Effects of Electrolyte on Laser-Induced Periodic Surface Structures with Picosecond Laser Pulses
Abstract
:1. Introduction
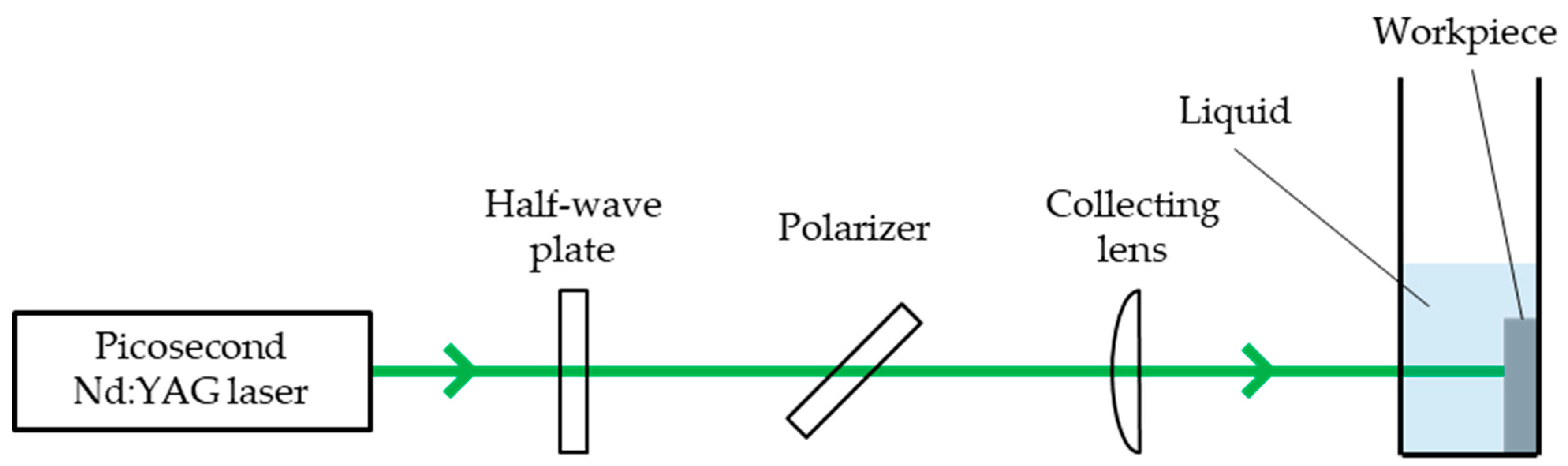
2. Experiment
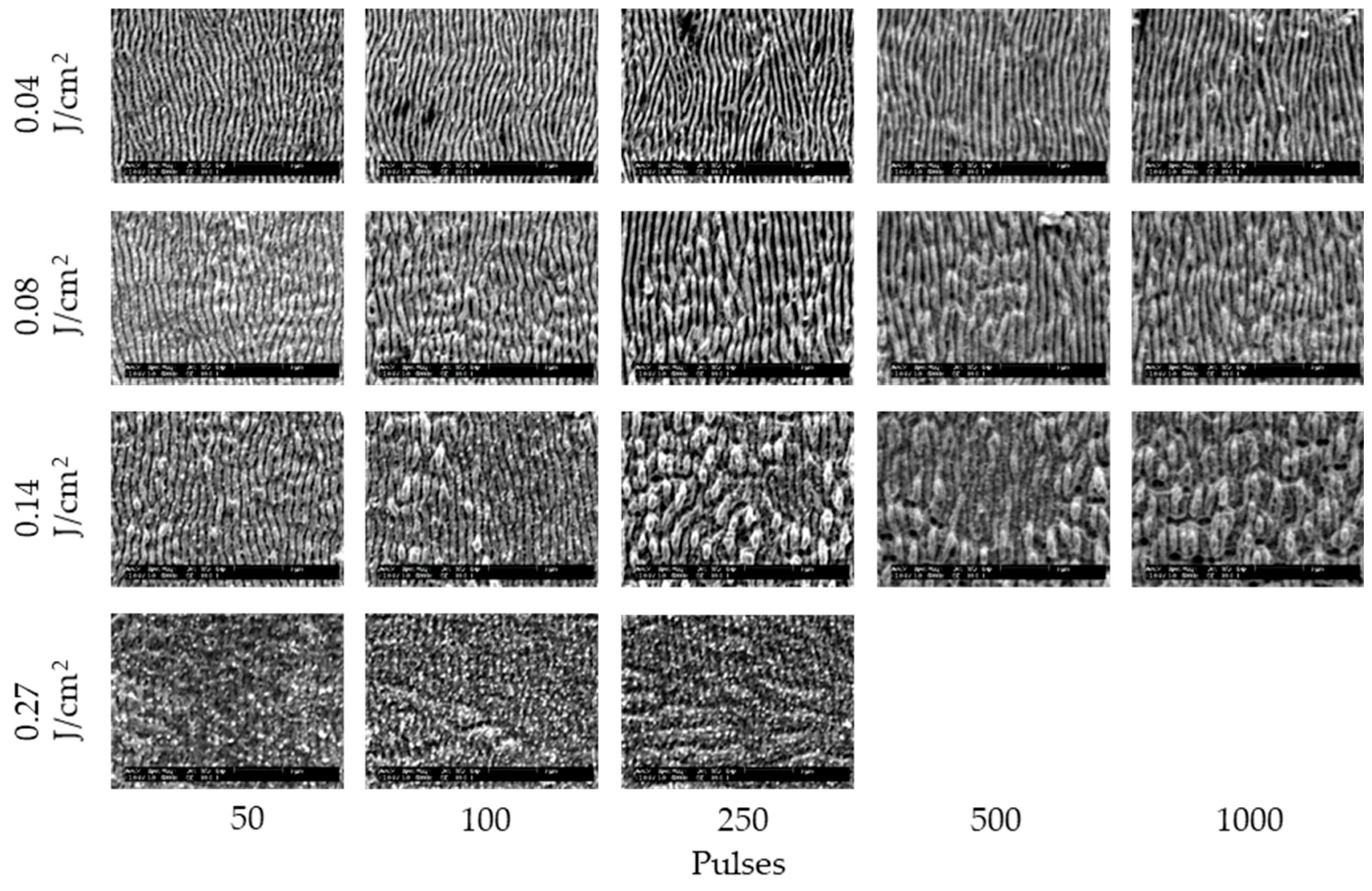
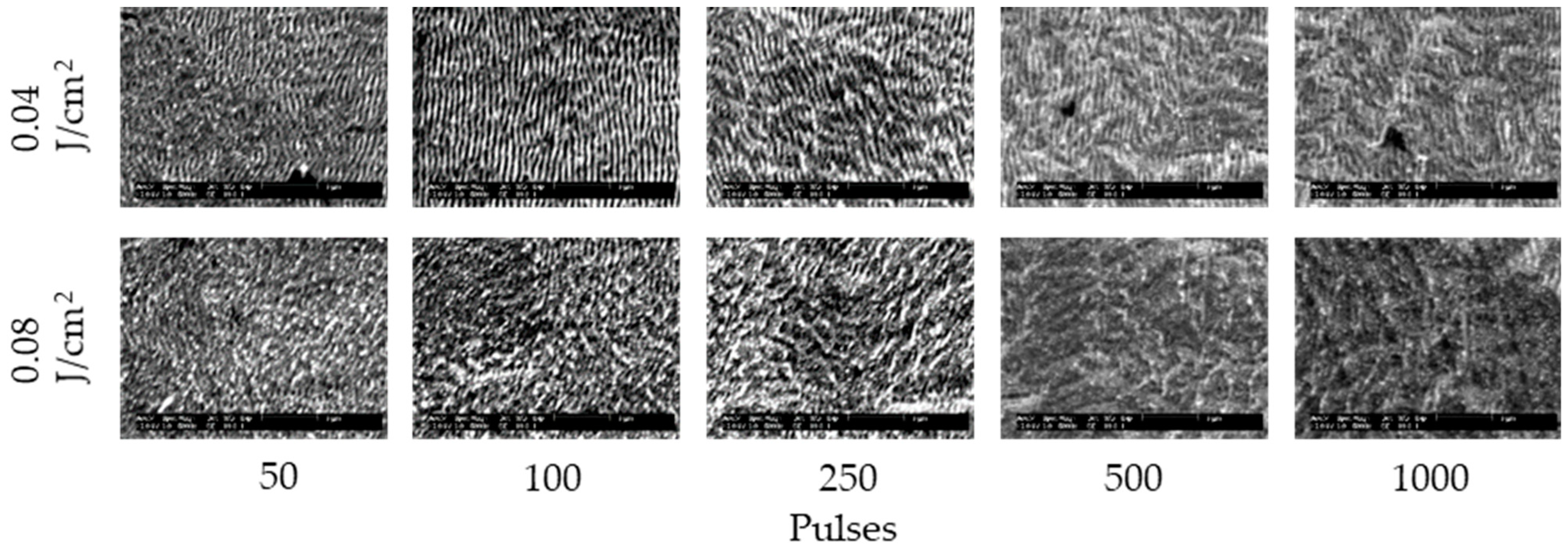
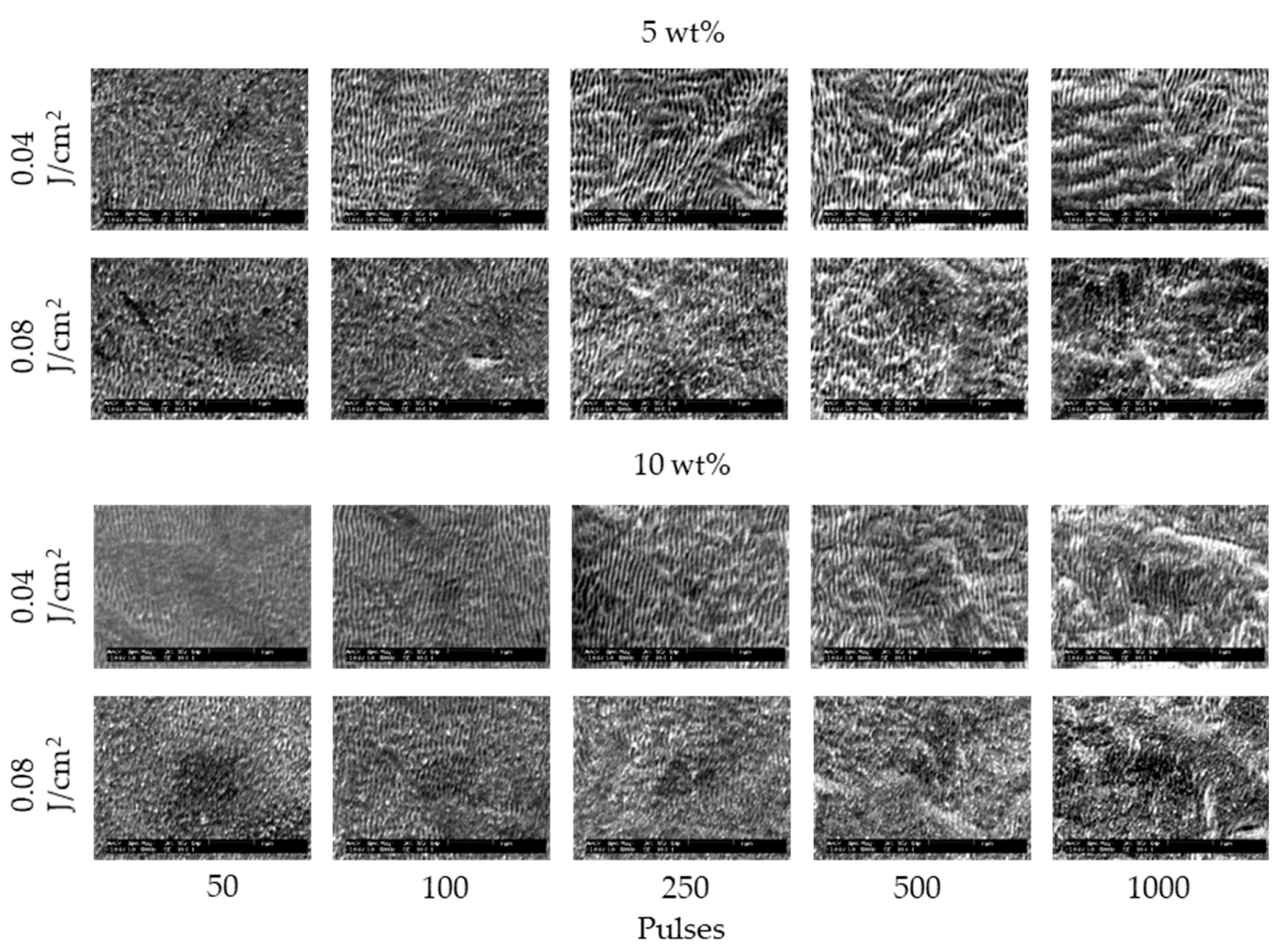
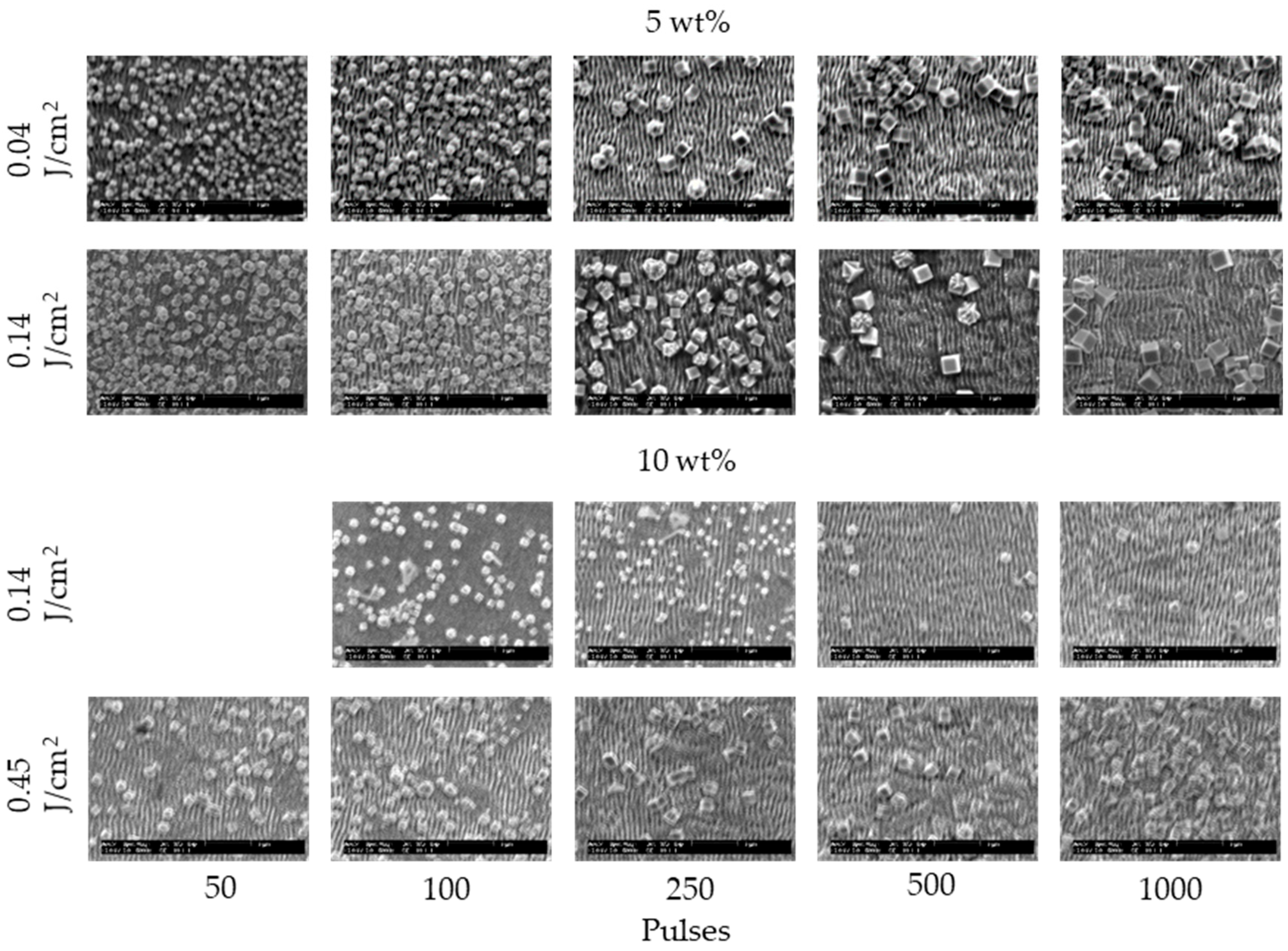
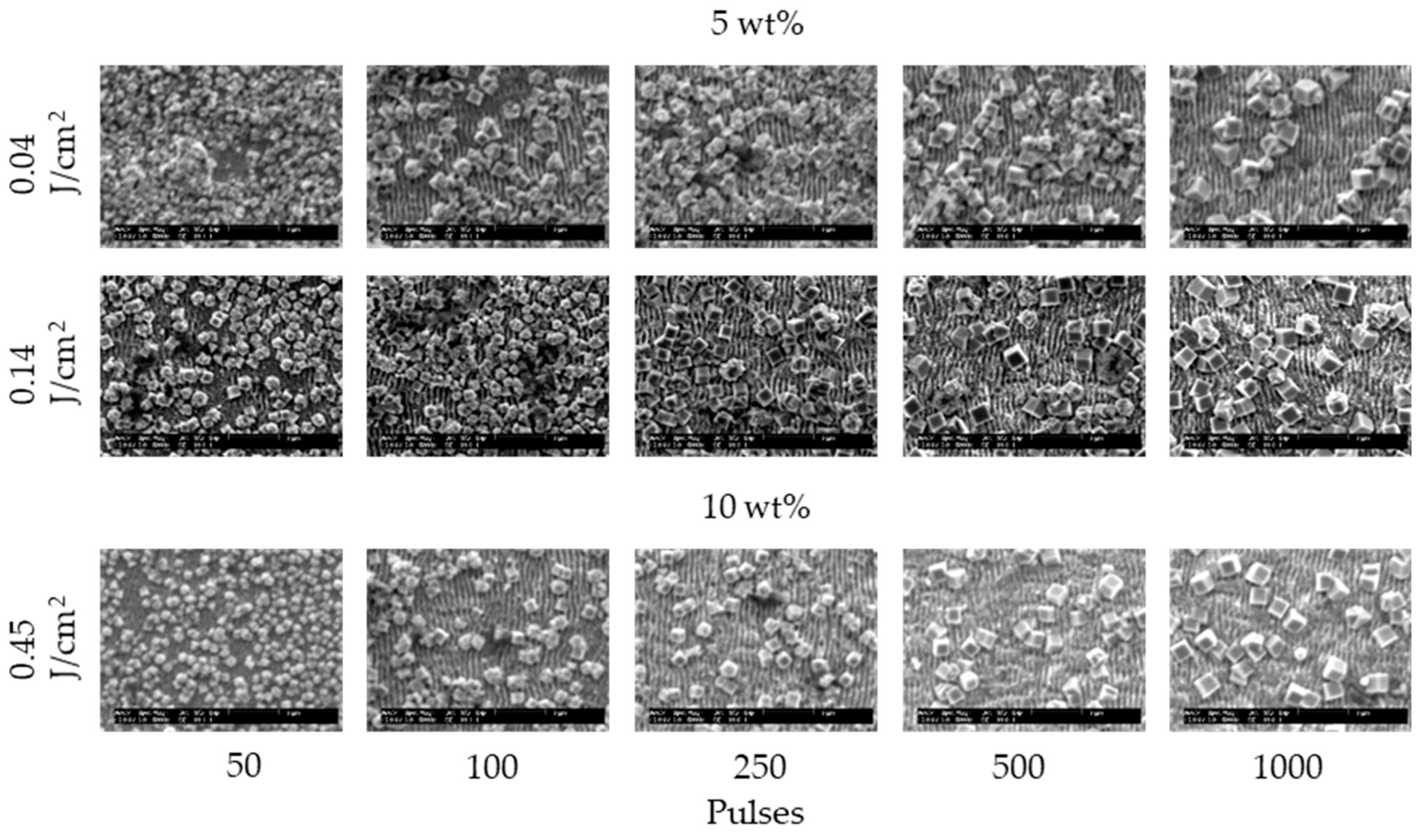
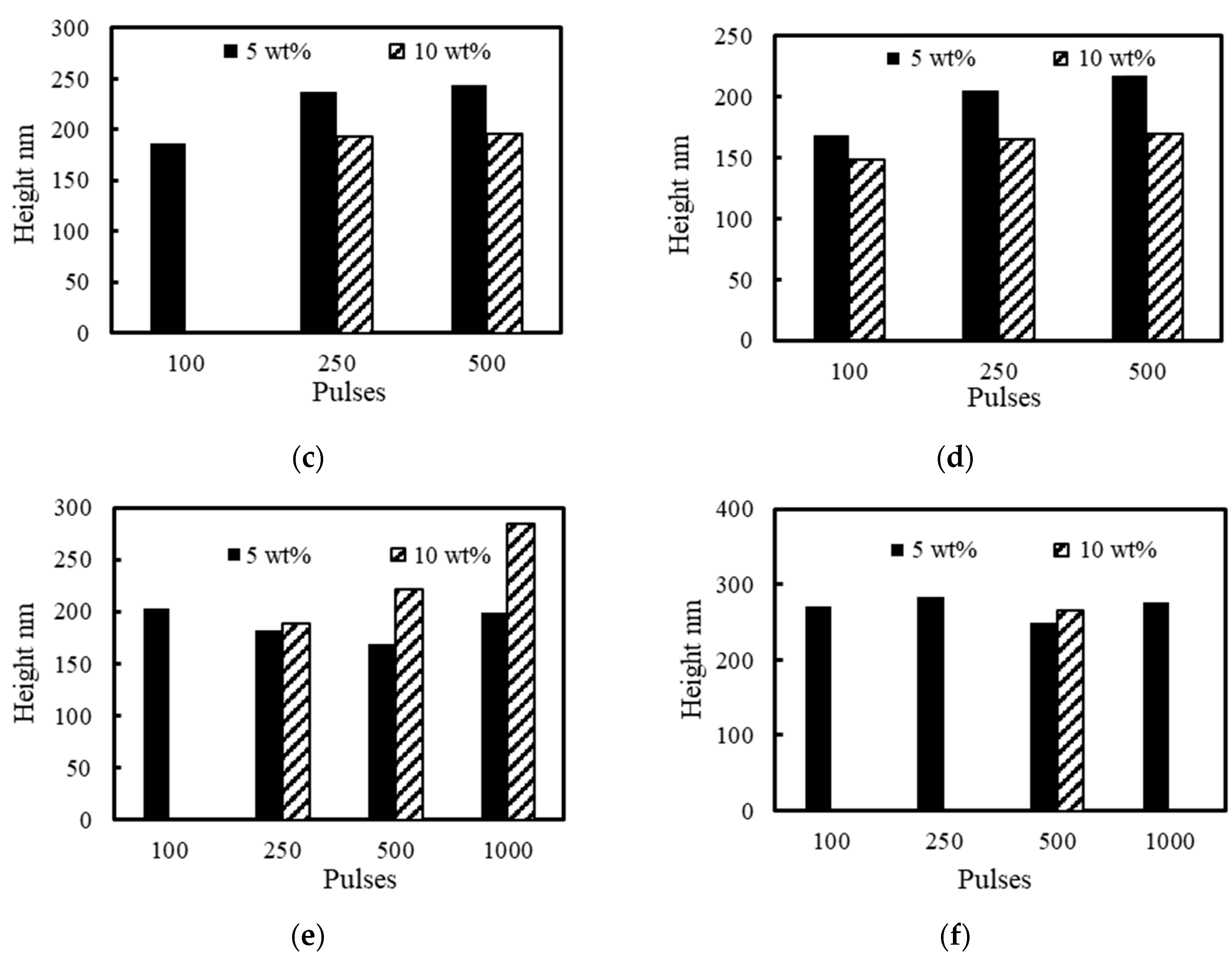
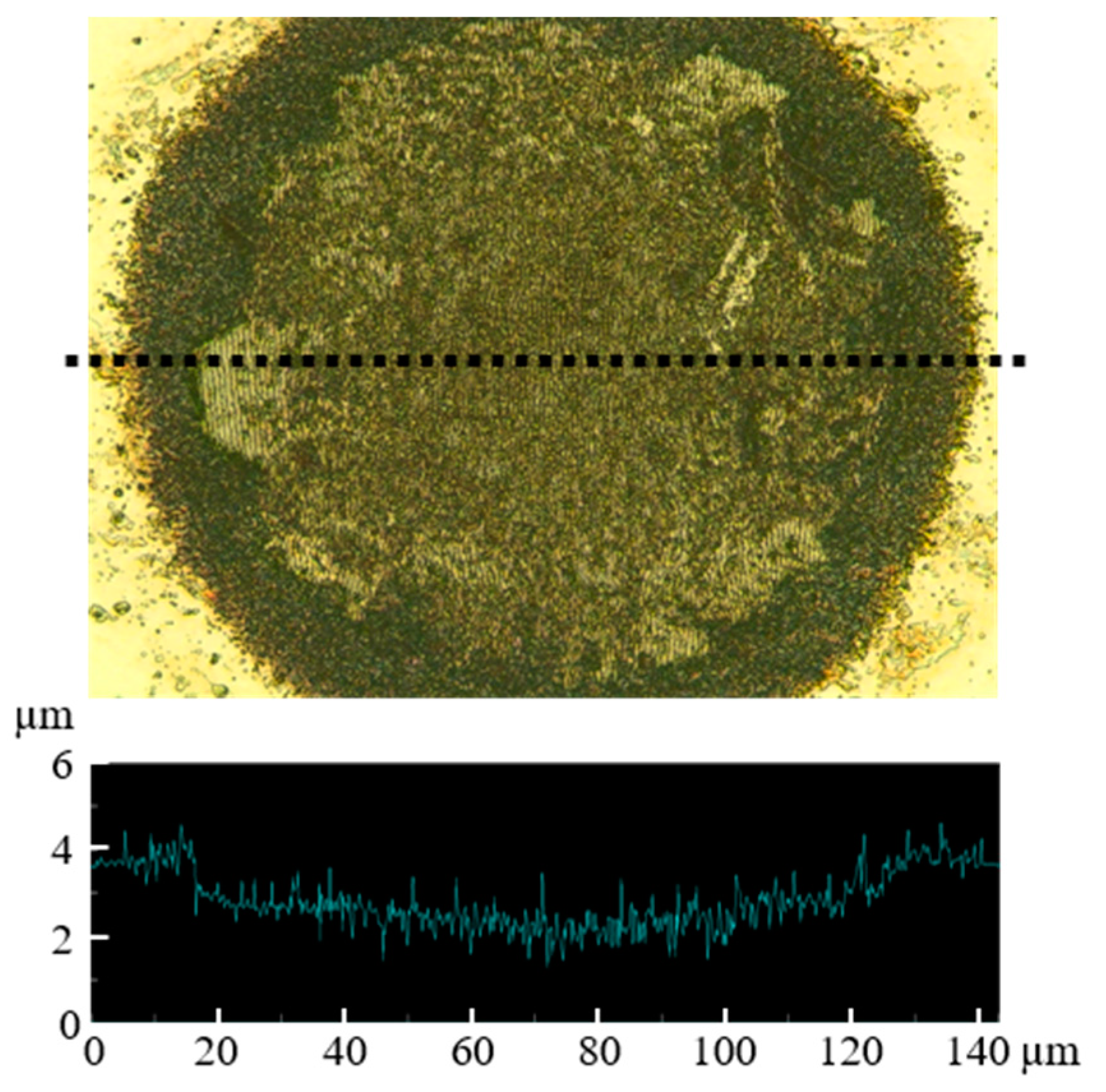
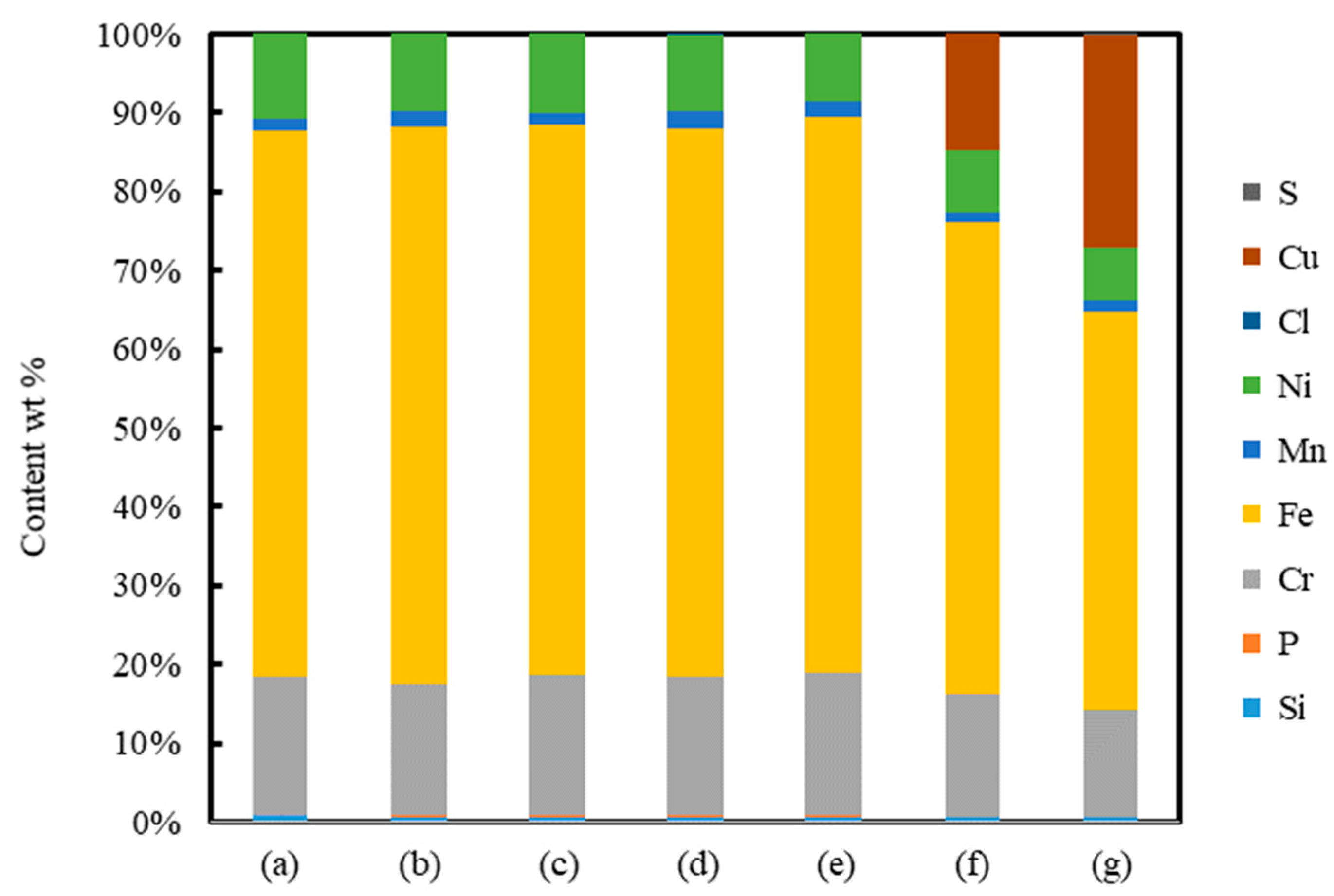
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, E.S.; Kim, S.M.; Lee, Y.Z. The effect of plateau honing on the friction and wear of cylinder liners. Wear 2018, 400, 207–212. [Google Scholar] [CrossRef]
- Ji, M.; Xu, J.; Chen, M.; El Mansori, M. Enhanced hydrophilicity and tribological behavior of dental zirconia ceramics based on picosecond laser surface texturing. Ceram. Int. 2020, 46, 7161–7169. [Google Scholar] [CrossRef]
- Shamsudin, S.; Ahmad, M.K.; Aziz, A.N.; Fakhriah, R.; Mohamad, F.; Ahmad, N.; Nafarizal, N.; Soon, C.F.; Ameruddin, A.S.; Faridah2, A.B.; et al. Hydrophobic rutile phase TiO2 nanostructure and its properties for self-cleaning application. AIP Conf. Proc. 2017, 1883, 1–9. [Google Scholar]
- Wang, H.; Zhuang, J.; Yu, J.; Qi, H.; Ma, Y.; Wang, H.; Guo, Z. Fabrication of Anti-Reflective Surface with Superhydrophobicity/High Oleophobicity and Enhanced Mechanical Durability via Nanosecond Laser Surface Texturing. Materials 2020, 13, 5691. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, W.; Tao, T.; Pan, A.; Mei, X. Multi-scale micro-nano structures prepared by laser cleaning assisted laser ablation for broadband ultralow reflectivity silicon surfaces in ambient air. Appl. Surf. Sci. 2020, 509, 145182. [Google Scholar] [CrossRef]
