Gold Carbide: A Predicted Nanotube Candidate from First Principle
Abstract
:1. Introduction
2. Methods
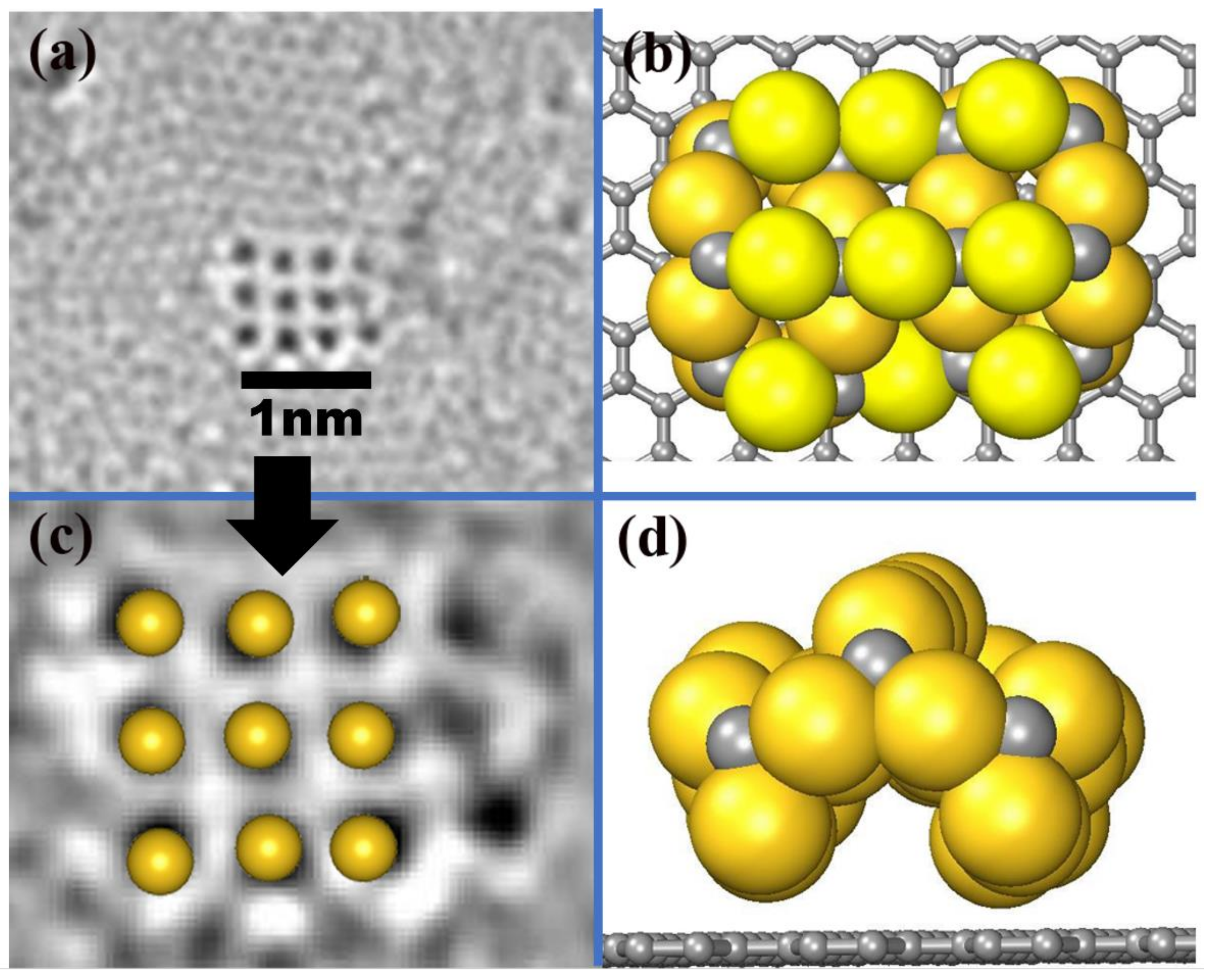
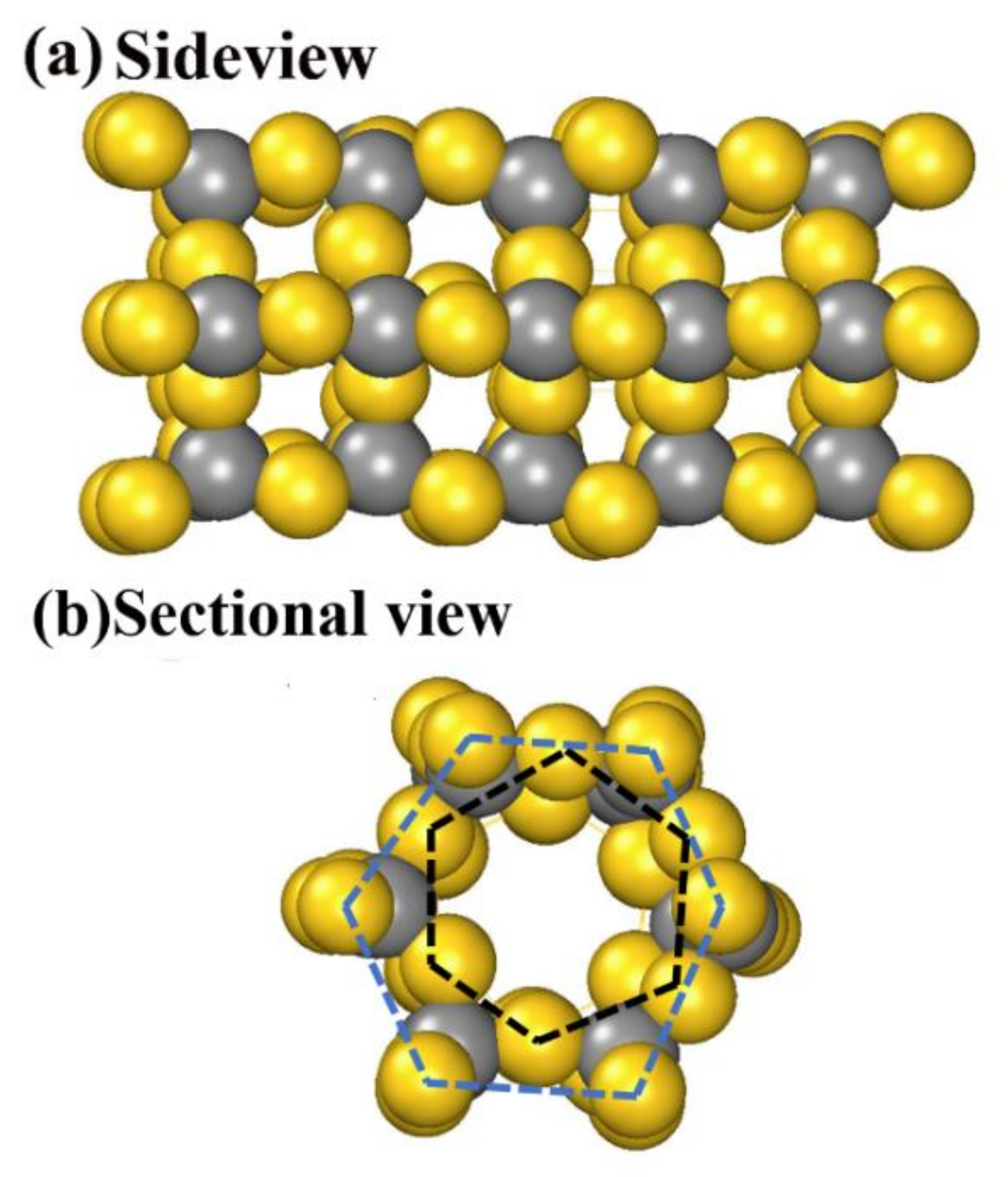
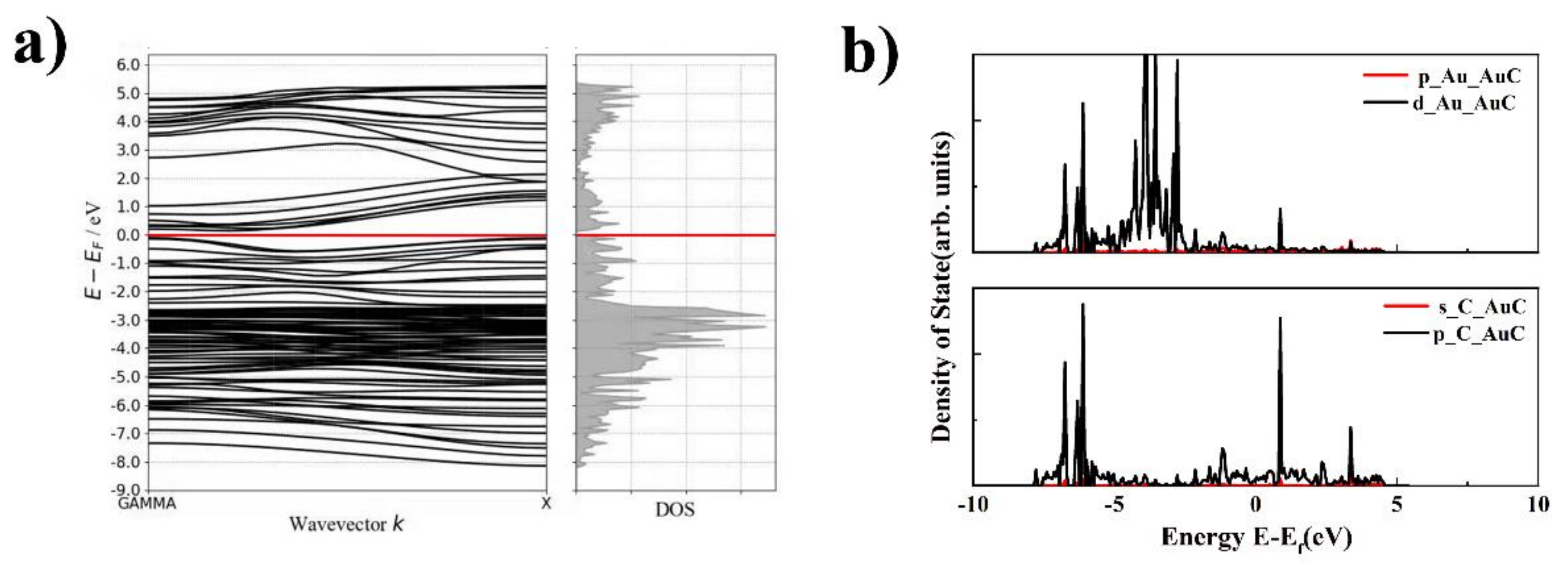
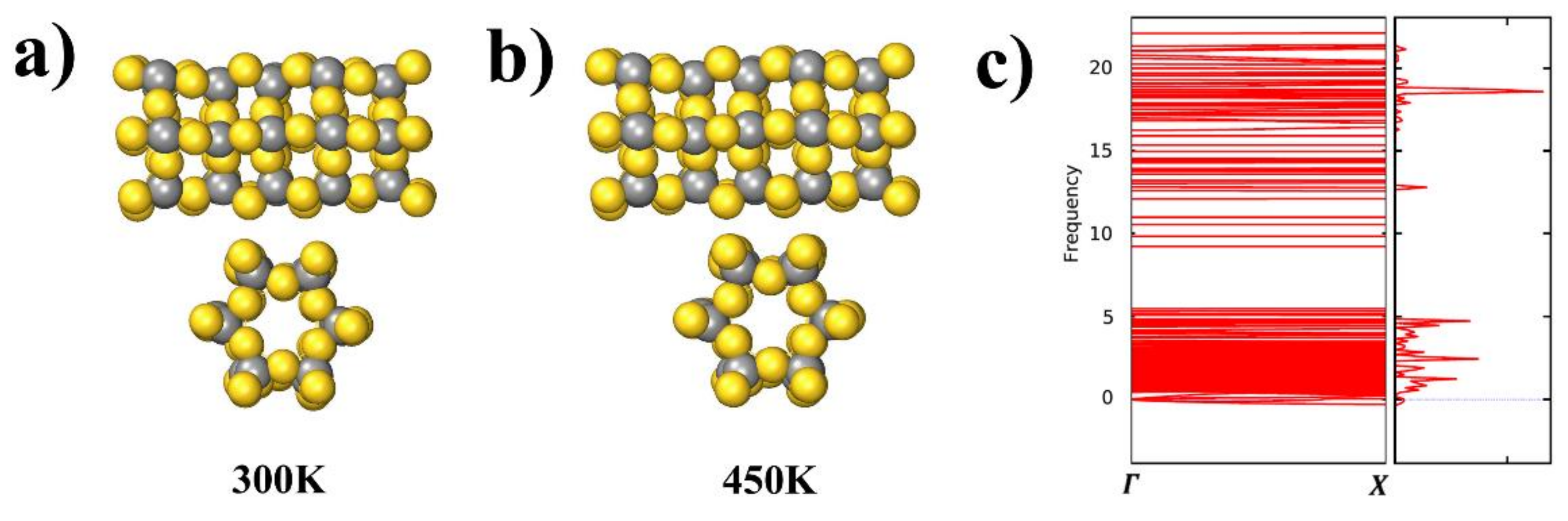
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, M.-F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and Breaking Mechanism of Multiwalled Carbon Nanotubes Under Tensile Load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef] [Green Version]
- Ruoff, R.S.; Tersoff, J.; Lorents, D.C.; Subramoney, S.; Chan, B. Radial deformation of carbon nanotubes by van der Waals forces. Nature 1993, 364, 514–516. [Google Scholar] [CrossRef]
- Palaci, I.; Fedrigo, S.; Brune, H.; Klinke, C.; Chen, M.; Riedo, E. Radial Elasticity of Multiwalled Carbon Nanotubes. Phys. Rev. Lett. 2005, 94, 175502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, I. Carbon nanotubes-properties and applications: A review. Carbon Lett. 2013, 14, 131–144. [Google Scholar]
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.W.; Ghaemi, H.F.; Thio, T. Electrical conductivity of individual carbon nanotubes. Nat. Cell Biol. 1996, 382, 54–56. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature 1996, 381, 678–680. [Google Scholar] [CrossRef]
- Chang, T.E.; Jensen, L.R.; Kisliuk, A.; Pipes, R.B.; Pyrz, R.; Sokolov, A.P. Microscopic mechanism of reinforcement in single-wall carbon nanotube/polypropylene nano-composite. Polymer 2005, 46, 439–444. [Google Scholar] [CrossRef]
