CdIn2S4/In(OH)3/NiCr-LDH Multi-Interface Heterostructure Photocatalyst for Enhanced Photocatalytic H2 Evolution and Cr(VI) Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of NiCr-LDH
2.2. Synthesis of CdIn2S4/In(OH)3/NiCr-LDH
2.3. Evaluation of Photocatalytic Performance
3. Results and Discussion
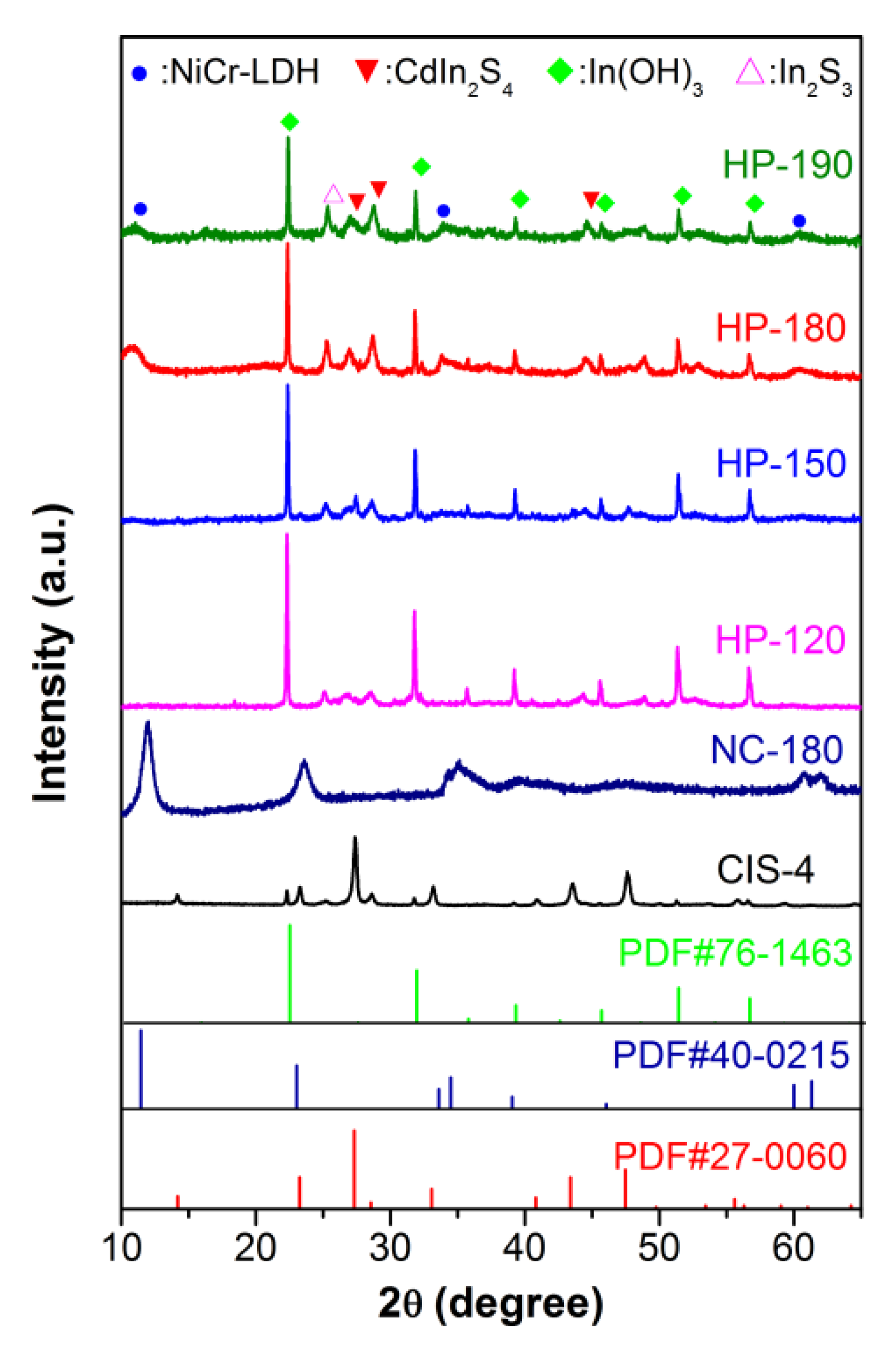
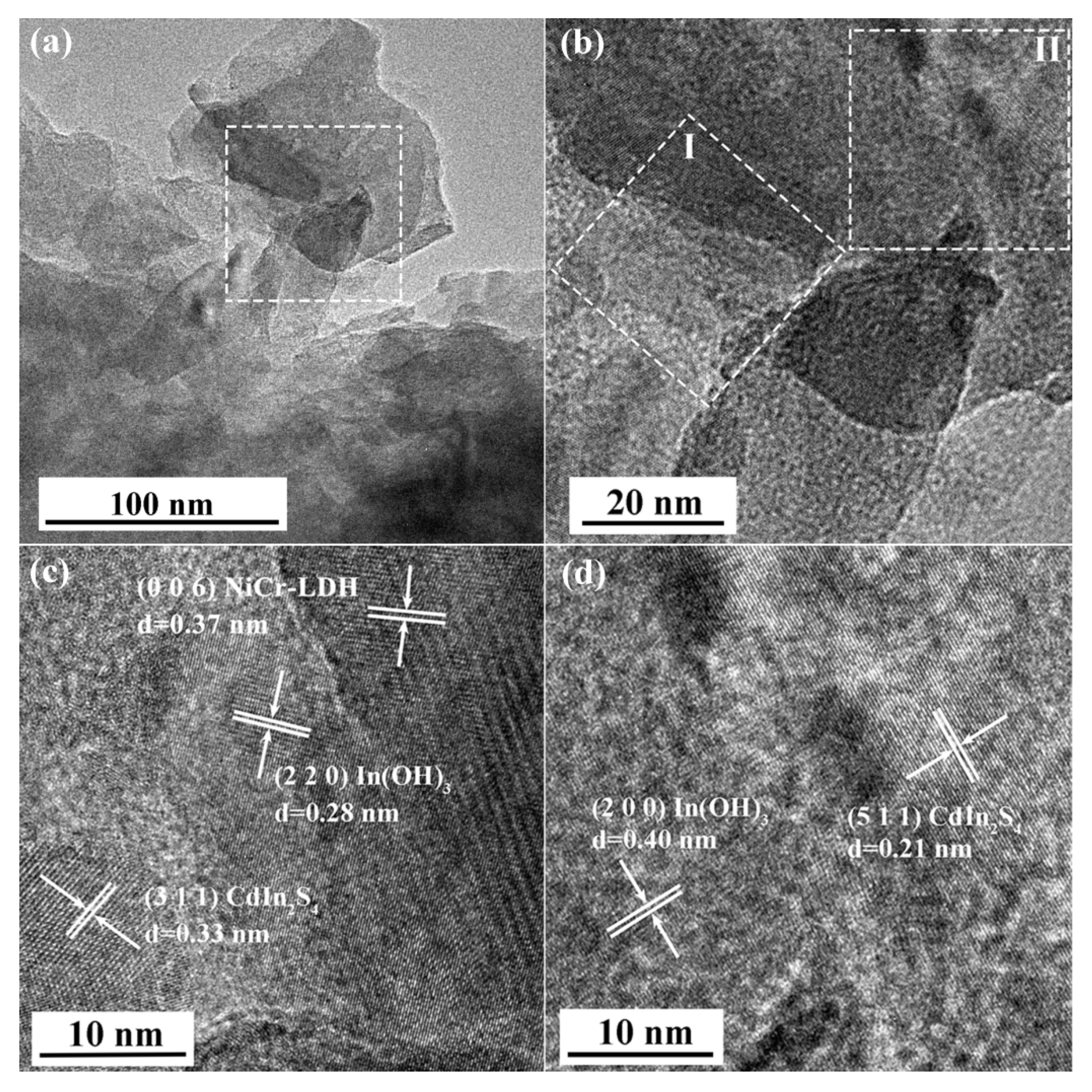
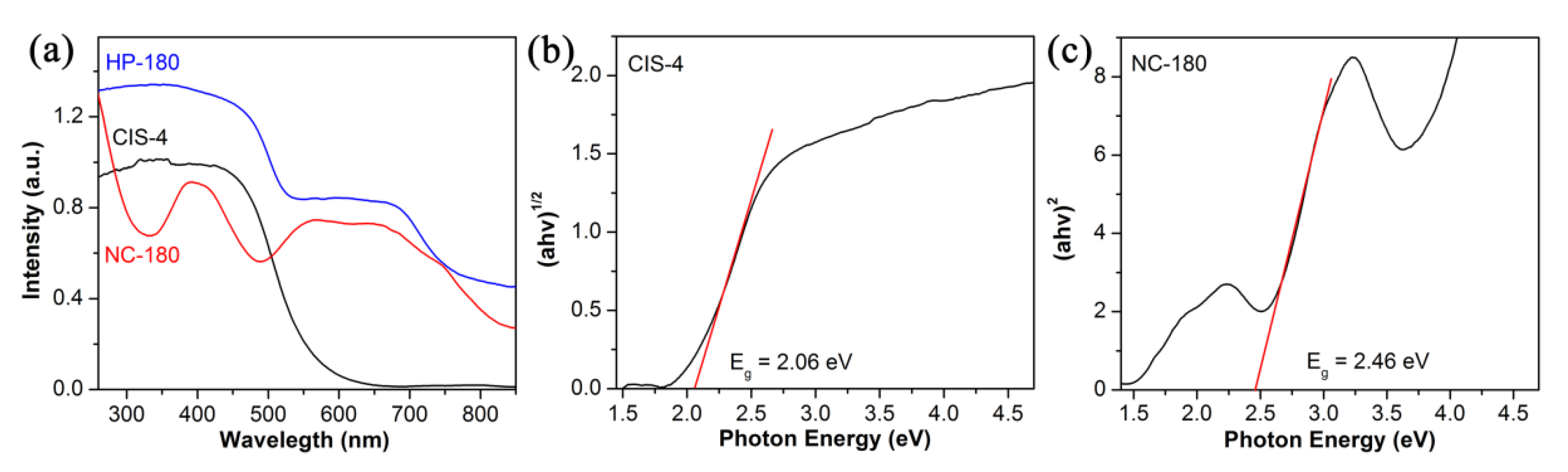
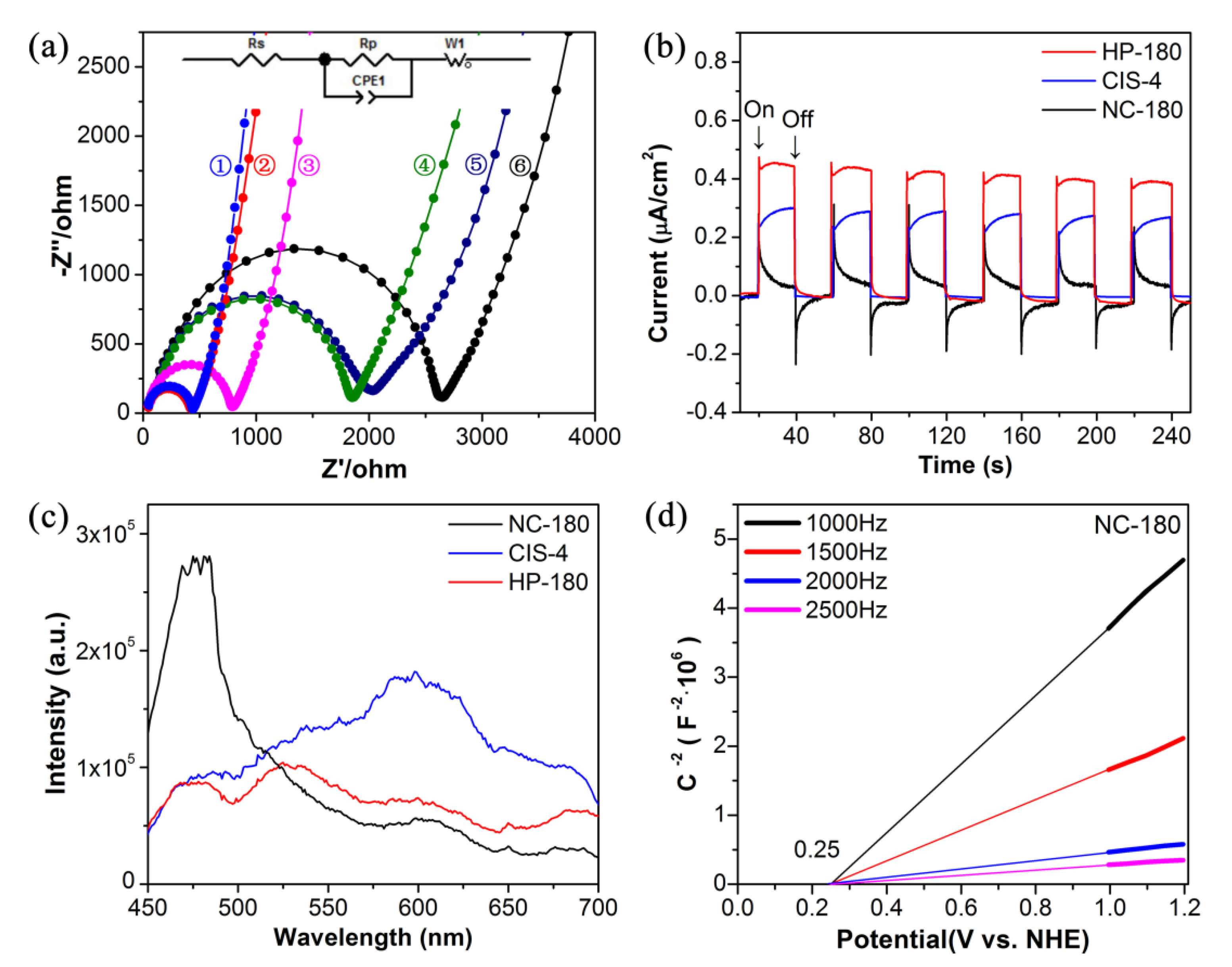
3.1. Characterization of Photocatalysts
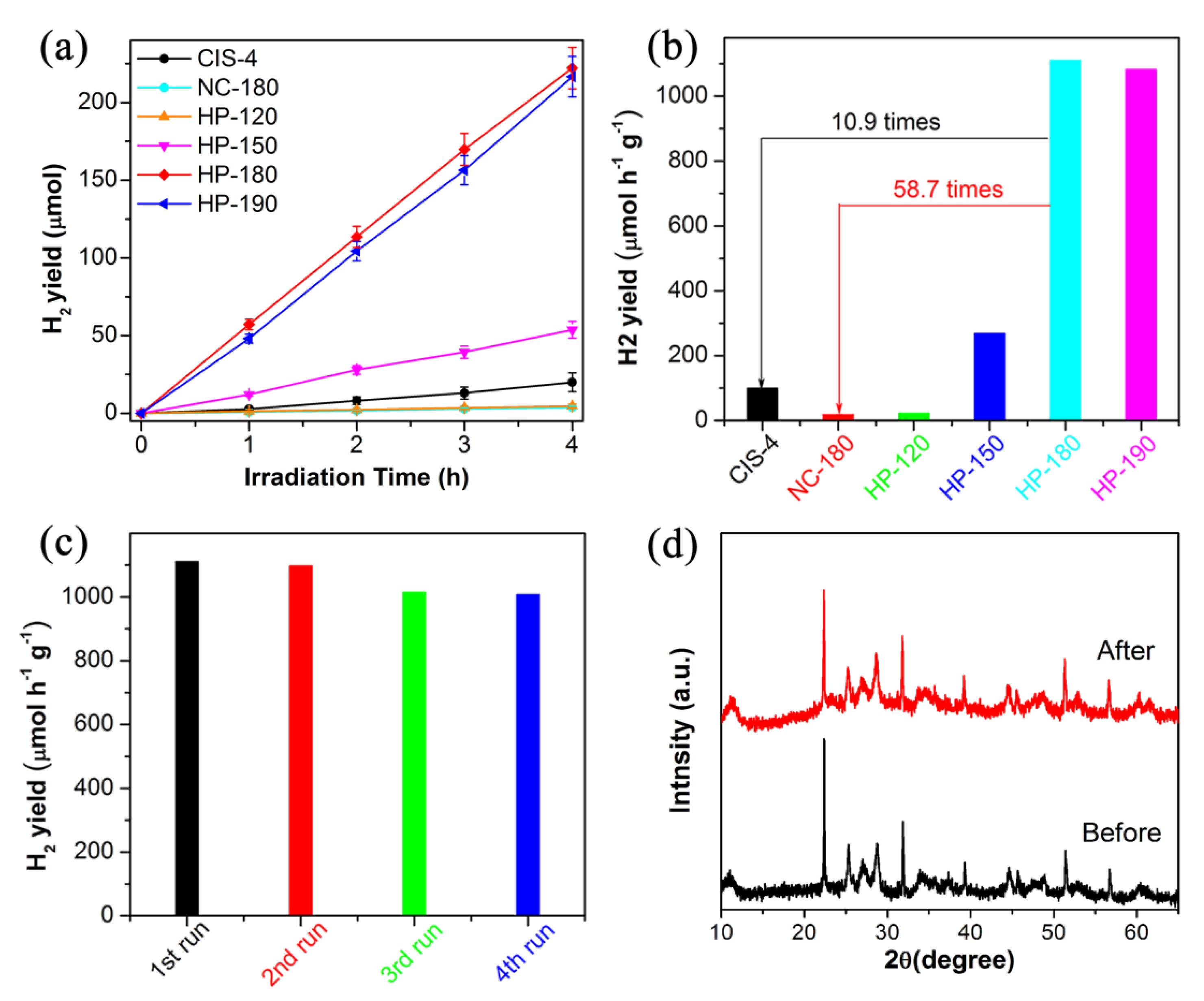
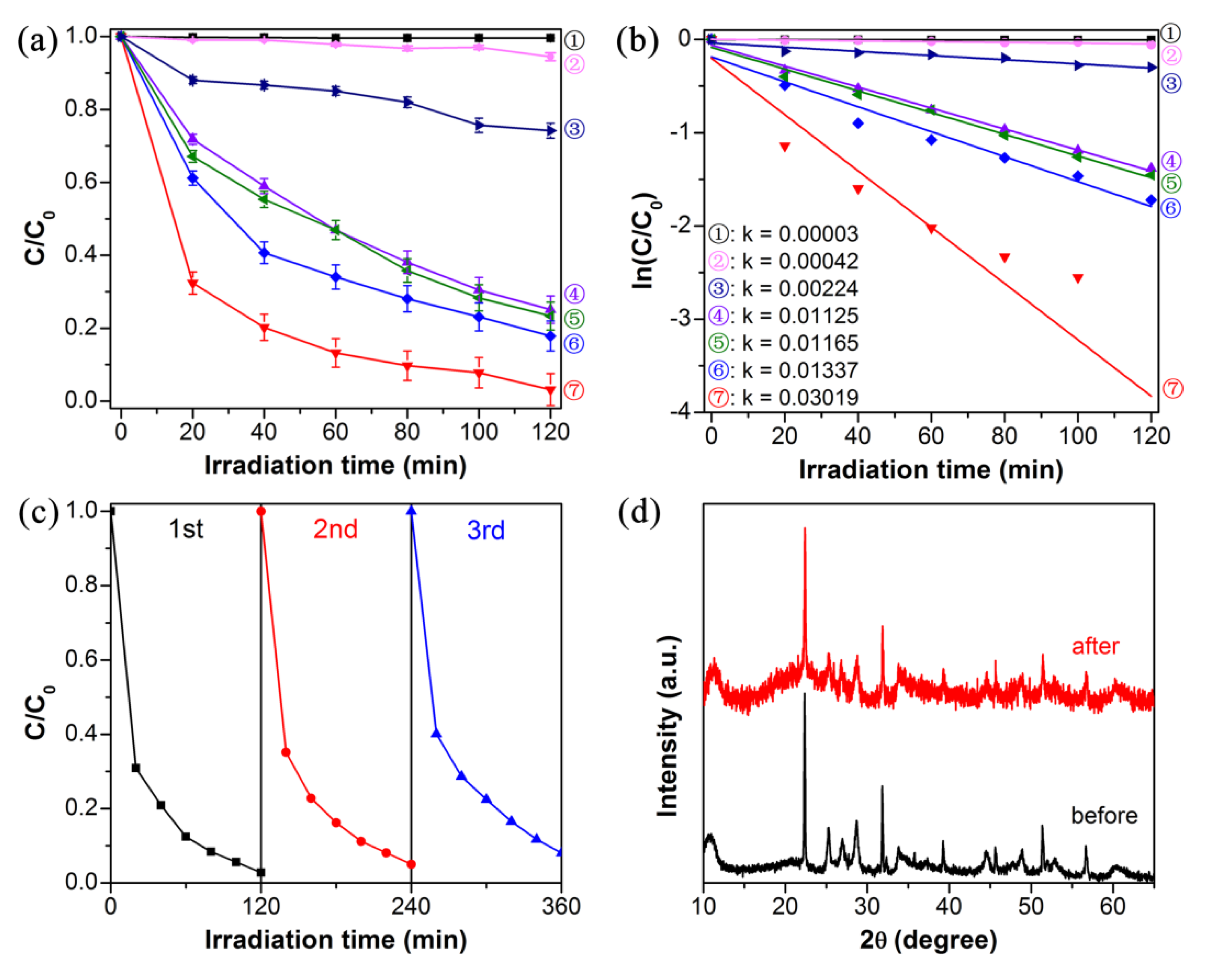
3.2. Photocatalytic Activity
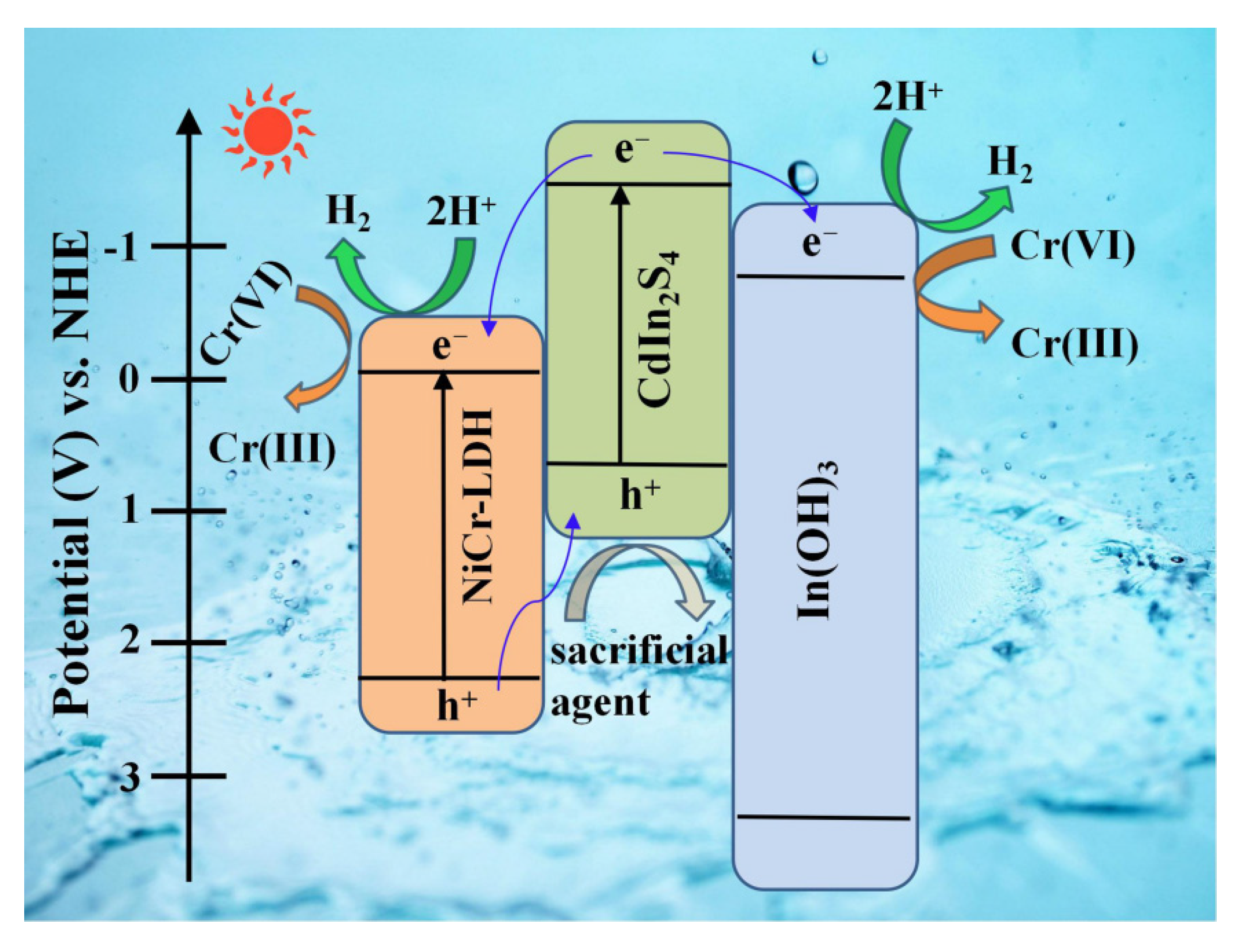
3.3. Plausible Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takata, T.; Jiang, J.Z.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrog. Energ. 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Wang, Y.; Diaz, D.F.R.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Sarma, G.K.; Gupta, S.S.; Bhattacharyya, K.G. Nanomaterials as versatile adsorbents for heavy metal ions in water: A review. Environ. Sci. Pollut. R. 2019, 26, 6245–6278. [Google Scholar] [CrossRef]
- Zhang, W.H.; Mohamed, A.R.; Ong, W.J. Z-Scheme photocatalytic systems for carbon dioxide reduction: Where are we now? Angew. Chem. Int. Edit. 2020, 59, 22894–22915. [Google Scholar] [CrossRef]
- Liang, Z.Z.; Shen, R.C.; Ng, Y.H.; Zhang, P.; Xiang, Q.J.; Li, X. A review on 2D MoS2 cocatalysts in photocatalytic H2 production. J. Mater. Sci. Technol. 2020, 56, 89–121. [Google Scholar] [CrossRef]
- Zhitkovich, A. Chromium in Drinking Water: Sources, Metabolism, and Cancer Risks. Chem. Res. Toxicol. 2011, 24, 1617–1629. [Google Scholar] [CrossRef]
- Zhao, Z.; An, H.; Lin, J.; Feng, M.; Murugadoss, V.; Ding, T.; Liu, H.; Shao, Q.; Mai, X.; Wang, N.; et al. Progress on the Photocatalytic Reduction Removal of Chromium Contamination. Chem. Rec. 2019, 19, 873–882. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Mathe, V.L. Photocatalytic hydrogen production using TiO2 nanogranules prepared by hydrothermal route. Chem. Phys. Lett. 2019, 731, 136582. [Google Scholar] [CrossRef]
