Chemically Roughened, Sputtered Au Films with Trace-Loaded Manganese Oxide for both On-Chip and Off-Chip High Frequency Supercapacitors
Abstract
:1. Introduction
2. Experimental
2.1. HFSC Electrode Fabrication
2.1.1. Au Film Growth
2.1.2. Oxidative Etching
2.2. HFSC Device Assembly
2.3. Material Characterization and Electrochemical Measurement
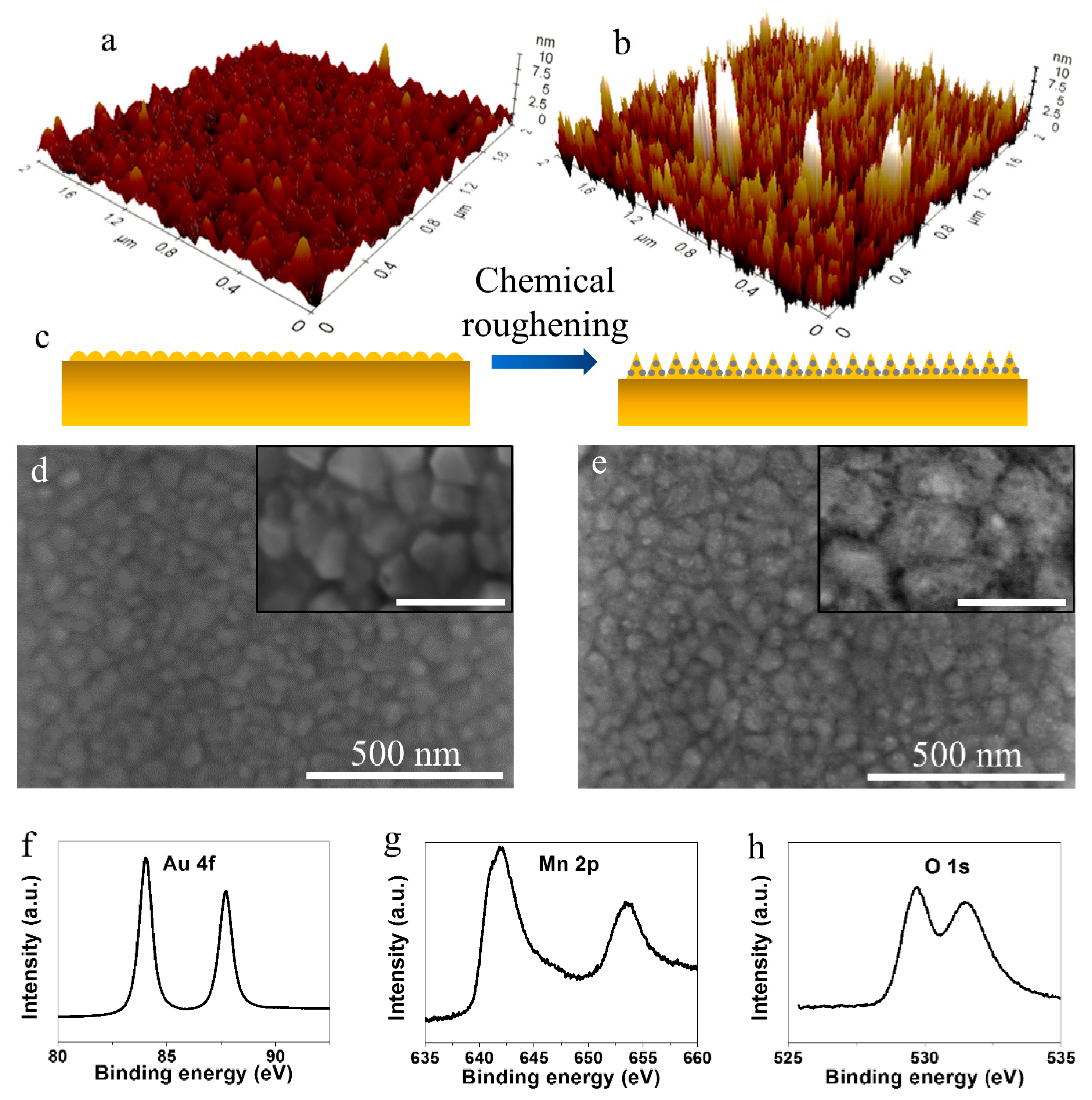
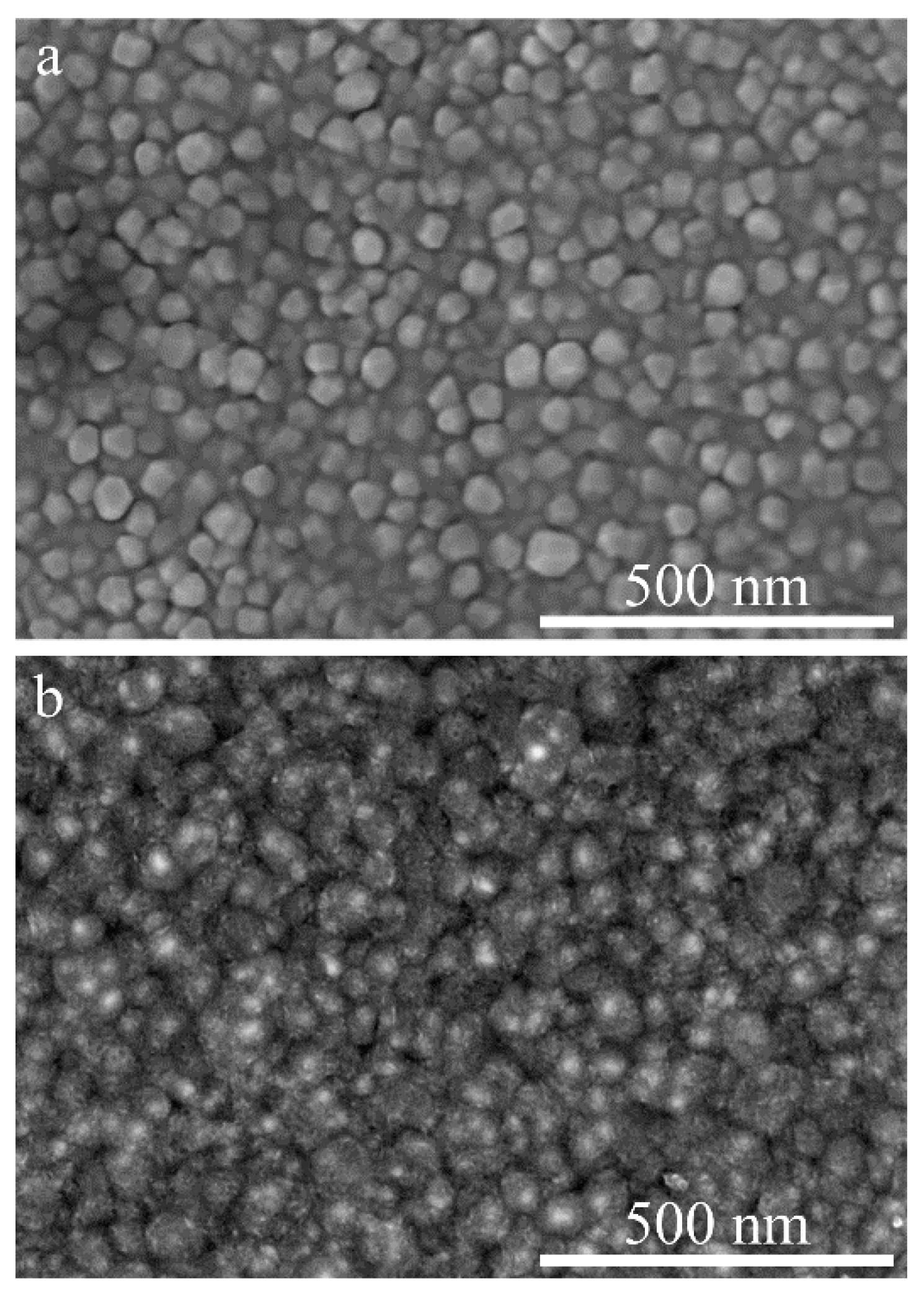
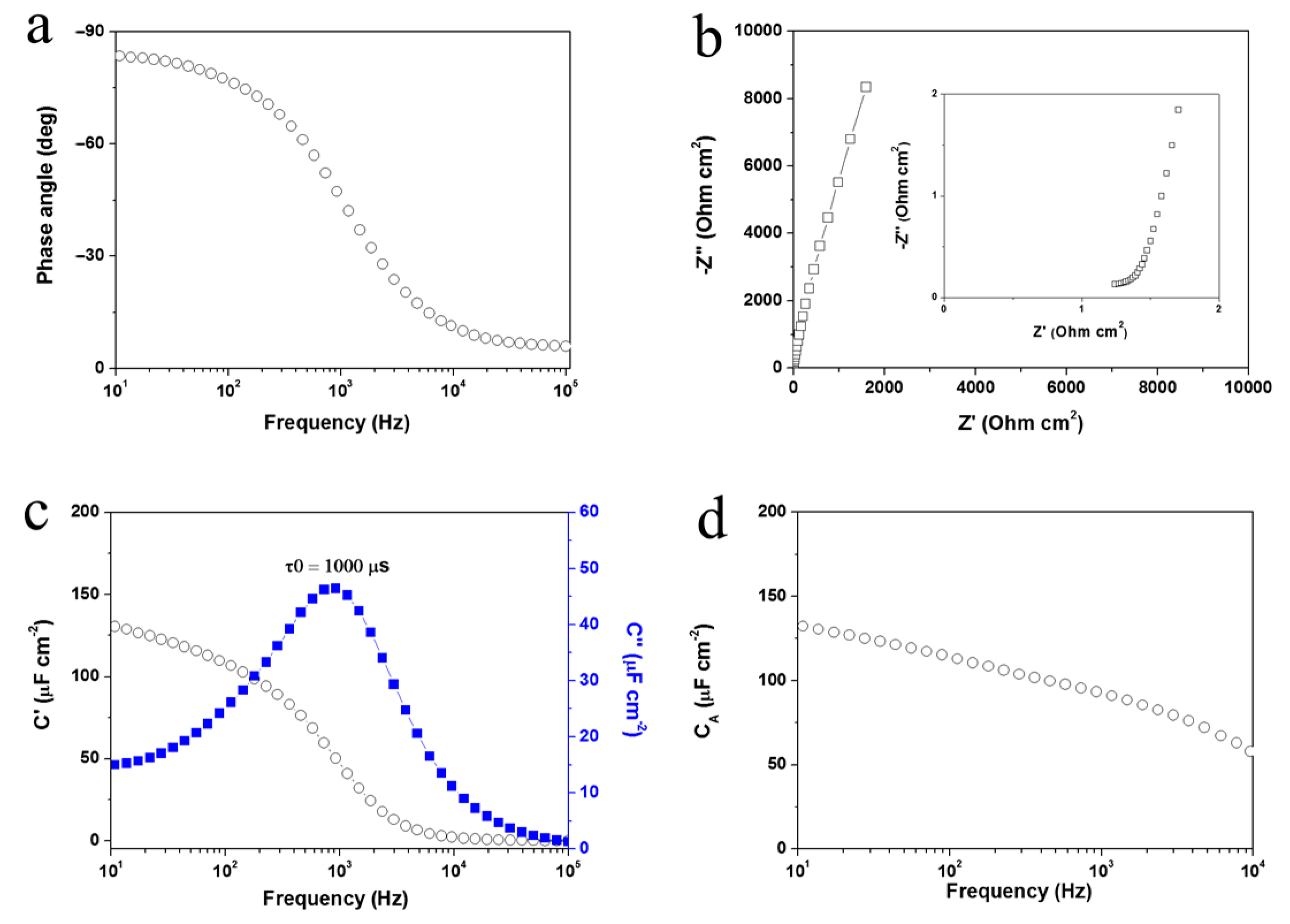
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Z.S.; Shen, J.D.; Liu, J.; Xu, X.J.; Liu, Z.B.; Hu, R.Z.; Yang, L.C.; Feng, Y.Z.; Shi, Z.C.; Ouyang, L.Z.; et al. Self-supported and flexible sulfur cathode enabled via synergistic confinement for high-energy-density lithium-sulfur batteries. Adv. Mater. 2019, 31, 1902228. [Google Scholar] [CrossRef] [PubMed]
- Si, W.P.; Yan, C.; Chen, Y.; Oswald, S.; Han, L.Y.; Schmidt, O.G. On chip, all solid-state and flexible micro-supercapacitors with high performance based on MnOx/Au multilayers. Energy Environ. Sci. 2013, 6, 3218–3223. [Google Scholar] [CrossRef]
- Pan, Z.H.; Qiu, Y.C.; Yang, J.; Ye, F.M.; Xu, Y.J.; Zhang, X.Y.; Liu, M.N.; Zhang, Y.G. Ultra-endurance flexible all-solid-state asymmetric supercapacitors based on three-dimensionally coated MnOx nanosheets on nanoporous current collectors. Nano Energy 2016, 26, 610–619. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.Y.; Islam, N.; Bayne, S.B. Towards kilohertz electrochemical capacitors for filtering and pulse energy harvesting. Nano Energy 2017, 39, 306–320. [Google Scholar] [CrossRef]
