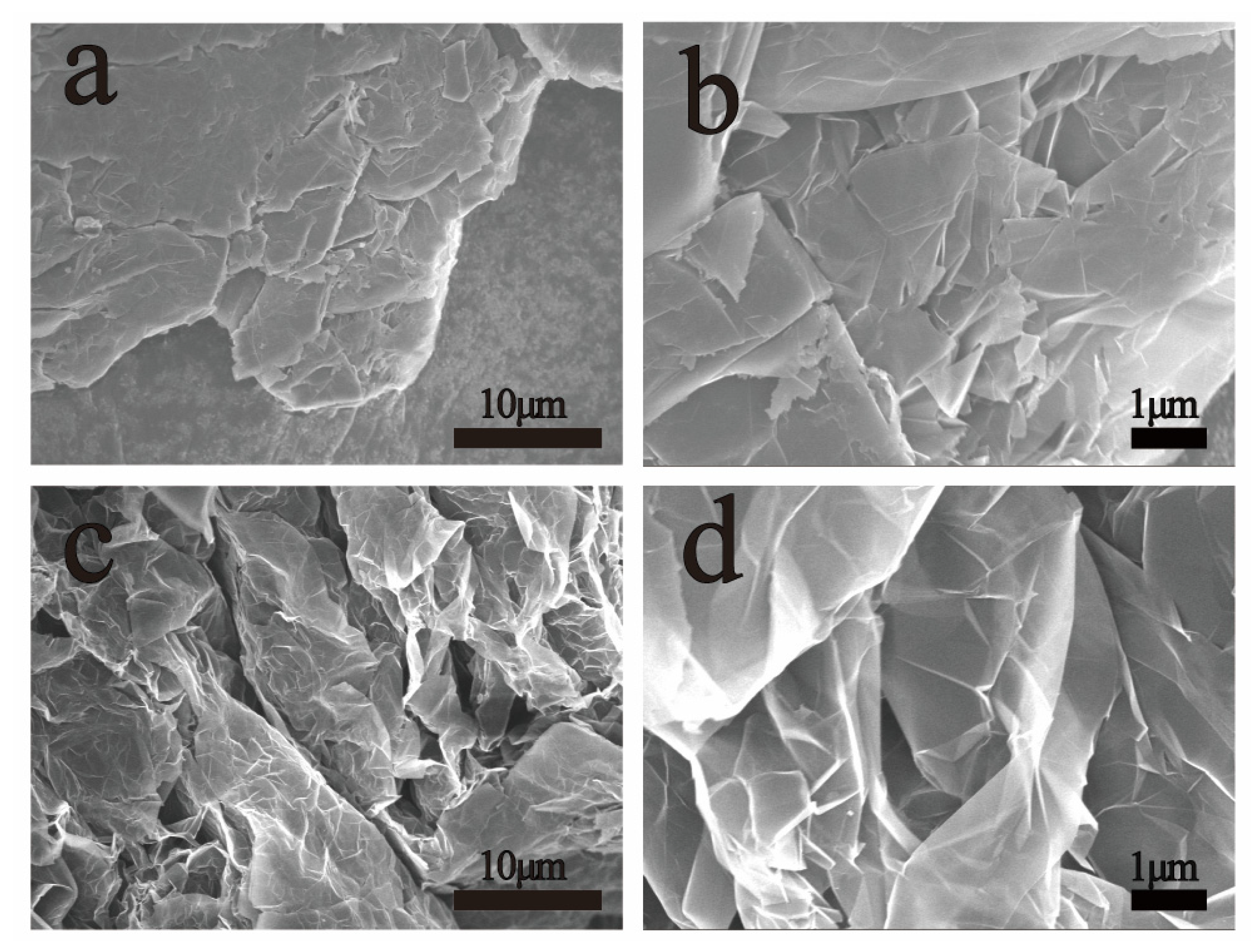
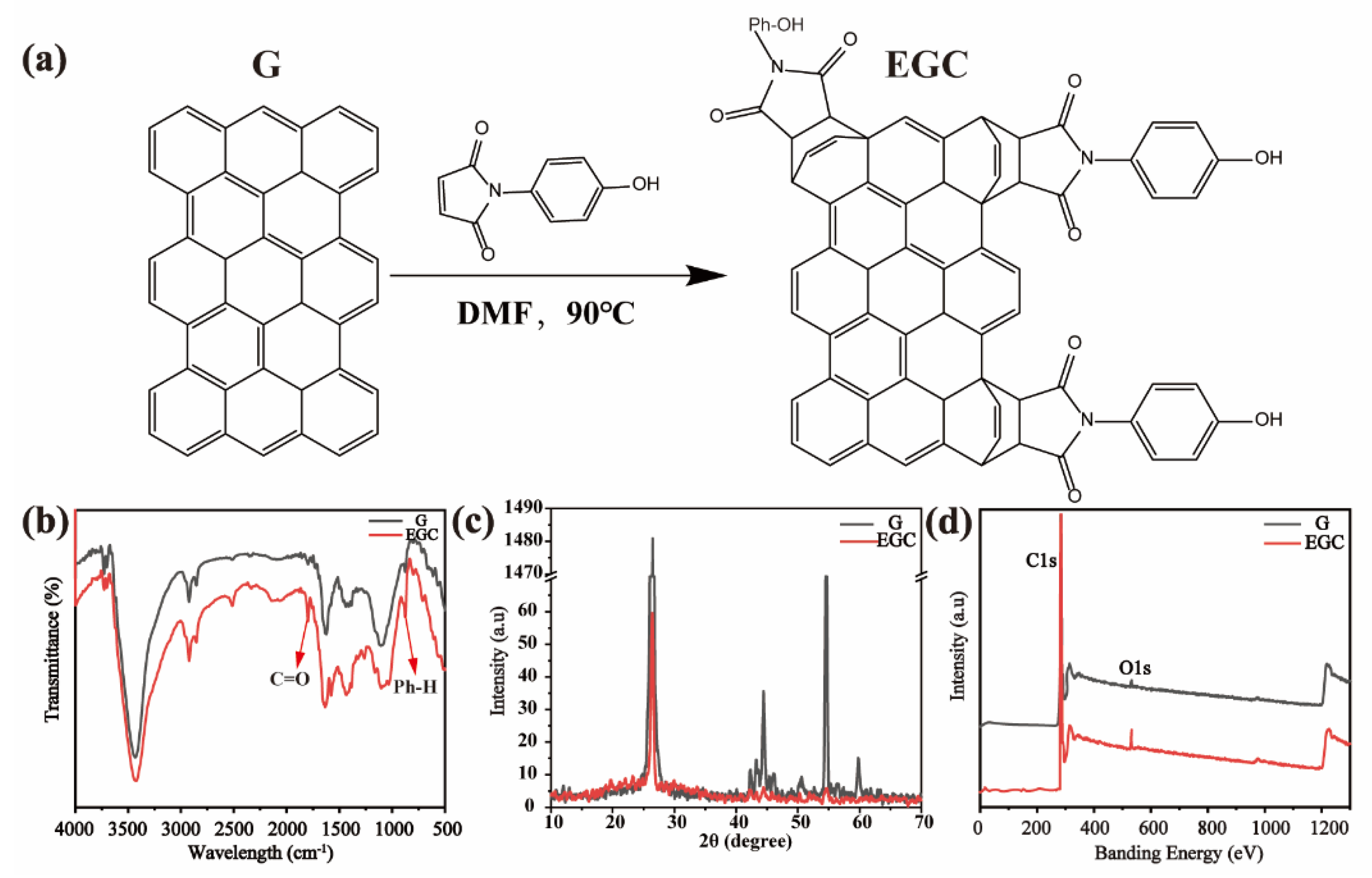
Characterization of Exfoliated Graphite Compound (EGC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Walimbe, P.; Chaudhari, M. State-of-the-art advancements in studies and applications of graphene: A comprehensive review. Mater. Today Sustain. 2019, 6, 100026. [Google Scholar] [CrossRef]
- Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C. Electronic confinement and coherence in patterned epitaxial graphene. Science 2006, 312, 1191–1196. [Google Scholar] [CrossRef] [Green Version]
- Chaika, A.N.; Aristov, V.Y.; Molodtsova, O.V. Graphene on cubic-SiC. Prog. Mater. Sci. 2017, 89, 1–30. [Google Scholar] [CrossRef]
- Beshkova, M.; Hultman, L.; Yakimova, R. Device applications of epitaxial graphene on silicon carbide. Vacuum 2016, 128, 186–197. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; König, M.; Bromley, C.J.; Yoon, B.; Treanor, M.; Garrido Torres, J.A. Ethene to graphene: Surface catalyzed chemical pathways, intermediates, and assembly. J. Phys. Chem. C 2017, 121, 9413–9423. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.J.; Mun, J.H.; Hwang, C.Y.; Cho, B.J. Monolayer graphene growth on sputtered thin film platinum. J. Appl. Phys. 2009, 106, 104309. [Google Scholar] [CrossRef] [Green Version]
- Edwards, R.S.; Coleman, K.S. Graphene film growth on polycrystalline metals. Acc. Chem Res. 2013, 46, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maria, B.; Artemii, I.; Dmitry, I. Direct growth of graphene film on piezoelectric La3Ga5.5Ta0.5O14 crystal. Phys. Status Solidi Rapid Res. Letterse. 2016, 7, 1–6. [Google Scholar]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, C.; Xie, D.; Wang, J.; Yang, Z.; Ren, G.; Zhu, Y. Ultrahigh conductive graphene paper based on ball-milling exfoliated graphene. Adv. Funct. Mater. 2017, 27, 1700240. [Google Scholar] [CrossRef]