- Samanta, A.; Wang, Q.; Singh, G.; Shaw, S.K.; Toor, F.; Ratner, A.; Ding, H. Nanosecond pulsed laser processing turns engineering metal alloys antireflective and superwicking. J. Manuf. Process. 2020, 54, 28–37. [Google Scholar] [CrossRef]
- Elena Sima, L.; Bonciu, A.; Baciu, M.; Anghel, I.; Dumitrescu, L.N.; Rusen, L.; Dinca, V. Bioinstructive Micro-Nanotextured Zirconia Ceramic Interfaces for Guiding and Stimulating an Osteogenic Response In Vitro. Nanomaterials 2020, 10, 2465. [Google Scholar] [CrossRef]
- Pawar, D.R.L.; Jeyapalina, S.; Bachus, K.N. Evaluation of soft-tissue response around laser microgrooved titanium percutaneous devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2031–2040. [Google Scholar] [CrossRef]
- Gnilitskyi, I.; Rota, A.; Gualtieri, E.; Valeri, S.; Orazi, L. Tribological Properties of High-Speed Uniform Femtosecond Laser Patterning on Stainless Steel. Lubricants 2019, 7, 83. [Google Scholar] [CrossRef] [Green Version]
- Murzin, S.P.; Balyakin, V.B.; Liedl, G.; Melnikov, A.A.; Fürbacher, R. Improving Tribological Properties of Stainless Steel Surfaces by Femtosecond Laser Irradiation. Coatings 2020, 10, 606. [Google Scholar] [CrossRef]