- Miller, J.R.; Outlaw, R.A.; Holloway, B.C. Graphene double-layer capacitor with ac line-filtering performance. Science 2010, 329, 1637–1639. [Google Scholar] [CrossRef]
- Islama, N.; Li, S.Q.; Ren, G.F.; Zu, Y.J.; Warzywoda, J.; Wang, S.; Fan, Z.Y. High-frequency electrochemical capacitors based on plasma pyrolyzed bacterial cellulose aerogel for current ripple filtering and pulse energy storage. Nano Energy 2017, 40, 107–114. [Google Scholar] [CrossRef]
- Zhang, C.G.; Du, H.Z.; Ma, K.; Yuan, Z.H. Ultrahigh-rate supercapacitor based on carbon nano-onion/graphene hybrid structure toward compact alternating current filter. Adv. Energy Mater. 2020, 10, 2002132. [Google Scholar] [CrossRef]
- Miller, J.R. Perspective on electrochemical capacitor energy storage. Appl. Surf. Sci. 2018, 460, 3–7. [Google Scholar] [CrossRef]
- Premathilake, D.; Outlaw, R.A.; Parler, S.G.; Butler, S.M.; Miller, J.R. Electric double layer capacitors for ac filtering made from vertically oriented graphene nanosheets on aluminum. Carbon 2017, 111, 231–237. [Google Scholar] [CrossRef]
- Rangom, Y.; Tang, X.; Nazar, L.F. Carbon nanotube-based supercapacitors with excellent ac line filtering and rate capability via improved interfacial impedance. ACS Nano 2015, 9, 7248–7255. [Google Scholar] [CrossRef]
