Full-Scale Pore Structure Characteristics and the Main Controlling Factors of Mesoproterozoic Xiamaling Shale in Zhangjiakou, Hebei, China
Abstract
:1. Introduction
2. Geological Setting
3. Samples and Experiments
3.1. Basic Geochemistry and Mineralogy
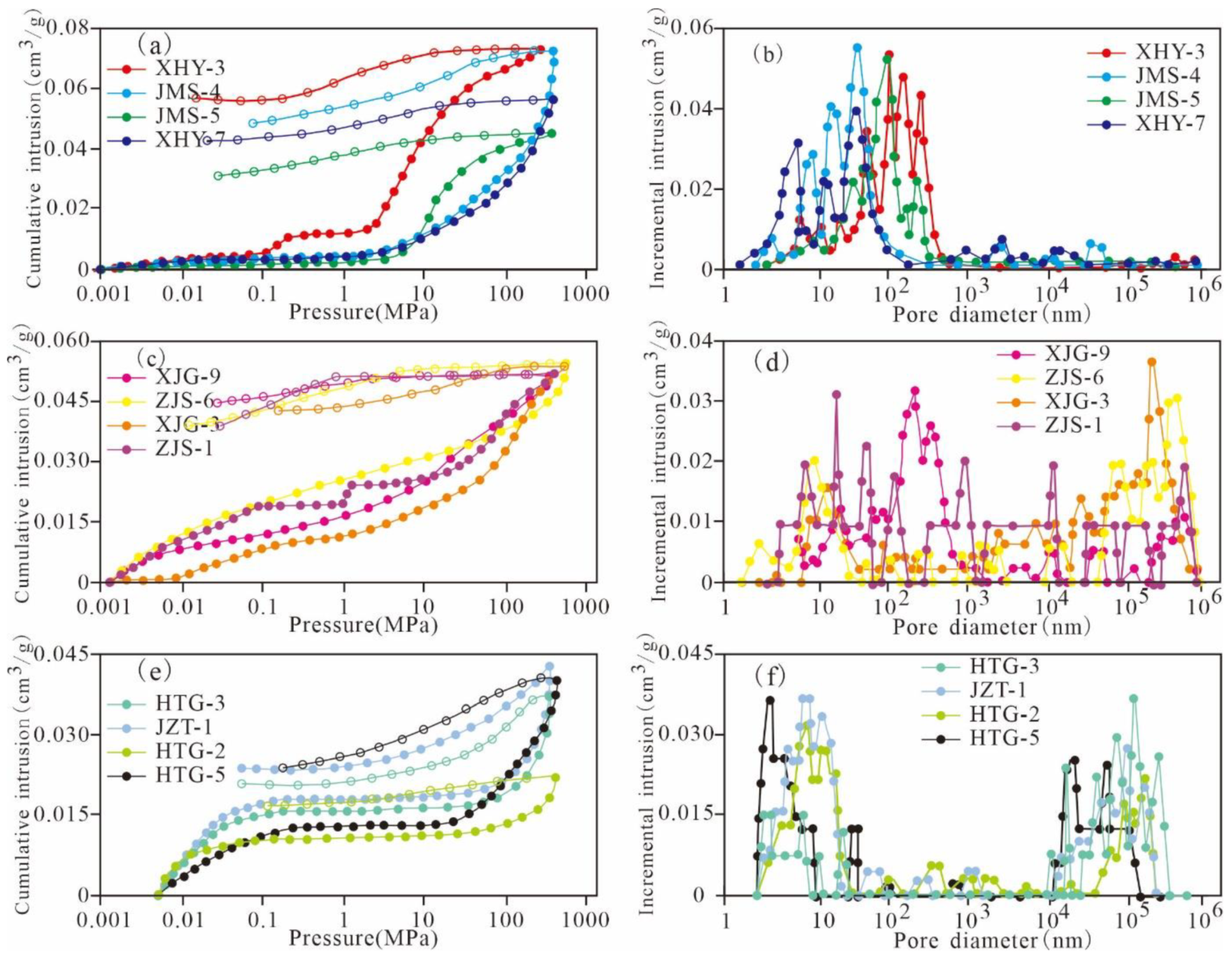
3.2. Mercury Injection Porosimetry
3.3. Residual Hydrocarbon Extraction
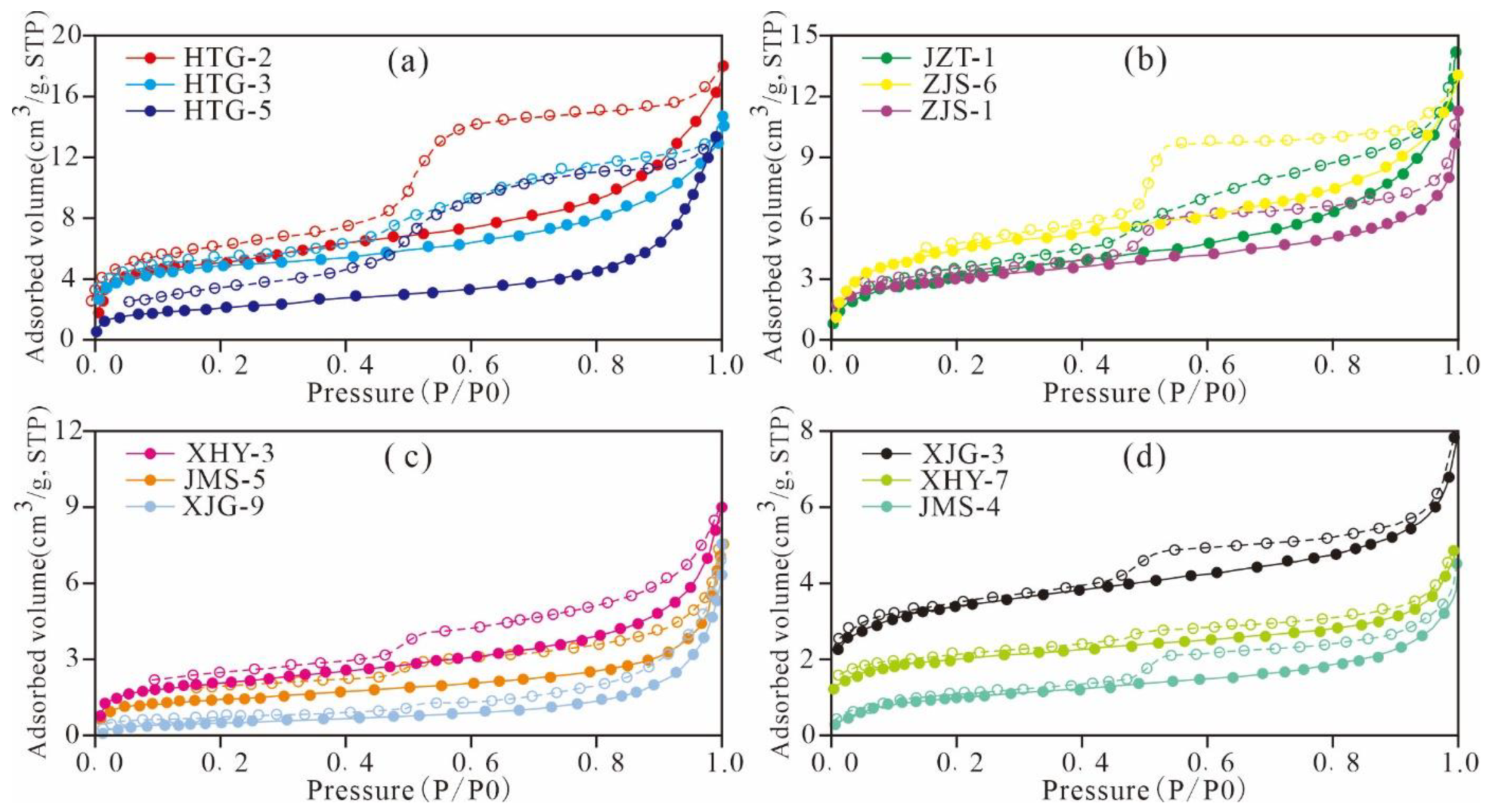
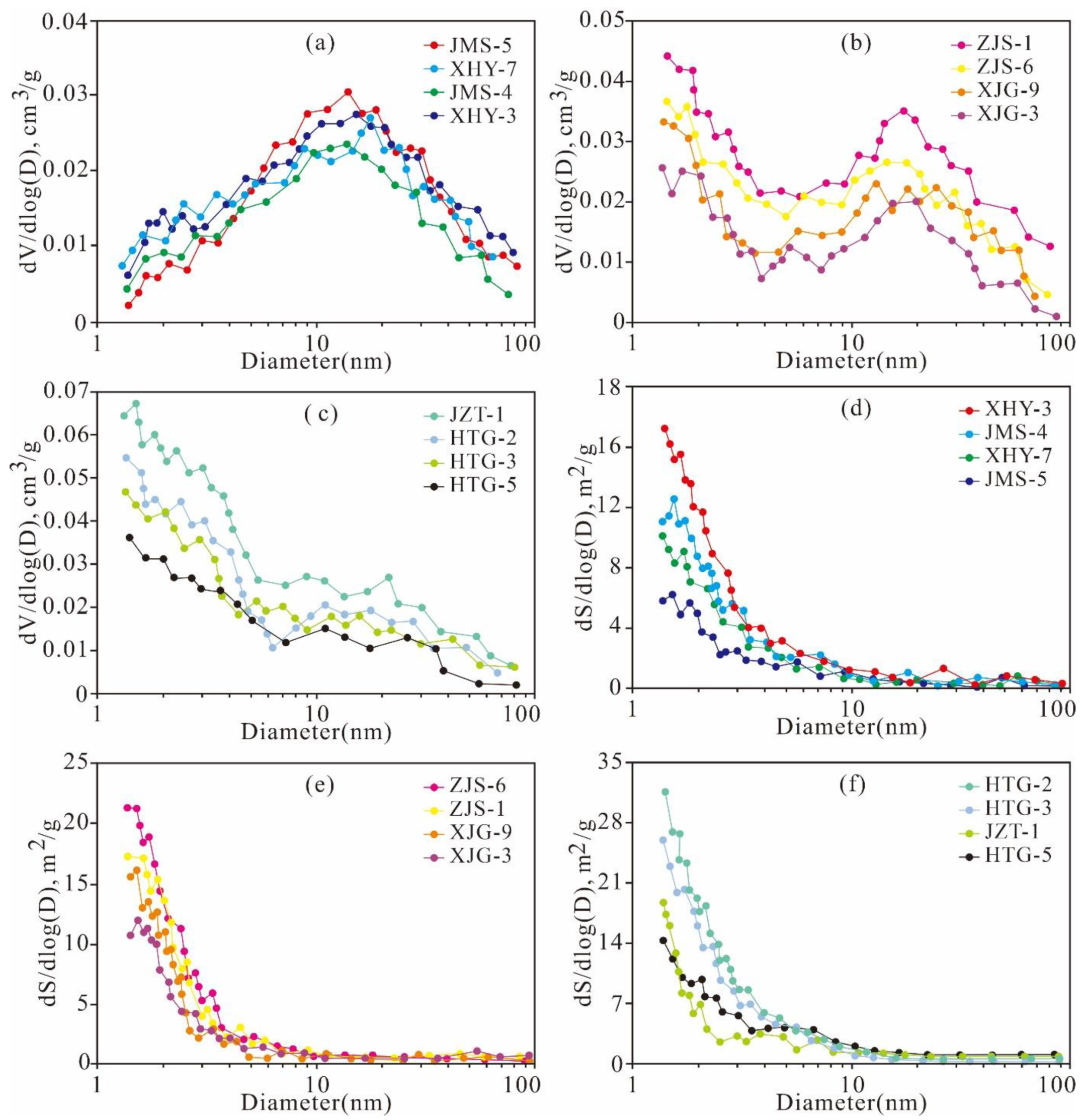