- Ong, W.-J.; Shak, K.P.Y. 2D/2D heterostructured photocatalysts: An emerging platform for artificial photosynthesis. Sol. RRL 2020, 4, 2000132. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Lu, J.; Feng, Y.; Meng, F.; Ma, C.; Yan, Y.; Meng, M. Synergy between van der waals heterojunction and vacancy in ZnIn2S4/g-C3N4 2D/2D photocatalysts for enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 277, 119254. [Google Scholar] [CrossRef]
- Xia, P.F.; Cao, S.W.; Zhu, B.C.; Liu, M.J.; Shi, M.S.; Yu, J.G.; Zhang, Y.F. Designing a 0D/2D S-Scheme Heterojunction over Polymeric Carbon Nitride for Visible-Light Photocatalytic Inactivation of Bacteria. Angew. Chem. Int. Edit. 2020, 59, 5218–5225. [Google Scholar] [CrossRef]
- Reddy, C.V.; Reddy, K.R.; Shetti, N.P.; Shim, J.; Aminabhavi, T.M.; Dionysiou, D.D. Hetero-nanostructured metal oxide-based hybrid photocatalysts for enhanced photoelectrochemical water splitting—A review. Int. J. Hydrog. Energ. 2020, 45, 18331–18347. [Google Scholar] [CrossRef]
- Ng, B.J.; Putri, L.K.; Kong, X.Y.; Teh, Y.W.; Pasbakhsh, P.; Chai, S.P. Z-Scheme photocatalytic systems for solar water splitting. Adv. Sci. 2020, 7, 1903171. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.L.; Zhang, L.Y.; Cheng, B.; Fan, J.J.; Yu, J.G. S-Scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Di, T.; Xu, Q.; Ho, W.; Tang, H.; Xiang, Q.; Yu, J. Review on metal sulphide-based Z-scheme photocatalysts. Chemcatchem 2019, 11, 1394–1411. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.-W. Spongy ball-like copper oxide nanostructure modified by reduced graphene oxide for enhanced photocatalytic hydrogen production. Mater. Res. Bull. 2021, 133, 111026. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.-W. Porous nanoplate-like tungsten trioxide/reduced graphene oxide catalyst for sonocatalytic degradation and photocatalytic hydrogen production. Surf. Interf. 2021, 24, 101075. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, H.-Y.; Li, J.-Y.; Liao, J.-F.; Zhang, H.-H.; Wang, X.-D.; Kuang, D.-B. Z-Scheme 2D/2D heterojunction of CsPbBr3/Bi2WO6 for improved photocatalytic CO2 reduction. Adv. Funct. Mater. 2020, 30, 2004293. [Google Scholar] [CrossRef]