- Zhao, M.; Nie, J.H.; Li, H.; Xia, M.Y.; Liu, M.Y.; Zhang, Z.L.; Liang, X.Q.; Qi, R.J.; Wang, Z.L.; Lu, X.M. High-frequency supercapacitors based on carbonized melamine foam as energy storage devices for triboelectric nanogenerators. Nano Energy 2019, 55, 447–453. [Google Scholar] [CrossRef]
- Wu, M.M.; Chi, F.Y.; Geng, H.Y.; Ma, H.Y.; Zhang, M.; Gao, T.T.; Li, C.; Qu, L.T. Arbitrary waveform AC line filtering applicable to hundreds of volts based on aqueous electrochemical capacitors. Nat. Commun. 2019, 10, 2855. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Kurra, N.; Maleski, K.; Lei, Y.J.; Liang, H.F.; Zhang, Y.Z.; Gogotsi, Y.; Alshareef, H.N. On-chip MXene microsupercapacitors for AC-line filtering applications. Adv. Energy Mater. 2019, 9, 1901061. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Miller, J.R.; Outlaw, R.A.; Butler, S.; Lian, K. Study of solid alkaline electrolyte under high temperatures and its application in electrochemical capacitors for AC line-filtering. J. Power Sources 2019, 417, 145–149. [Google Scholar] [CrossRef]
- Xu, S.X.; Liu, W.; Hu, B.M.; Wang, X.H. Circuit-integratable high-frequency micro supercapacitors with filter/oscillator demonstrations. Nano Energy 2019, 58, 803–810. [Google Scholar] [CrossRef]