- Jin, F.-L.; Park, S.-J. Recent Advances in Carbon-Nanotube-Based Epoxy Composites. Carbon Lett. 2013, 14, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wepasnick, K.A.; Smith, B.A.; Bitter, J.L.; Fairbrother, D.H. Chemical and structural characterization of carbon nanotube surfaces. Anal. Bioanal. Chem. 2010, 396, 1003–1014. [Google Scholar] [CrossRef]
- Mathews, A.J.; Watters, L.L. The Carbide of Gold. J. Am. Chem. Soc. 1900, 22, 108–111. [Google Scholar] [CrossRef] [Green Version]
- Feuerstein, T.J.; Poß, M.; Seifert, T.P.; Bestgen, S.; Feldmann, C.; Roesky, P.W. A highly luminescent octanuclear gold(i) carbide cluster. Chem. Commun. 2017, 53, 9012–9015. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.C.; Rodríguez, L. Applications of gold(i) alkynyl systems: A growing field to explore. Chem. Soc. Rev. 2011, 40, 5442–5456. [Google Scholar] [CrossRef] [PubMed]
- Che, C.M.; Chao, H.Y.; Miskowski, V.M.; Li, Y.; Cheung, K.K. Luminescent mu-ethynediyl and mu-butadiynediyl binuclear gold(I) complexes: Observation of (3)(pi pi*) emissions from bridging C(n)(2-) units. J. Am. Chem. Soc. 2001, 123, 4985–4991. [Google Scholar] [CrossRef]
- McArdle, C.P.; Van, S.; Jennings, M.C.; Puddephatt, R.J. Gold(I) Macrocycles and Topologically Chiral [2]Catenanes. J. Am. Chem. Soc. 2002, 124, 3959–3965. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, A. Gold in Chains: Self-Assembly of a Gold(I) Catenane. Angew. Chem. Int. Ed. 1995, 34, 2107–2109. [Google Scholar] [CrossRef]