- Chu, D.K.; Yin, K.; Dong, X.R.; Luo, Z.; Duan, J.A. Femtosecond laser fabrication of robust underwater superoleophobic and anti-oil surface on sapphire. Aip Adv. 2017, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Yan, J. Generating Nanodot Structures on Stainless-Steel Surfaces by Cross Scanning of a Picosecond Pulsed Laser. Nanomanuf. Metrol. 2020, 3, 105–111. [Google Scholar] [CrossRef]
- Žemaitis, A.; Mimidis, A.; Papadopoulos, A.; Gečys, P.; Račiukaitis, G.; Stratakis, E.; Gedvilas, M. Controlling the wettability of stainless steel from highly-hydrophilic to super-hydrophobic by femtosecond laser-induced ripples and nanospikes. RSC Adv. 2020, 10, 37956–37961. [Google Scholar] [CrossRef]
- Lee, K.; Ki, H. Femtosecond laser patterning based on the control of surface reflectance. Appl. Surf. Sci. 2019, 494, 187–195. [Google Scholar] [CrossRef]
- Nigo, F.; Hashida, M.; Tsukamoto, M.; Sakabe, S.; Kusaba, M. Reflectance and crystallinity of silicon solar cells with LIPSS produced by XeCl excimer laser pulses. Appl. Phys. A 2020, 126, 129. [Google Scholar] [CrossRef]
- Shinonaga, T.; Tsukamoto, M. Creation of New Functional Biomaterials by Periodic Nanostructures Formation with Femtosecond Laser. J. Jpn Soc. Precis. Eng. 2015, 81, 726–730. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Yao, S.; Zhang, H.; Cai, M.; Liu, W.; Pan, R.; Chen, C.; Wang, X.; Wang, L.; Zhong, M. Biocompatible nano-ripples structured surfaces induced by femtosecond laser to rebel bacterial colonization and biofilm formation. Opt. Laser Technol. 2020, 124, 105973. [Google Scholar] [CrossRef]