- Han, Q.; Li, L.; Gao, W.; Shen, Y.; Wang, L.; Zhang, Y.; Wang, X.; Shen, Q.; Xiong, Y.; Zhou, Y.; et al. Elegant construction of ZnIn2S4/BiVO4 hierarchical heterostructures as direct Z-Scheme photocatalysts for efficient CO2 photoreduction. ACS Appl. Mater. Inter. 2021, 13, 15092–15100. [Google Scholar] [CrossRef]
- Ng, S.-F.; Lau, M.Y.L.; Ong, W.-J. Engineering layered double hydroxide–based photocatalysts toward artificial photosynthesis: State-of-the-art progress and prospects. Sol. RRL 2021, 5, 2000535. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Shi, R.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Efficient photocatalytic nitrogen fixation over Cuδ+-modified defective ZnAl-layered double hydroxide nanosheets. Adv. Energy Mater. 2020, 10, 1901973. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Meng, Y.; Pan, G.; Ni, Z.; Xia, S. Layered double hydroxides-based photocatalysts and visible-light driven photodegradation of organic pollutants: A review. Chem. Eng. J. 2020, 392, 123684. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Wei, J.-J.; Zeng, G.-M.; Zhang, H.-Q.; Tan, X.-F.; Ma, C.; Li, X.-C.; Li, Z.-H.; Zhang, C. A review on strategies to LDH-based materials to improve adsorption capacity and photoreduction efficiency for CO2. Coordin. Chem. Rev. 2019, 386, 154–182. [Google Scholar] [CrossRef]
- Sideris, P.J.; Nielsen, U.G.; Gan, Z.; Grey, C.P. Mg/Al ordering in layered double hydroxides revealed by multinuclear NMR spectroscopy. Science 2008, 321, 113. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Yu, X.; Liu, Q.; Liu, G.; Tang, H. Internal electric field induced S–scheme heterojunction MoS2/CoAl LDH for enhanced photocatalytic hydrogen evolution. J. Colloid Interf. Sci. 2021, 585, 470–479. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Li, Y.; Zhang, L.; Wang, A.; Xiao, N.; Gao, Y.; Li, N.; Song, W.; Ge, L.; et al. Novel photocatalyst incorporating Ni-Co layered double hydroxides with P-doped CdS for enhancing photocatalytic activity towards hydrogen evolution. Appl. Catal. B Environ. 2019, 254, 145–155. [Google Scholar] [CrossRef]