- Cohen, Y.; Bernshtein, V.; Armon, E.; Bekkerman, A.; Kolodney, E. Formation and emission of gold and silver carbide cluster ions in a single C60− surface impact at keV energies: Experiment and calculations. J. Chem. Phys. 2011, 134, 124701. [Google Scholar] [CrossRef]
- Okamoto, H.; Massalski, T.B. The Au-C (Gold-Carbon) system. Bull. Alloy. Phase Diagr. 1984, 5, 378–379. [Google Scholar] [CrossRef]
- Westenfelder, B.; Biskupek, J.; Meyer, J.C.; Kurasch, S.; Lin, X.; Scholz, F.; Gross, A.; Kaiser, U. Bottom-up formation of robust gold carbide. Sci. Rep. 2015, 5, 8890–8891. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; He, K.; Robertson, A.W.; Kirkland, A.I.; Warner, J.H. Atomic Structure and Dynamics of Epitaxial 2D Crystalline Gold on Graphene at Elevated Temperatures. ACS Nano 2016, 10, 10418–10427. [Google Scholar] [CrossRef]
- Silveri, F.; Quesne, M.G.; Roldan, A.; de Leeuw, N.H.; Catlow, C.R.A. Hydrogen adsorption on transition metal carbides: A DFT study. Phys. Chem. Chem. Phys. 2019, 21, 5335–5343. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Kong, Z.; Li, N.; Zhao, X.; Sun, C. Proposing the prospects of Ti3CN transition metal carbides (MXenes) as anodes of Li-ion batteries: A DFT study. Phys. Chem. Chem. Phys. 2016, 18, 32937–32943. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, C.R.; Thompson, G.B. A computational search for the zeta phase in the tantalum carbides. J. Am. Ceram. Soc. 2018, 102, 1454–1462. [Google Scholar] [CrossRef]