- Okamuro, K.; Hashida, M.; Miyasaka, Y.; Ikuta, Y.; Tokita, S.; Sakabe, S. Laser fluence dependence of periodic grating structures formed on metal surfaces under femtosecond laser pulse irradiation. Phys. Rev. B 2010, 82, 165417. [Google Scholar] [CrossRef] [Green Version]
- Maruo, S.; Nakamura, O.; Kawata, S. Evanescent-wave holography: By use of surface-plasmon resonance. Appl. Opt. 1997, 36, 2343–2346. [Google Scholar] [CrossRef]
- Sakabe, S.; Hashida, M.; Tokita, S.; Namba, S.; Okamuro, K. Mechanism for self-formation of periodic grating structures on a metal surface by a femtosecond laser pulse. Phys. Rev. B 2009, 79, 4. [Google Scholar] [CrossRef] [Green Version]
- Gemini, L.; Hashida, M.; Miyasaka, Y.; Inoue, S.; Limpouch, J.; Mocek, T.; Sakabe, S. Periodic surface structures on titanium self-organized upon double femtosecond pulse exposures. Appl. Surf. Sci. 2015, 336, 349–353. [Google Scholar] [CrossRef]
- Kodama, S.; Yamaguchi, H.; Shimada, K.; Mizutani, M.; Kuriyagawa, T. Control of short-pulsed laser induced periodic surface structures with machining—Picosecond laser nanotexturing with magnetic abrasive finishing. Precis. Eng. J. Int. Soc. Precis. Eng. Nanotechnol. 2019, 60, 428–436. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Zhang, A. Electrochemical dissolution behavior of Ti-45Al-2Mn-2Nb+0.8 vol% TiB2 XD alloy in NaCl and NaNO3 solutions. Corros. Sci. 2019, 157, 357–369. [Google Scholar] [CrossRef]