- He, H.X.; Zhao, T.M.; Guan, H.Y.; Zhong, T.Y.; Zeng, H.; Xing, L.L.; Zhang, Y.; Xue, X.Y. A water-evaporation-induced self-charging hybrid power unit for application in the Internet of Things. Sci. Bull. 2019, 64, 1409–1417. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Mueller, L.; Hoffmann, M.; Chen, X.Y. Taper silicon nano-scaffold regulated compact integration of 1D nanocarbons for improved on-chip supercapacitor. Nano Energy 2017, 41, 618–625. [Google Scholar] [CrossRef]
- Lu, P.; Ohlckers, P.; Mueller, L.; Leopold, S.; Hoffmann, M.; Grigoras, K.; Ahopelto, J.; Prunnila, M.; Chen, X.Y. Nano fabricated silicon nanorod array with titanium nitride coating for on-chip supercapacitors. Electrochem. Commun. 2016, 70, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Monga, A.; Pal, B. Influence of oxidative etching of Au nanostructures by KMnO4 on its surface morphology. J. Ind. Eng. Chem. 2015, 31, 223–230. [Google Scholar] [CrossRef]
- Liu, J.S.; Hu, Y. Characterization of manganese oxides/Au nanocompsite thin films for supercapacitor. Int. J. Electrochem. Sci. 2013, 8, 9231–9241. [Google Scholar]
- Abdur, R.; Kim, K.; Kim, J.H.; Lee, J. Electrochemical behavior of manganese oxides on flexible substrates for thin film supercapacitors. Electrochim. Acta 2015, 153, 184–189. [Google Scholar] [CrossRef]