- Nayak, S.; Swain, G.; Parida, K. Enhanced photocatalytic activities of RhB degradation and H2 evolution from in situ formation of the electrostatic heterostructure MoS2/NiFe LDH nanocomposite through the Z-Scheme mechanism via p–n heterojunctions. ACS Appl. Mater. Inter. 2019, 11, 20923–20942. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, Y.; Liu, J.; Chen, T.; Liu, Q.; Zhu, Y.; Niu, L.; Li, C.; Liu, X.; Sun, C.Q.; et al. Constructing NiFe-LDH wrapped Cu2O nanocube heterostructure photocatalysts for enhanced photocatalytic dye degradation and CO2 reduction via Z-scheme mechanism. J. Alloy. Compd. 2020, 831, 154723. [Google Scholar] [CrossRef]
- Li, X.; Yu, Z.; Shao, L.; Zeng, H.; Liu, Y.; Feng, X. A novel strategy to construct a visible-light-driven Z-scheme (ZnAl-LDH with active phase/g-C3N4) heterojunction catalyst via polydopamine bridge (a similar “bridge” structure). J. Hazard. Mater. 2020, 386, 121650. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, S.; Zhou, R.; Li, Y.; He, Z.; Ding, H.; Chen, D.; Ao, W. A review: Synthesis, modification and photocatalytic applications of ZnIn2S4. J. Mater. Sci. Technol. 2021, 78, 1–19. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Xu, J.; Shao, Y.; Wu, J.; Xu, X.; Pan, Y.; Ju, H.; Zhu, J.; Xie, Y. Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers. Nat. Energy 2019, 4, 690–699. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, Y.; Teo, W.L.; Xie, A.; Guo, Y.; Dai, Y.; Zhou, W.; Jana, D.; Xian, Q.; Dong, W.; et al. Ultrathin ZnIn2S4 nanosheets anchored on Ti3C2TX MXene for photocatalytic H2 evolution. Angew. Chem. Int. Edit. 2020, 59, 11287–11292. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.-W. Ultrasound assisted synthesis of highly active nanoflower-like CoMoS4 electrocatalyst for oxygen and hydrogen evolution reactions. Ultrason. Sonochem. 2021, 72, 105454. [Google Scholar] [CrossRef]
- Zhuge, Z.; Liu, X.; Chen, T.; Gong, Y.; Li, C.; Niu, L.; Xu, S.; Xu, X.; Alothman, Z.A.; Sun, C.Q.; et al. Highly efficient photocatalytic degradation of different hazardous contaminants by CaIn2S4-Ti3C2Tx Schottky heterojunction: An experimental and mechanism study. Chem. Eng. J. 2021, 421, 127838. [Google Scholar] [CrossRef]