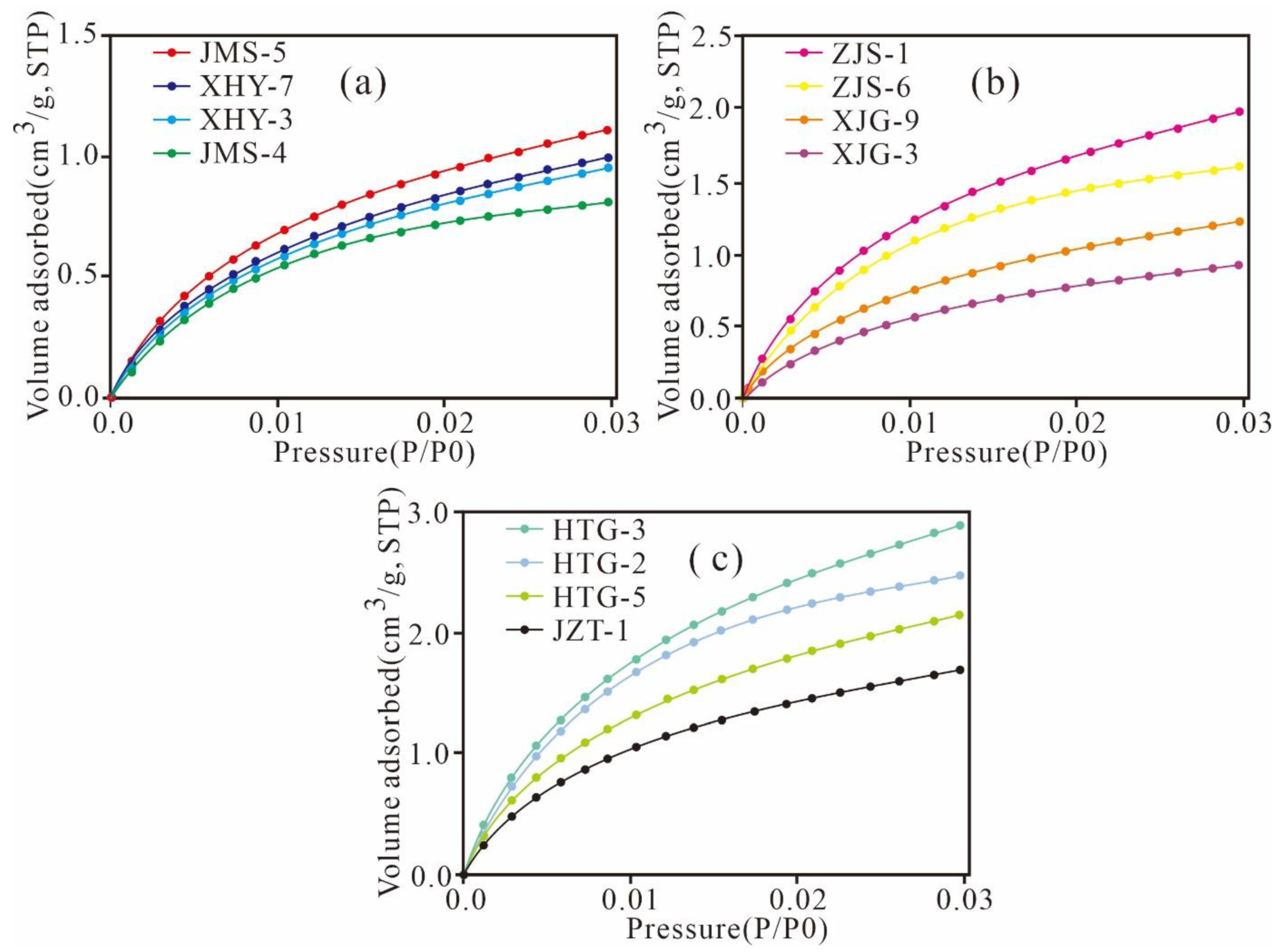
3.4. Low-Pressure N2 and CO2 Adsorption
3.5. Determination of Total Porosity
4. Results
4.1. Basic Organic Geochemistry and Mineralogy
4.2. Pore Characterization Based on MIP