- Li, X.; Shen, J.; Wu, C.; Wu, K. Ball-mill-exfoliated graphene: Tunable electrochemistry and phenol sensing. Small 2019, 15, 1805567. [Google Scholar] [CrossRef]
- Su, C.; Lu, A.; Xu, Y.; Chen, F.; Khlobystov, A.N.; Li, L. High-quality thin graphene films from fast electrochemical exfoliation. ACS Nano 2011, 5, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Tripathi, C.C. Electrochemical exfoliation of graphite into graphene for flexible supercapacitor application. Mater. Today Proc. 2018, 5, 1125–1130. [Google Scholar] [CrossRef]
- Tang, H.; He, P.; Huang, T.; Cao, Z.; Zhang, P.; Wang, G. Electrochemical method for large size and few-layered water-dispersible graphene. Carbon 2019, 143, 559–563. [Google Scholar] [CrossRef]
- Qian, W.; Hao, R.; Hou, Y.; Tian, Y.; Shen, C.; Gao, H. Solvothermal-assisted exfoliation process to produce graphene with high yield and high quality. Nano Res. 2009, 2, 706–712. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.-M.; Jeon, I.-Y.; Baek, J.-B. Mechanochemically driven solid-state Diels–Alder reaction of graphite into graphene nanoplatelets. Chem. Sci. 2013, 4, 4273. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.M.; Baek, J.B. A solvent-free Diels-Alder reaction of graphite into functionalized graphene nanosheets. Chem. Commun. 2014, 50, 14651. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Yang, S.; Huang, T.; Sun, J.; Wang, H.; Pu, X. One-step fast electrochemical fabrication of water-dispersible graphene. Carbon 2017, 111, 617–621. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Zhu, J.; Li, L.; Du, X.; Sun, X. Electrochemically exfoliated high-yield graphene in ambient temperature molten salts and its application for flexible solid-state supercapacitors. Carbon 2018, 127, 392–403. [Google Scholar] [CrossRef]
- Brodie, B.C. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar]