- Gao, B.; Dong, S.; Liu, J.; Liu, L.; Feng, Q.; Tan, N.; Liu, T.; Bo, L.; Wang, L. Identification of intermediates and transformation pathways derived from photocatalytic degradation of five antibiotics on ZnIn2S4. Chem. Eng. J. 2016, 304, 826–840. [Google Scholar] [CrossRef]
- Pei, C.-Y.; Chen, Y.-G.; Wang, L.; Chen, W.; Huang, G.-B. Step-scheme WO3/CdIn2S4 hybrid system with high visible light activity for tetracycline hydrochloride photodegradation. Appl. Surf. Sci. 2021, 535, 147682. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, D.; Li, N.; Li, H.; Xu, Q.; He, J.; Lu, J. Construction of hollow In2S3/CdIn2S4 heterostructures with high efficiency for Cr(vi) reduction. Environ. Sci. Nano 2021, 8, 1389–1397. [Google Scholar] [CrossRef]
- Chen, W.; Chang, L.; Ren, S.-B.; He, Z.-C.; Huang, G.-B.; Liu, X.-H. Direct Z-scheme 1D/2D WO2.72/ZnIn2S4 hybrid photocatalysts with highly-efficient visible-light-driven photodegradation towards tetracycline hydrochloride removal. J. Hazard. Mater. 2020, 384, 121308. [Google Scholar] [CrossRef]
- Guo, J.; Wang, L.; Wei, X.; Alothman, Z.A.; Albaqami, M.D.; Malgras, V.; Yamauchi, Y.; Kang, Y.; Wang, M.; Guan, W.; et al. Direct Z-scheme CuInS2/Bi2MoO6 heterostructure for enhanced photocatalytic degradation of tetracycline under visible light. J. Hazard. Mater. 2021, 415, 125591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Construction of Hierarchical Hollow Co9S8/ZnIn2S4 Tubular Heterostructures for Highly Efficient Solar Energy Conversion and Environmental Remediation. Angew. Chem. Int. Edit. 2020, 59, 8255–8261. [Google Scholar] [CrossRef]
- Li, Y.; Hou, Y.; Fu, Q.; Peng, S.; Hu, Y.H. Oriented growth of ZnIn2S4/In(OH)3 heterojunction by a facile hydrothermal transformation for efficient photocatalytic H2 production. Appl. Catal. B Environ. 2017, 206, 726–733. [Google Scholar] [CrossRef]