5. Discussion
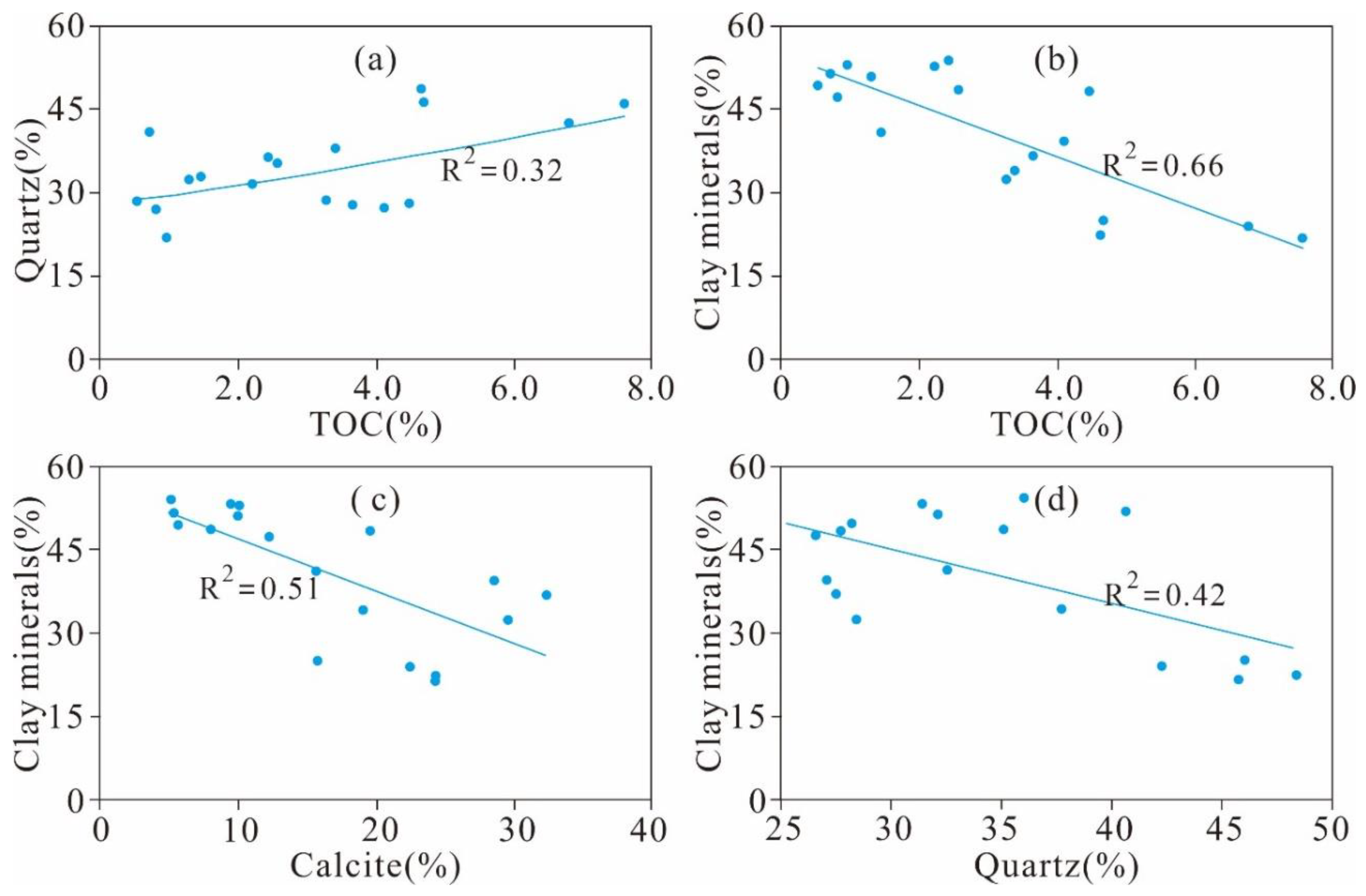
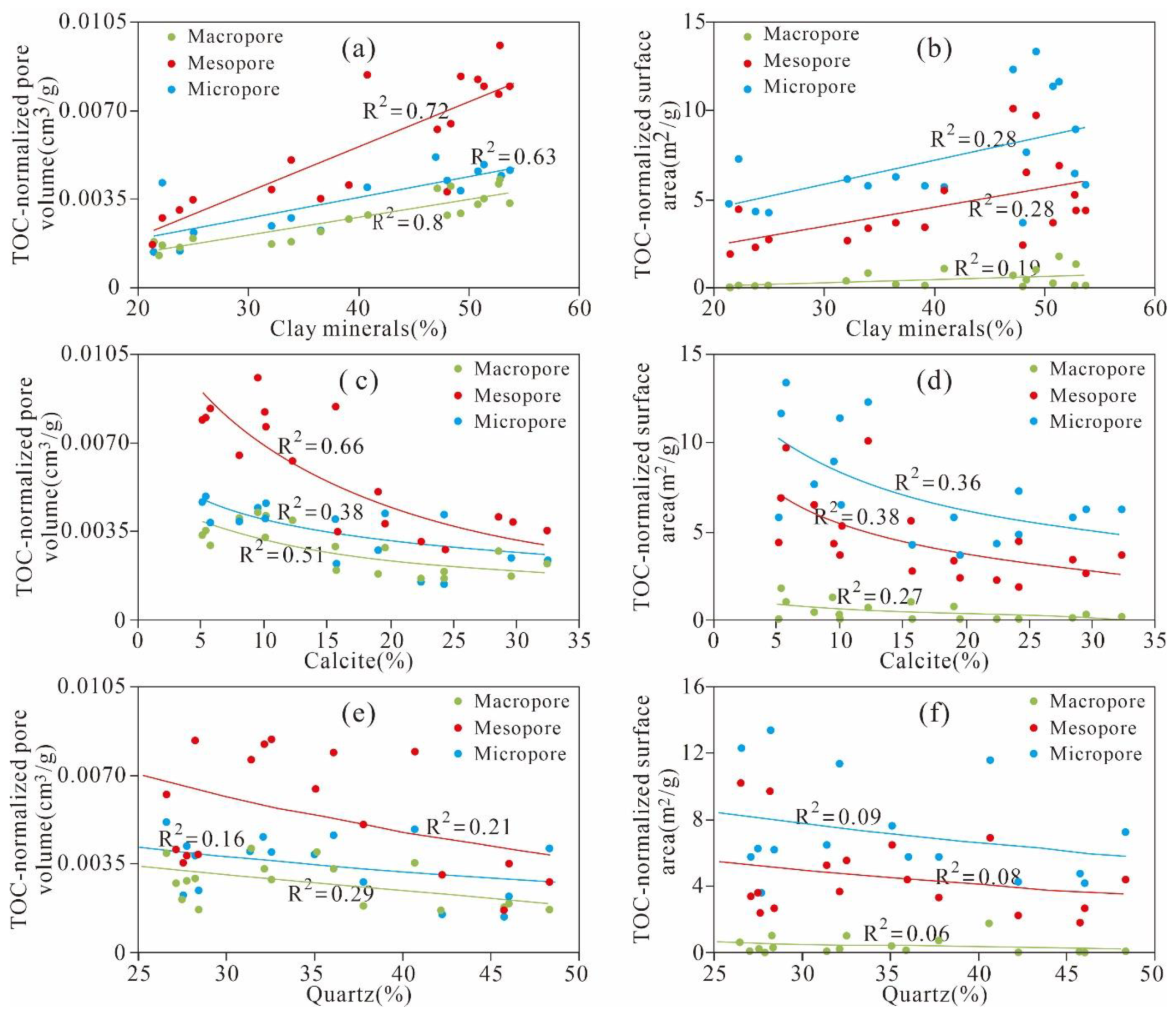
5.1. Relationship between TOC and Quartz and Clay Minerals
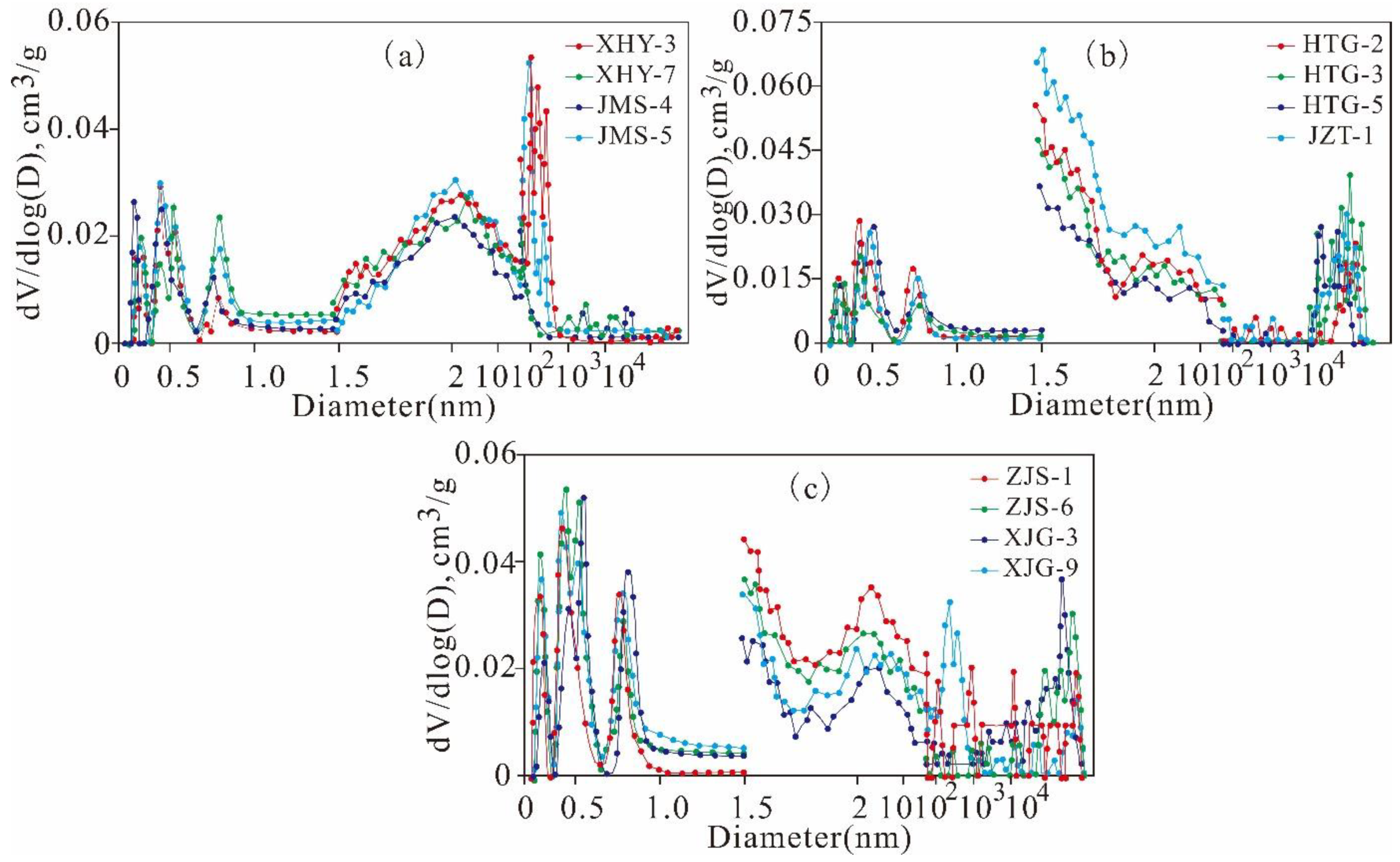