- Liu, Y.; Kelly, T.G.; Chen, J.G.; Mustain, W.E. Metal Carbides as Alternative Electrocatalyst Supports. ACS Catal. 2013, 3, 1184–1194. [Google Scholar] [CrossRef] [Green Version]
- Tran, C.-C.; Mohan, O.; Banerjee, A.; Mushrif, S.H.; Kaliaguine, S. A Combined Experimental and DFT Investigation of Selective Hydrodeoxygenation of Guaiacol over Bimetallic Carbides. Energy Fuels 2020, 34, 16265–16273. [Google Scholar] [CrossRef]
- Figueras, M.; Gutiérrez, R.A.; Prats, H.; Viñes, F.; Ramírez, P.J.; Illas, F.; Rodriguez, J.A. Boosting the activity of transition metal carbides towards methane activation by nanostructuring. Phys. Chem. Chem. Phys. 2020, 22, 7110–7118. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthm, J. Uller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Lin, X.; Dasgupta, A.; Xie, F.; Schimmel, T.; Evers, F.; Groß, A. Exchange processes in the contact formation of Pb electrodes. Electrochim. Acta 2014, 140, 505–510. [Google Scholar] [CrossRef]
- Song, L.; Tian, X.; Yang, Y.; Qin, J.; Li, H.; Lin, X. Probing the Microstructure in Pure Al & Cu Melts: Theory Meets Experiment. Front. Chem. 2020, 8, 607. [Google Scholar] [CrossRef]
- Song, L.; Tian, X.; Shao, A.; Li, L.; Zhang, Y.; Li, H.; Lin, X. The structure of metallic melts in binary homogenous alloys: A thermodynamical understanding from the Wulff cluster model. Phys. Chem. Chem. Phys. 2020, 22, 23237–23245. [Google Scholar] [CrossRef]