- Geng, M.; Peng, Y.; Zhang, Y.; Guo, X.; Yu, F.; Yang, X.; Xie, G.; Dong, W.; Liu, C.; Li, J.; et al. Hierarchical ZnIn2S4: A promising cocatalyst to boost visible-light-driven photocatalytic hydrogen evolution of In(OH)3. Int. J. Hydrog. Energ. 2019, 44, 5787–5798. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, T.; Shan, D.; Zhu, Y.; Gao, G.; Liu, Y.; Liu, J.; Liu, M.; You, W. ZnIn2S4/In(OH)3 hollow microspheres fabricated by one-step l-cysteine-mediated hydrothermal growth for enhanced hydrogen production and MB degradation. Int. J. Hydrog. Energ. 2020, 45, 13975–13984. [Google Scholar] [CrossRef]
- Ye, L.; Wen, Z.; Li, Z.; Huang, H. Hierarchical Architectured Ternary Nanostructures Photocatalysts with In(OH)3 Nanocube on ZnIn2S4/NiS Nanosheets for Photocatalytic Hydrogen Evolution. Solar RRL 2020, 4, 2000027. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, X.; Zhao, N.; Li, X.; Lu, B.; Zhang, X.; Yu, H. Cocatalyst designing: A binary noble-metal-free cocatalyst system consisting of ZnIn2S4 and In(OH)3 for efficient visible-light photocatalytic water splitting. RSC Adv. 2018, 8, 4979–4986. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yan, X.; Zhao, N.; Zhao, J.; Lu, B.; Zhang, X.; Zhang, X.; Yu, H. Facile synthesis of ternary CdIn2S4/In(OH)3/Zn2GeO4 nanocomposite with enhanced visible-light photocatalytic H2 evolution. J. Photoch. Photobio. A 2018, 360, 298–305. [Google Scholar] [CrossRef]
- Tan, Y.-X.; Chai, Z.-M.; Wang, B.-H.; Tian, S.; Deng, X.-X.; Bai, Z.-J.; Chen, L.; Shen, S.; Guo, J.-K.; Cai, M.-Q.; et al. Boosted photocatalytic oxidation of toluene into benzaldehyde on CdIn2S4-CdS: Synergetic effect of compact heterojunction and S-vacancy. ACS Catal. 2021, 11, 2492–2503. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, S.; Li, B.; Yan, H.; He, S.; Tian, L.; Shi, W.; Ma, J.; Wei, M.; Evans, D.G.; et al. A family of visible-light responsive photocatalysts obtained by dispersing CrO6 octahedra into a hydrotalcite matrix. Chem. Eur. J. 2011, 17, 13175–13181. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xia, Y.; Li, H.; Wang, X.; Yu, Y.; Jiao, X.; Chen, D. Highly active deficient ternary sulfide photoanode for photoelectrochemical water splitting. Nat. Commun. 2020, 11, 3078. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, C.; Zheng, Y.; Zhang, G.; Guo, C.; Li, C.M. Spatially separating redox centers on Z-Scheme ZnIn2S4/BiVO4 hierarchical heterostructure for highly efficient photocatalytic hydrogen evolution. Small 2020, 16, 2002988. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Bhardwaj, M.; Yadav, P.; Ogale, S. g-C3N4/NiAl-LDH 2D/2D hybrid heterojunction for high-performance photocatalytic reduction of CO2 into renewable fuels. ACS Appl. Mater. Inter. 2018, 10, 2667–2678. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, D.P.; Nayak, S.; Reddy, K.H.; Martha, S.; Parida, K. Fabrication of a Co(OH)2/ZnCr LDH “p–n” heterojunction photocatalyst with enhanced separation of charge carriers for efficient visible-light-driven H2 and O2 evolution. Inorg. Chem. 2018, 57, 3840–3854. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rivas, F.; Pastor, A.; de Miguel, G.; Cruz-Yusta, M.; Pavlovic, I.; Sánchez, L. Cr3+ substituted Zn-Al layered double hydroxides as UV–Vis light photocatalysts for NO gas removal from the urban environment. Sci. Total. Environ. 2020, 706, 136009. [Google Scholar] [CrossRef]
- Gao, F.; Lei, R.; Huang, X.; Yuan, J.; Jiang, C.; Feng, W.; Zhang, L.; Liu, P. In situ etching growth of defective ZnS nanosheets anchored vertically on layered-double-hydroxide microflowers for accelerated photocatalytic activity. Appl. Catal. B Environ. 2021, 292, 120187. [Google Scholar] [CrossRef]
- Meng, F.; Qin, Y.; Lu, J.; Lin, X.; Meng, M.; Sun, G.; Yan, Y. Biomimetic design and synthesis of visible-light-driven g-C3N4 nanotube @polydopamine/NiCo-layered double hydroxides composite photocatalysts for improved photocatalytic hydrogen evolution activity. J. Colloid Interf. Sci. 2021, 584, 464–473. [Google Scholar] [CrossRef]
- He, J.-Y.; Zhang, D.; Wang, X.-J.; Zhao, J.; Li, Y.-P.; Liu, Y.; Li, F.-T. Phosphorylation of NiAl-layered double hydroxide nanosheets as a novel cocatalyst for photocatalytic hydrogen evolution. Int. J. Hydrog. Energ. 2021, 46, 18977–18987. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Waterhouse, G.I.N.; Zheng, L.; Cao, X.; Teng, F.; Wu, L.-Z.; Tung, C.-H.; O’Hare, D.; Zhang, T. Layered-Double-Hydroxide Nanosheets as Efficient Visible-Light-Driven Photocatalysts for Dinitrogen Fixation. Adv. Mater. 2017, 29, 1703828. [Google Scholar] [CrossRef]