5.2. Full-Scale Pore Structure Characteristics
5.3. Control Effects on Pore Structure
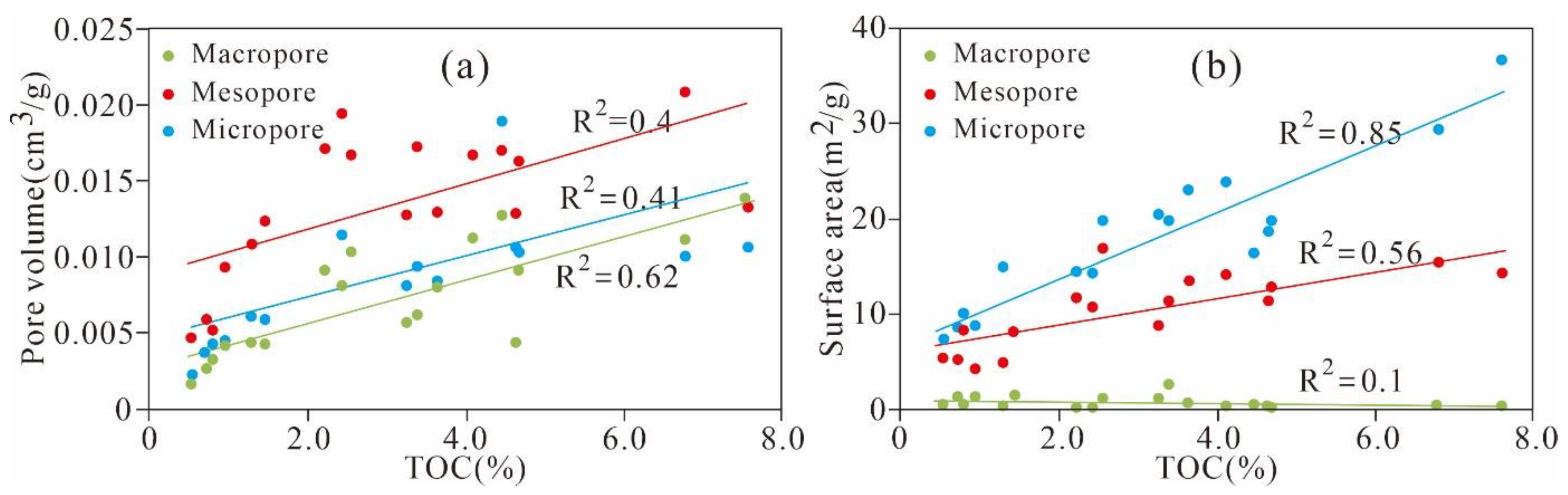
5.3.1. Porosity and Controlling Factors
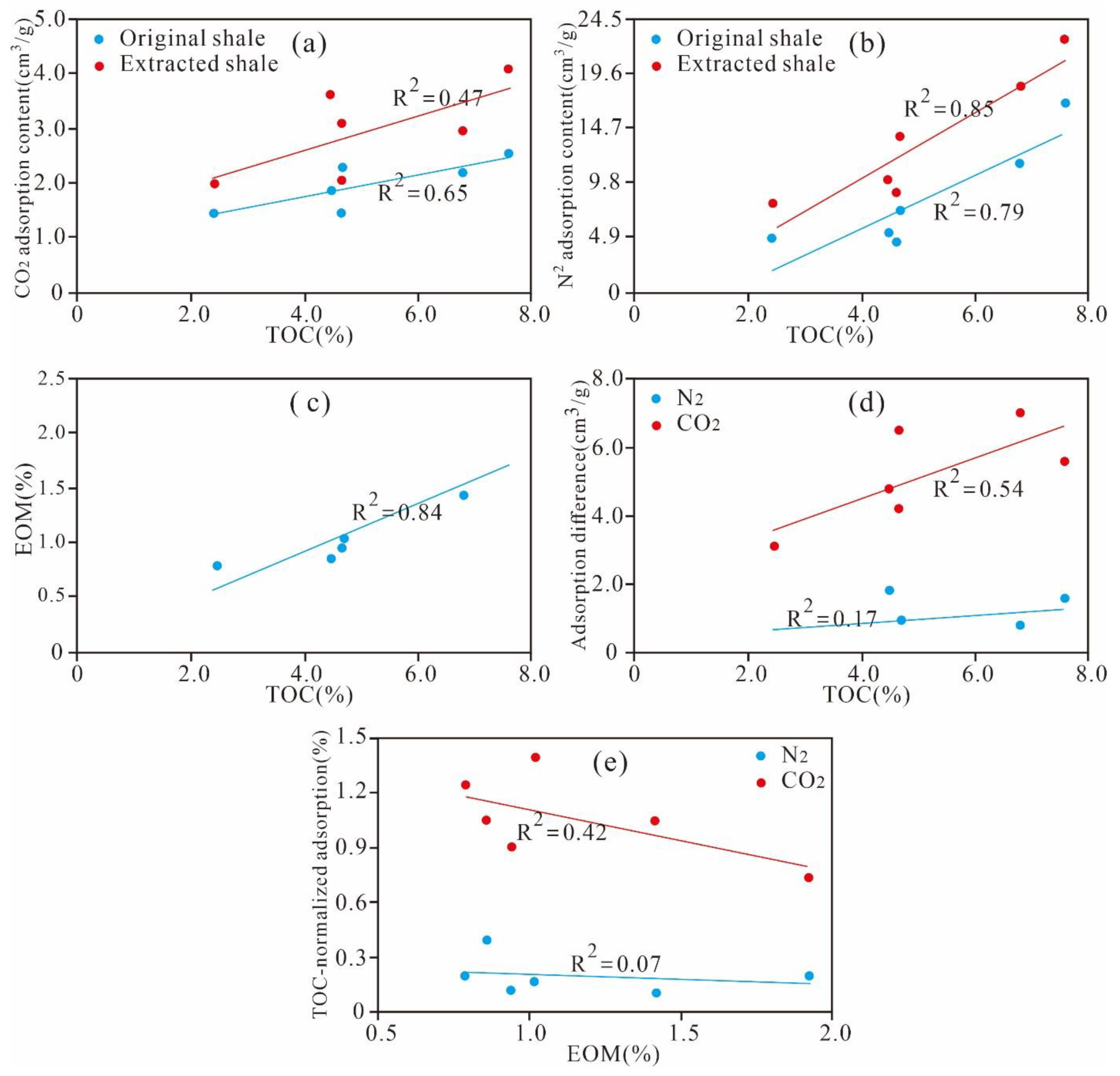
5.3.2. Effect of TOC Content on Pore Structure