- Ayyappan, S.; Sivakumar, K. Investigation of electrochemical machining characteristics of 20MnCr5 alloy steel using potassium dichromate mixed aqueous NaCl electrolyte and optimization of process parameters. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2015, 229, 1984–1996. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.W.; Wang, J.M. Investigation of a novel hybrid process of laser drilling assisted with jet electrochemical machining. Opt. Lasers Eng. 2009, 47, 1242–1249. [Google Scholar] [CrossRef]
- Long, Y.H.; Shi, T.L.; Xiong, L.C. Excimer laser electrochemical etching n-Si in the KOH solution. Opt. Lasers Eng. 2010, 48, 570–574. [Google Scholar] [CrossRef]
- De Silva, A.K.M.; Pajak, P.T.; McGeough, J.A.; Harrison, D.K. Thermal effects in laser assisted jet electrochemical machining. Cirp Ann. Manuf. Technol. 2011, 60, 243–246. [Google Scholar] [CrossRef]
- Long, Y.H.; Li, Q.Y.; Zhong, Z.X.; Xiong, L.C.; Shi, T.L. Experimental study on the processes of laser-enhanced electrochemical micromachining stainless steel. Optik 2015, 126, 1826–1829. [Google Scholar] [CrossRef]
- Duan, W.; Mei, X.; Fan, Z.; Li, J.; Wang, K.; Zhang, Y. Electrochemical corrosion assisted laser drilling of micro-hole without recast layer. Optik 2020, 202, 163577. [Google Scholar] [CrossRef]
- Saxena, K.K.; Qian, J.; Reynaerts, D. A tool-based hybrid laser-electrochemical micromachining process: Experimental investigations and synergistic effects. Int. J. Mach. Tools Manuf. 2020, 155, 103569. [Google Scholar] [CrossRef]
- Kobayashi, T.; Wakabayashi, T.; Takushima, Y.; Yan, J.W. Formation behavior of laser-induced periodic surface structures on stainless tool steel in various media. Precis. Eng. J. Int. Soc. Precis. Eng. Nanotechnol. 2019, 57, 244–252. [Google Scholar] [CrossRef]