- Staudenmaier, L. VerfahrenzurDarstellung der graphitslure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.O.R. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Sarkar, S.; Elena, B.; Robert, C. Chemistry at the Dirac Point: Diels-Alder reactivity of graphene. Acc. Chem. Res. 2012, 45, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Bekyarova, E.; Sandip, N.; Robert, C. Diels-Alder chemistry of graphite and graphene: Graphene as diene and dienophile. J. Am. Chem. Soc. 2011, 133, 3324–3327. [Google Scholar] [CrossRef]
- Cao, Y.; Osuna, S.; Liang, Y.; Robert, C.; Houk, K. Diels-Alder reactions of graphene: Computational predictions of products and sites of reaction. J. Am. Chem. Soc. 2013, 135, 17643–17649. [Google Scholar] [CrossRef]
- Feng, Z.; Zuo, H.; Hu, J.; Yu, B.; Ning, N.; Tian, M.; Zhang, L. In Situ Exfoliation of Graphite into Graphene Nanosheets in Elastomer Composites Based on Diels–Alder Reaction during Melt Blending. Ind. Eng. Chem. Res. 2019, 58, 13182–13189. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, K.; Ouyang, Q.; Gui, Q.; Chen, X. One-step functionalization of graphene via Diels—Alder reaction for improvement of dispersibility. Front. Mater. Sci. 2020, 14, 198–210. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhou, Q.; Wang, C.; Li, Q.; Wang, C.; Fang, Y. Toward intrinsic graphene surfaces: A systematic study on thermal annealing and wet-chemical treatment of SiO2-supported graphene devices. Nano Lett. 2011, 11, 767–771. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, X.; Zhang, X.; Xia, X. Synthesis and curing properties of a novel curing agent based on N-(4-hydroxyphenyl) maleimide and dicyclopentadiene moieties. J. Appl. Polym. Sci. 2011, 120, 56–61. [Google Scholar] [CrossRef]
- Razeghi, M.; Pircheraghi, G. TPU/graphene nanocomposites: Effect of graphene functionality on the morphology of separated hard domains in thermoplastic polyurethane. Polymer 2018, 148, 169–180. [Google Scholar] [CrossRef]