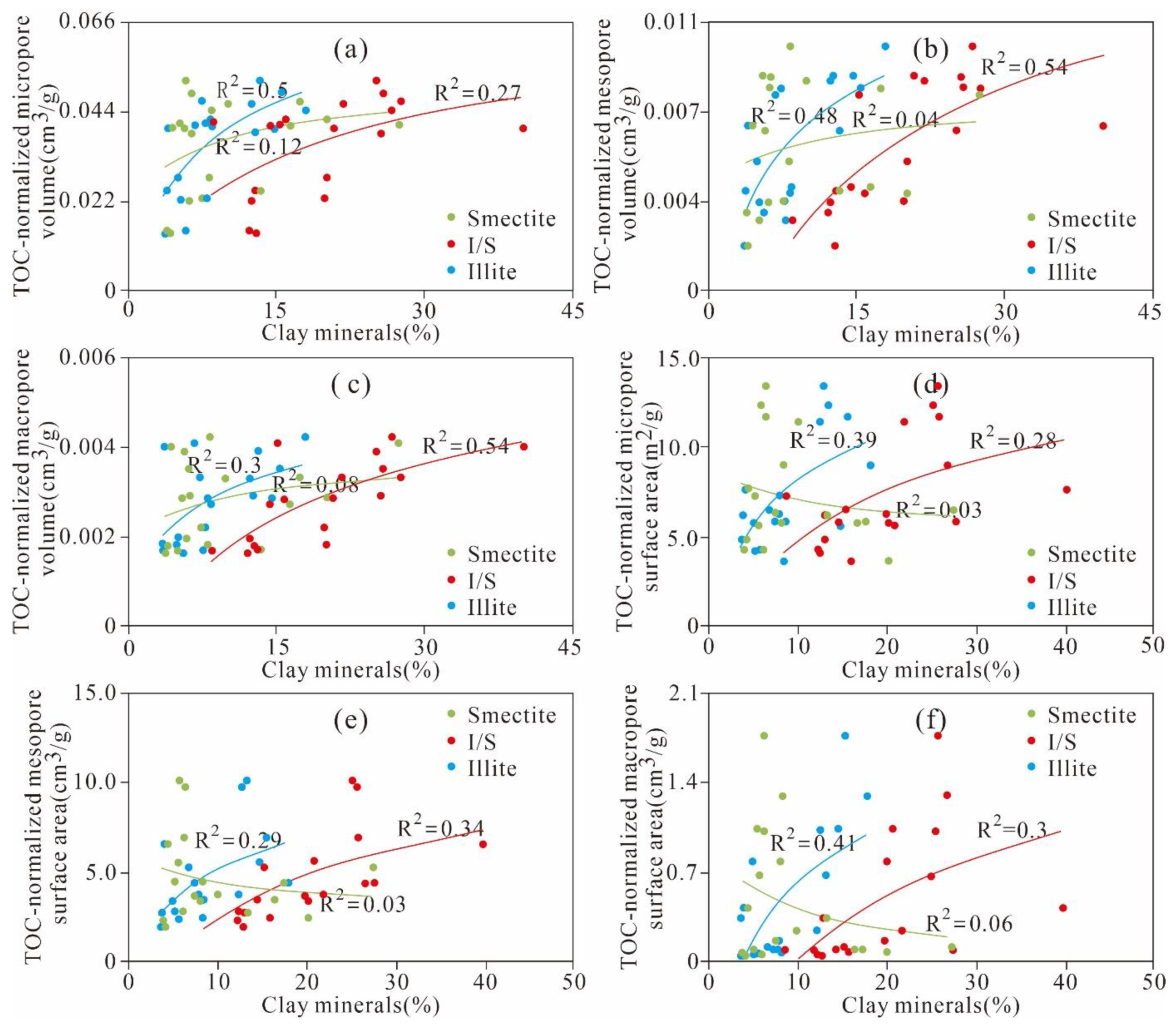
5.3.3. Effect of Mineral Composition on Pore Structure
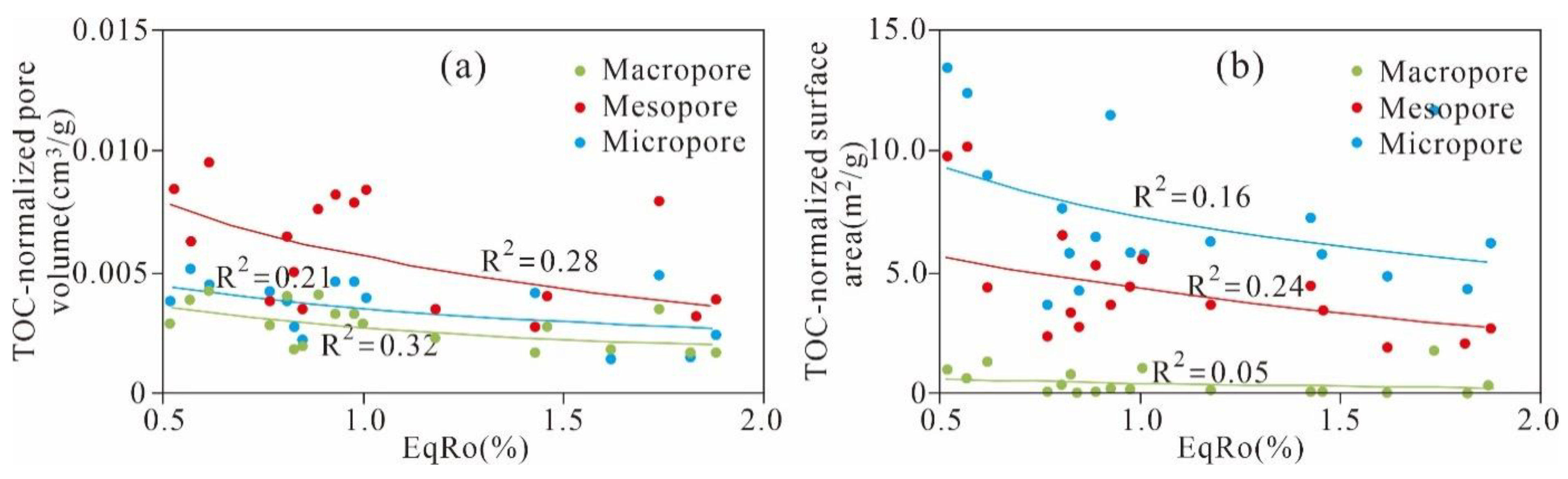
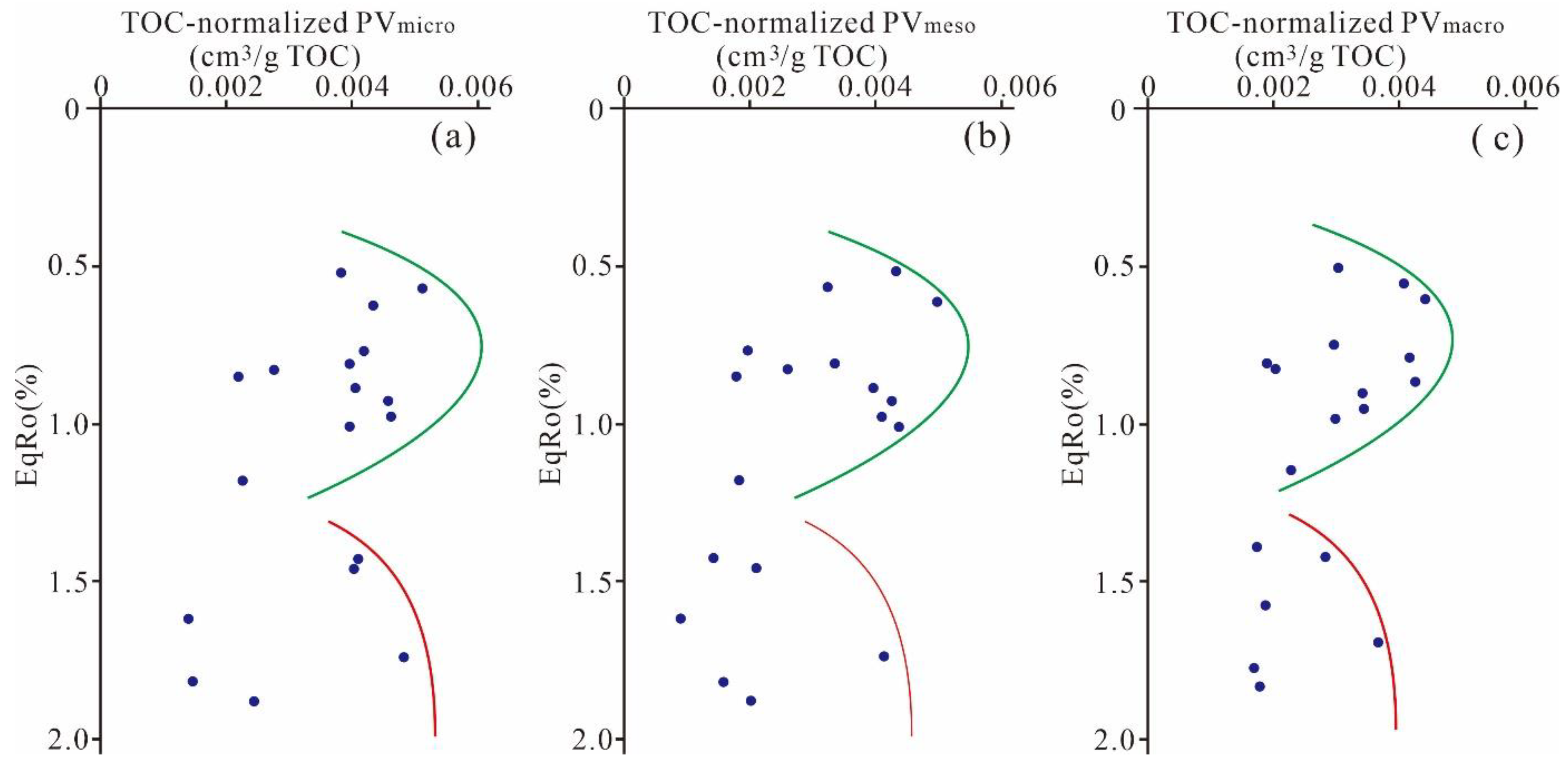
5.3.4. Effect of Maturation on Pore Evolution