- Sallee, N.; Cromer, M.; Vittori, O. Electroplating of Copper on Aluminum with Direct and Pulsed Currents. Can. Metall. Q. 1994, 33, 155–162. [Google Scholar] [CrossRef]
- Behera, A.K.; Mallik, A. Ultrasound assisted electroplating of nano-composite thin film of Cu matrix with electrochemically in-house synthesized few layer graphene nano-sheets as reinforcement. J. Alloys Compd. 2018, 750, 587–598. [Google Scholar] [CrossRef]
- Rosa-Ortiz, S.M.; Khorramshahi, F.; Takshi, A. Study the impact of CuSO4 and H2SO4 concentrations on lateral growth of hydrogen evolution assisted copper electroplating. J. Appl. Electrochem. 2019, 49, 1203–1210. [Google Scholar] [CrossRef]
- Saeki, I.; Harada, T.; Tanaka, I.; Ando, T.; Gan, L.; Murakami, H. Electroplating of Copper on Low Carbon Steel from Alkaline Citrate Complex Baths. ISIJ Int. 2020, 60, 2031–2037. [Google Scholar] [CrossRef]
- Ge, Y.C.; Zhu, Z.W.; Wang, D.Y. Electrochemical Dissolution Behavior of the Nickel-Based Cast Superalloy K423A in NaNO3 Solution. Electrochim. Acta 2017, 253, 379–389. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, N.S. Electrochemical Milling of TB6 Titanium Alloy in NaNO3 Solution. J. Electrochem. Soc. 2019, 166, E35–E49. [Google Scholar] [CrossRef]
- Berthe, L.; Fabbro, R.; Peyre, P.; Tollier, L.; Bartnicki, E. Shock waves from a water-confined laser-generated plasma. J. Appl. Phys. 1997, 82, 2826–2832. [Google Scholar] [CrossRef]
- Itina, T.E. On Nanoparticle Formation by Laser Ablation in Liquids. J. Phys. Chem. C 2011, 115, 5044–5048. [Google Scholar] [CrossRef]