- Udayabhaskar, R.; Mangalaraja, R.V.; Pandiyarajan, T.; Karthikeyan, B.; Mansilla, H.D.; Contreras, D. Spectroscopic investigation on graphene-copper nanocomposites with strong UV emission and high catalytic activity. Carbon 2017, 124, 256–262. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Gudkov, M.V.; Baidakova, M.V.; Brunkov, P.N. Unveiling a facile approach for large-scale synthesis of N-doped graphene with tuned electrical properties. 2D Mater. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Saveliev, S.D.; Stolyarova, D.Y.; Brzhezinskaya, M.; Brunkov, P.N. Modulating nitrogen species via N-doping and post annealing of graphene derivatives: XPS and XAS examination. Carbon 2021, 182, 593–604. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Q.; Zhang, Q.; Zhang, D.; Shi, Y.; Jiang, C.; Shi, X. Preliminary separation and purification of resveratrol from extract of peanut (Arachis hypogaea) sprouts by macroporous adsorption resins. Food Chem. 2014, 145, 1–7. [Google Scholar] [CrossRef] [PubMed]


| Adsorbent | Qmax, e (mg/g) | Adsorbent | Qmax, e (mg/g) |
|---|---|---|---|
| ADS-5 | 24.00 | HPD-600 | 12.67 |
| ADS-7 | 16.05 | S-8 | 24.02 |
| ADS-17 | 23.36 | X-5 | 17.35 |
| ADS-21 | 25.31 | D101 | 14.68 |
| NKA-9 | 20.65 | Ab-8 | 22.67 |
| NKA-11 | 18.00 | Our work | 292.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Ouyang, Q.; Gui, Q.; Chen, X. Ultrasonic-Assisted Diels–Alder Reaction Exfoliation of Graphite into Graphene with High Resveratrol Adsorption Capacity. Nanomaterials 2021, 11, 3060. https://doi.org/10.3390/nano11113060
Zhang J, Ouyang Q, Gui Q, Chen X. Ultrasonic-Assisted Diels–Alder Reaction Exfoliation of Graphite into Graphene with High Resveratrol Adsorption Capacity. Nanomaterials. 2021; 11(11):3060. https://doi.org/10.3390/nano11113060
Chicago/Turabian StyleZhang, Jinxing, Qi Ouyang, Qilin Gui, and Xiaonong Chen. 2021. "Ultrasonic-Assisted Diels–Alder Reaction Exfoliation of Graphite into Graphene with High Resveratrol Adsorption Capacity" Nanomaterials 11, no. 11: 3060. https://doi.org/10.3390/nano11113060