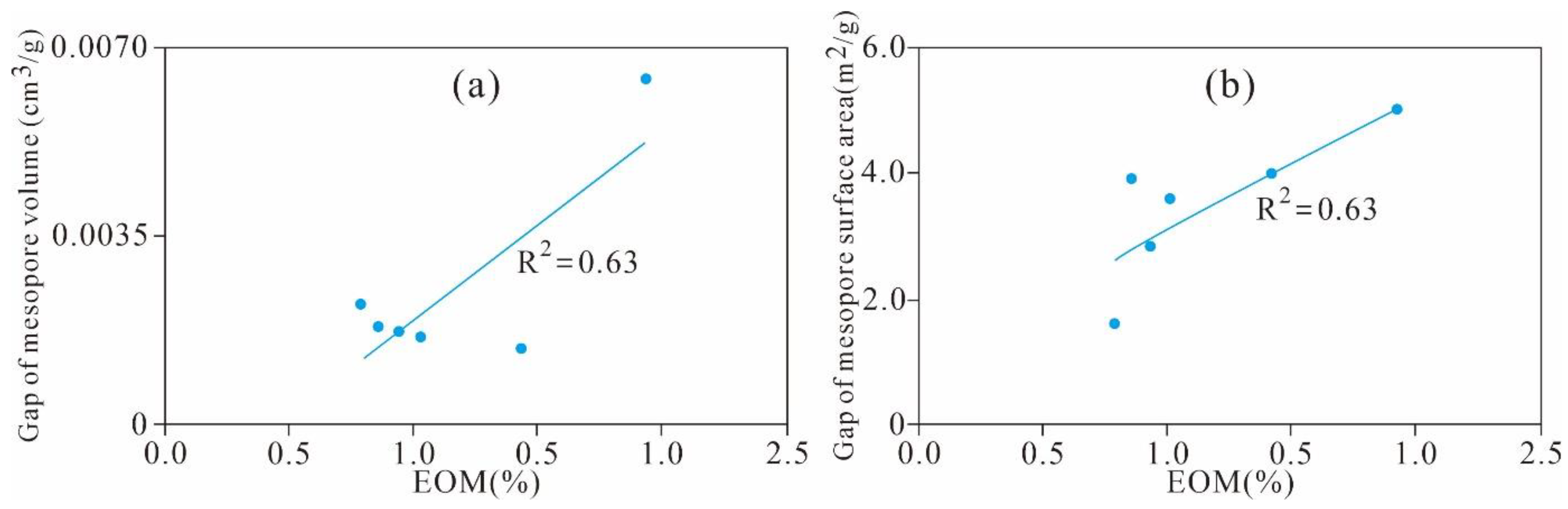
5.4. Effect of Residual Hydrocarbon on the Pore Structure
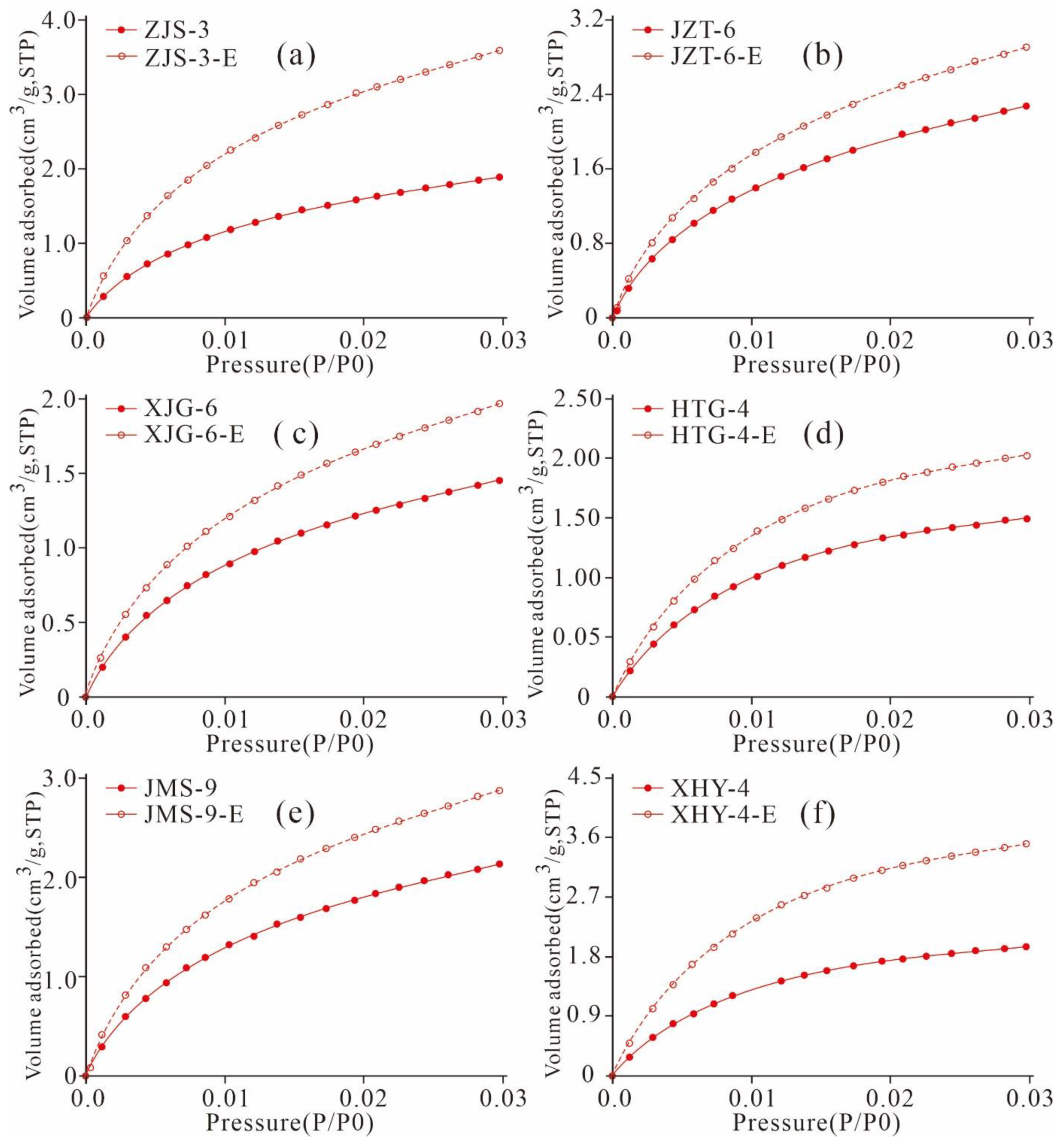
5.4.1. Adsorption Desorption Curve Characteristics