- Wang, Q.S.; Jiang, L.; Sun, J.Y.; Pan, C.J.; Han, W.N.; Wang, G.Y.; Zhang, H.; Grigoropoulos, C.P.; Lu, Y.F. Enhancing the expansion of a plasma shockwave by crater-induced laser refocusing in femtosecond laser ablation of fused silica. Photonics Res. 2017, 5, 488–493. [Google Scholar] [CrossRef]
- Nguyen, T.T.P.; Tanabe-Yamagishi, R.; Ito, Y. Impact of liquid layer thickness on the dynamics of nano- to sub-microsecond phenomena of nanosecond pulsed laser ablation in liquid. Appl. Surf. Sci. 2019, 470, 250–258. [Google Scholar] [CrossRef]
- Simakin, A.V.; Voronov, V.V.; Shafeev, G.A. Nanoparticle formation during laser ablation of solids in liquids. Phys. Wave Phenom. 2007, 15, 218–240. [Google Scholar] [CrossRef]
- Sasaki, K. Temporally-Resolved Measurements on Growth Processes of Nanoparticles in Laser Ablation. Rev. Laser Eng. 2010, 38, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Soliman, W.; Takada, N.; Sasaki, K. Growth Processes of Nanoparticles in Liquid-Phase Laser Ablation Studied by Laser-Light Scattering. Appl. Phys. Express 2010, 3, 3. [Google Scholar] [CrossRef]
- Bashkatov, A.; Genina, E. Water refractive index in dependence on temperature and wavelength: A simple approximation. Proc. SPIE Int. Soc. Opt. Eng. 2003, 5068. [Google Scholar] [CrossRef]
- Otipka, P.; Vlček, J.; Lesnak, M.; Talík, A. Designing of refractive index of NaCl solution using SPR. Proc. SPIE 2008, 4, 374–379. [Google Scholar] [CrossRef]