- Song, L.; Tian, X.; Shao, A.; Hua, M.; Li, L.; Li, H.; Lin, X. The structure of metallic melts in eutectic alloys based on the Wulff cluster model: Theory meets ex-periment. Phys. Chem. Chem. Phys. 2021, 23, 3606–3614. [Google Scholar] [CrossRef] [PubMed]
- Groß, A. Theoretical Surface Science; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Song, L.; Tian, X.; Jiang, H.; Yu, W.; Zhao, Z.; Zheng, H.; Qin, J.; Lin, X. Vacancies effect on the mechanical properties in B2 FeAl intermetallic by the first-principles study. Philos. Mag. 2019, 99, 2703–2717. [Google Scholar] [CrossRef]
- Persson, K. Materials Data on Au (SG:225) by Materials Project. 2016. Available online: https://materialsproject.org/materials/mp-81/ (accessed on 1 August 2021).
- Persson, K. Materials Data on C (SG:67) by Materials Project. 2014. Available online: https://materialsproject.org/materials/mp-568286/ (accessed on 1 August 2021).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Song, L.; Shao, A.; Hua, M.; Tian, X. Gold Carbide: A Predicted Nanotube Candidate from First Principle. Nanomaterials 2021, 11, 3182. https://doi.org/10.3390/nano11123182
Lin X, Song L, Shao A, Hua M, Tian X. Gold Carbide: A Predicted Nanotube Candidate from First Principle. Nanomaterials. 2021; 11(12):3182. https://doi.org/10.3390/nano11123182
Chicago/Turabian StyleLin, Xiaohang, Lin Song, Anchen Shao, Minghao Hua, and Xuelei Tian. 2021. "Gold Carbide: A Predicted Nanotube Candidate from First Principle" Nanomaterials 11, no. 12: 3182. https://doi.org/10.3390/nano11123182