- Liu, H.; Guo, H.; Liu, B.; Liang, M.; Lv, Z.; Adair, K.R.; Sun, X. Few-Layer MoSe2 Nanosheets with Expanded (002) Planes Confined in Hollow Carbon Nanospheres for Ultrahigh-Performance Na-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1707480. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.Y.; Lu, Y.; Lou, X.W.D. Formation of Hierarchical In2S3–CdIn2S4 Heterostructured Nanotubes for Efficient and Stable Visible Light CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 17305–17308. [Google Scholar] [CrossRef] [PubMed]
- Xiaofei, Y.; Lin, T.; Xiaolong, Z.; Hua, T.; Qinqin, L.; Guisheng, L. Interfacial optimization of g-C3N4-base Z-scheme heterojunction toward synergistic enhancement of solar-driven photocatalytic oxygen evolution. Appl. Catal. B Environ. 2019, 244, 240–249. [Google Scholar]
- Ma, D.; Shi, J.-W.; Zou, Y.; Fan, Z.; Shi, J.; Cheng, L.; Sun, D.; Wang, Z.; Niu, C. Multiple carrier-transfer pathways in a flower-like In2S3/CdIn2S4/In2O3 ternary heterostructure for enhanced photocatalytic hydrogen production. Nanoscale 2018, 10, 7860–7870. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, R.; Gong, Y.; Li, C.; Niu, L.; Liu, X. CdIn2S4/In(OH)3/NiCr-LDH Multi-Interface Heterostructure Photocatalyst for Enhanced Photocatalytic H2 Evolution and Cr(VI) Reduction. Nanomaterials 2021, 11, 3122. https://doi.org/10.3390/nano11113122
Fu R, Gong Y, Li C, Niu L, Liu X. CdIn2S4/In(OH)3/NiCr-LDH Multi-Interface Heterostructure Photocatalyst for Enhanced Photocatalytic H2 Evolution and Cr(VI) Reduction. Nanomaterials. 2021; 11(11):3122. https://doi.org/10.3390/nano11113122
Chicago/Turabian StyleFu, Rao, Yinyan Gong, Can Li, Lengyuan Niu, and Xinjuan Liu. 2021. "CdIn2S4/In(OH)3/NiCr-LDH Multi-Interface Heterostructure Photocatalyst for Enhanced Photocatalytic H2 Evolution and Cr(VI) Reduction" Nanomaterials 11, no. 11: 3122. https://doi.org/10.3390/nano11113122