- Fujiwara, S.; Nishimoto, Y.; Arakawa, F. Fluctuation of Refractive Index of Aqueous Sodium Chloride Solution and Oxygen Effect. Anal. Sci. 1985, 1, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Graber, T.A.; Taboada, M.E.; Cartón, A.; Bolado, S. Liquid−Liquid Equilibrium of the Poly (ethylene glycol) + Sodium Nitrate + Water System at 298.15 K. J. Chem. Eng. Data 2000, 45, 182–184. [Google Scholar] [CrossRef]
- Mamyrbekova, A.K. Concentration dependences of the density, viscosity, and refraction index of Cu(NO3)2 3H2O solutions in DMSO at 298 K. Russ. J. Phys. Chem. A 2013, 87, 414–417. [Google Scholar] [CrossRef]
- Wani, N.V.; Biradar, U.V.; Dongarge, S.M. Refractive Index of Copper Sulfate Pentahydrate from Aqueous Solution. Int. J. Phys. Appl. 2017, 9, 11–18. [Google Scholar]
- Kodama, S.; Shimada, K.; Mizutani, M.; Kuriyagawa, T. Effects of Pulse Duration and Heat on Laser-Induced Periodic Surface Structures. Int. J. Autom. Technol. 2020, 14, 552–559. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodama, S.; Natsu, W. Effects of Electrolyte on Laser-Induced Periodic Surface Structures with Picosecond Laser Pulses. Nanomaterials 2021, 11, 327. https://doi.org/10.3390/nano11020327
Kodama S, Natsu W. Effects of Electrolyte on Laser-Induced Periodic Surface Structures with Picosecond Laser Pulses. Nanomaterials. 2021; 11(2):327. https://doi.org/10.3390/nano11020327
Chicago/Turabian StyleKodama, Shuhei, and Wataru Natsu. 2021. "Effects of Electrolyte on Laser-Induced Periodic Surface Structures with Picosecond Laser Pulses" Nanomaterials 11, no. 2: 327. https://doi.org/10.3390/nano11020327