- Chigane, M.; Ishikawa, M. Manganese oxide thin film preparation by potentiostatic electrolyses and electrochromism. J. Electrochem. Soc. 2000, 147, 2246–2251. [Google Scholar] [CrossRef]
- Taberna, P.L.; Simon, P.; Fauvarque, J.F. Electrochemical characteristics and impedance spectroscopy studies of carbon-carbon supercapacitors. J. Electrochem. Soc. 2003, 150, A292–A300. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Taberna, P.; Simon, P.; Fabre, N.; Mesnilgrente, F.; Conedera, V.; Durou, H. Elaboration of a microstructured inkjet-printed carbon electrochemical capacitor. J. Power Sources 2010, 195, 1266–1269. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, P.; Xue, H.; Liu, W.; Feng, Z.; Sun, Q. Chemically Roughened, Sputtered Au Films with Trace-Loaded Manganese Oxide for both On-Chip and Off-Chip High Frequency Supercapacitors. Nanomaterials 2021, 11, 257. https://doi.org/10.3390/nano11020257
Lu P, Xue H, Liu W, Feng Z, Sun Q. Chemically Roughened, Sputtered Au Films with Trace-Loaded Manganese Oxide for both On-Chip and Off-Chip High Frequency Supercapacitors. Nanomaterials. 2021; 11(2):257. https://doi.org/10.3390/nano11020257
Chicago/Turabian StyleLu, Pai, Haitao Xue, Wentao Liu, Zhongbao Feng, and Qiang Sun. 2021. "Chemically Roughened, Sputtered Au Films with Trace-Loaded Manganese Oxide for both On-Chip and Off-Chip High Frequency Supercapacitors" Nanomaterials 11, no. 2: 257. https://doi.org/10.3390/nano11020257