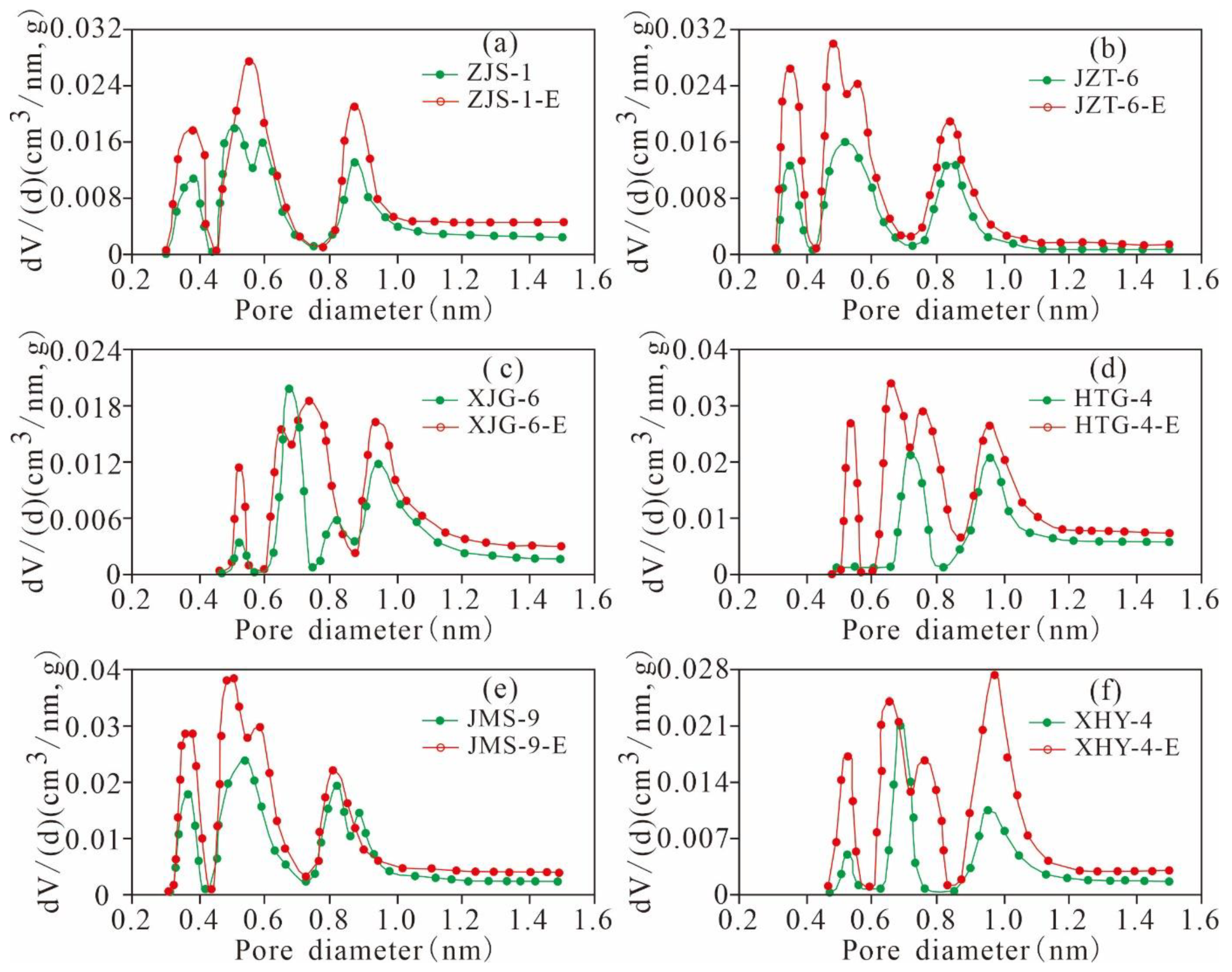
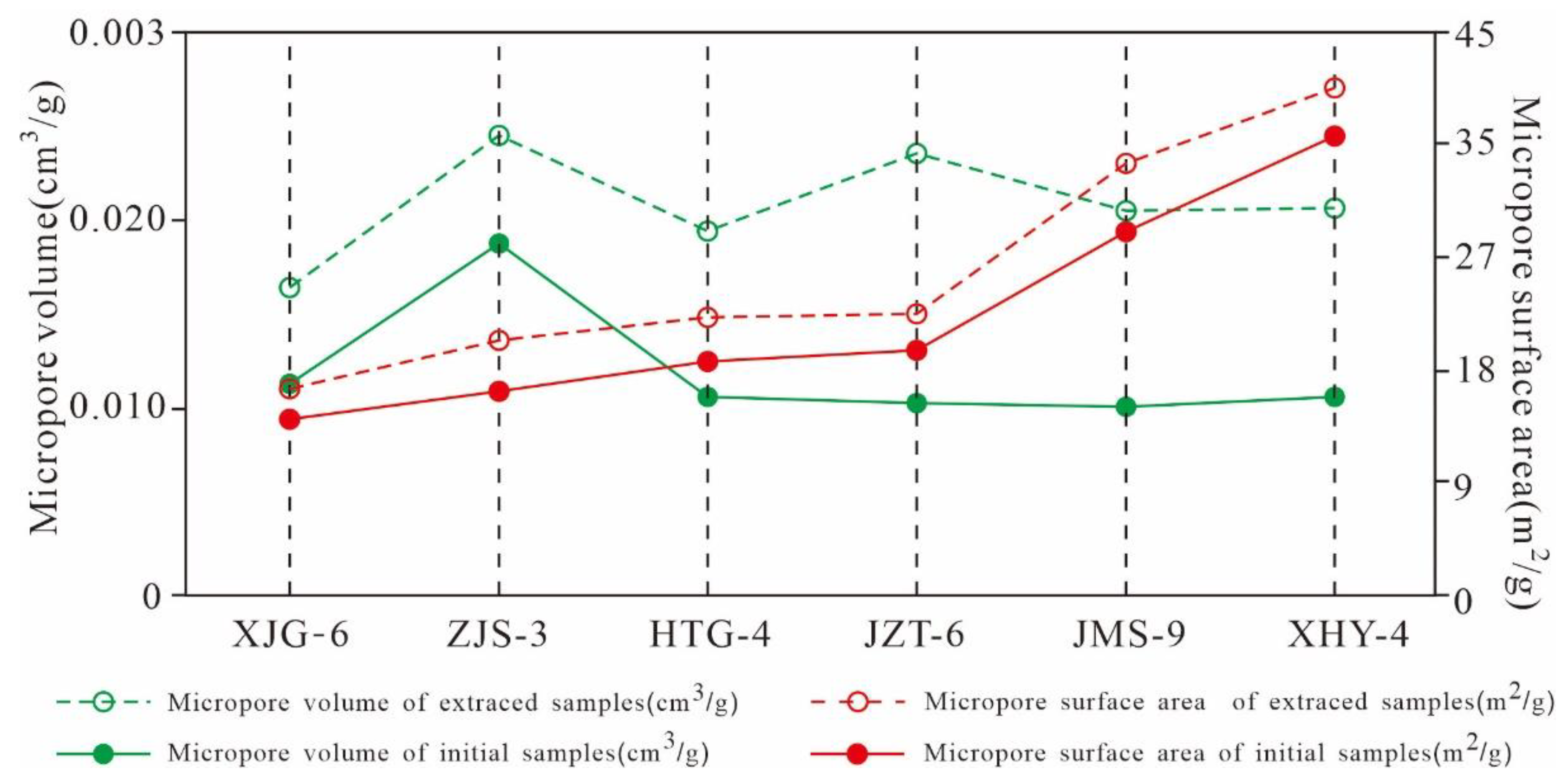
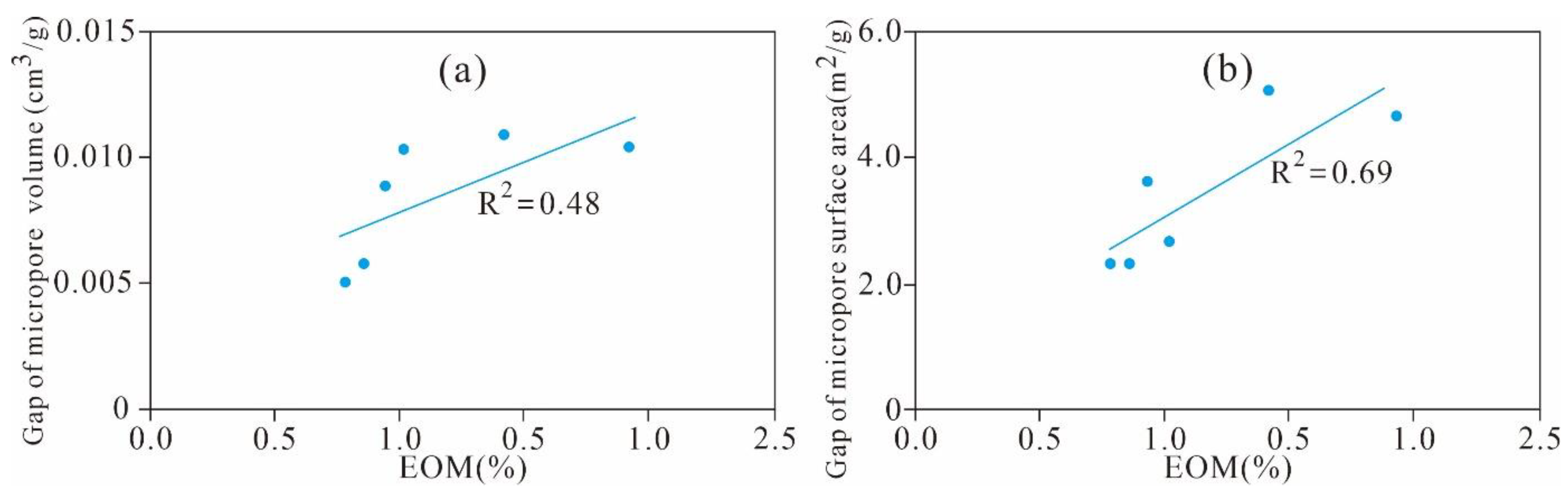
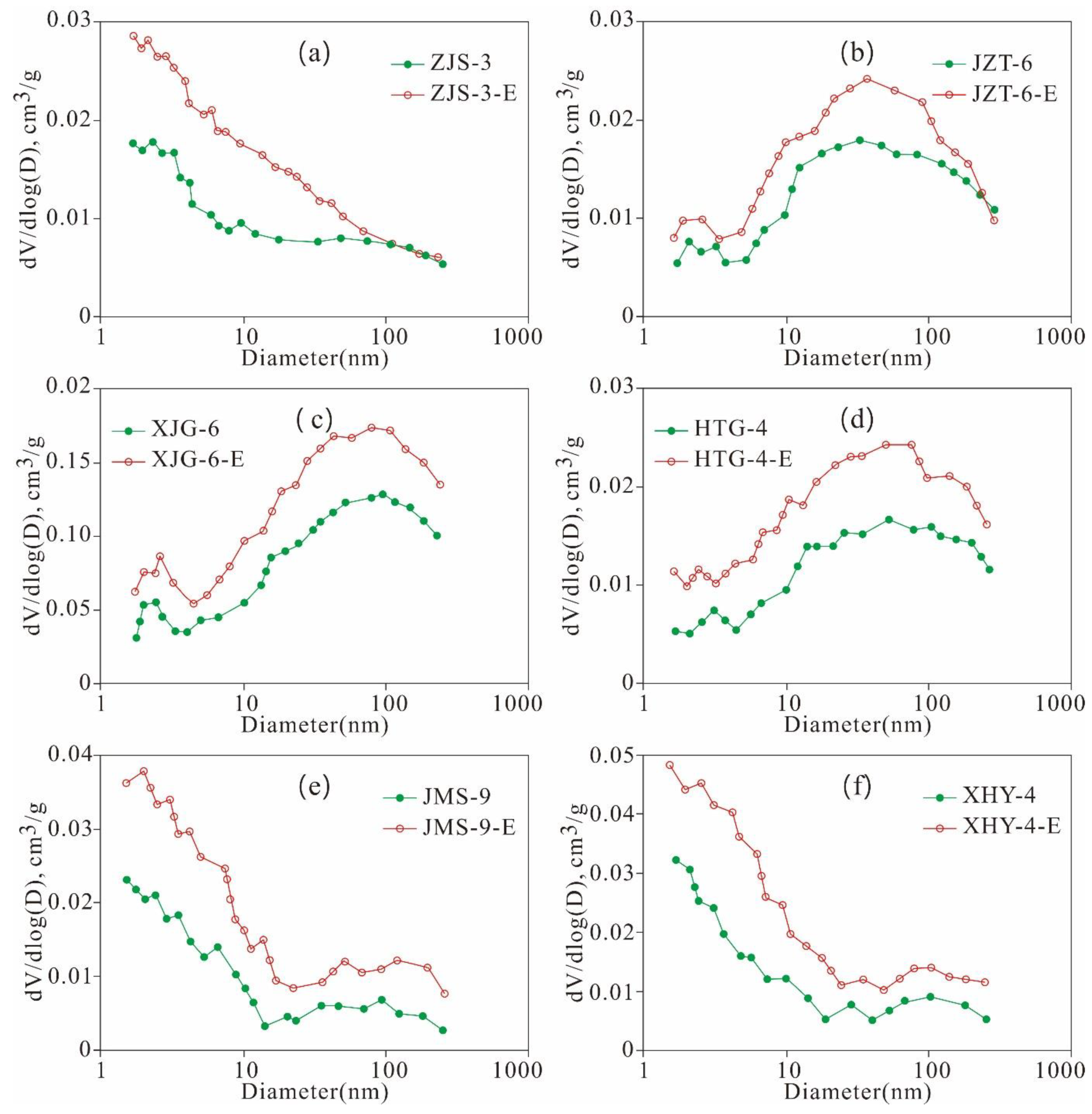
5.4.2. Variation Characteristics of the Micropore Structures
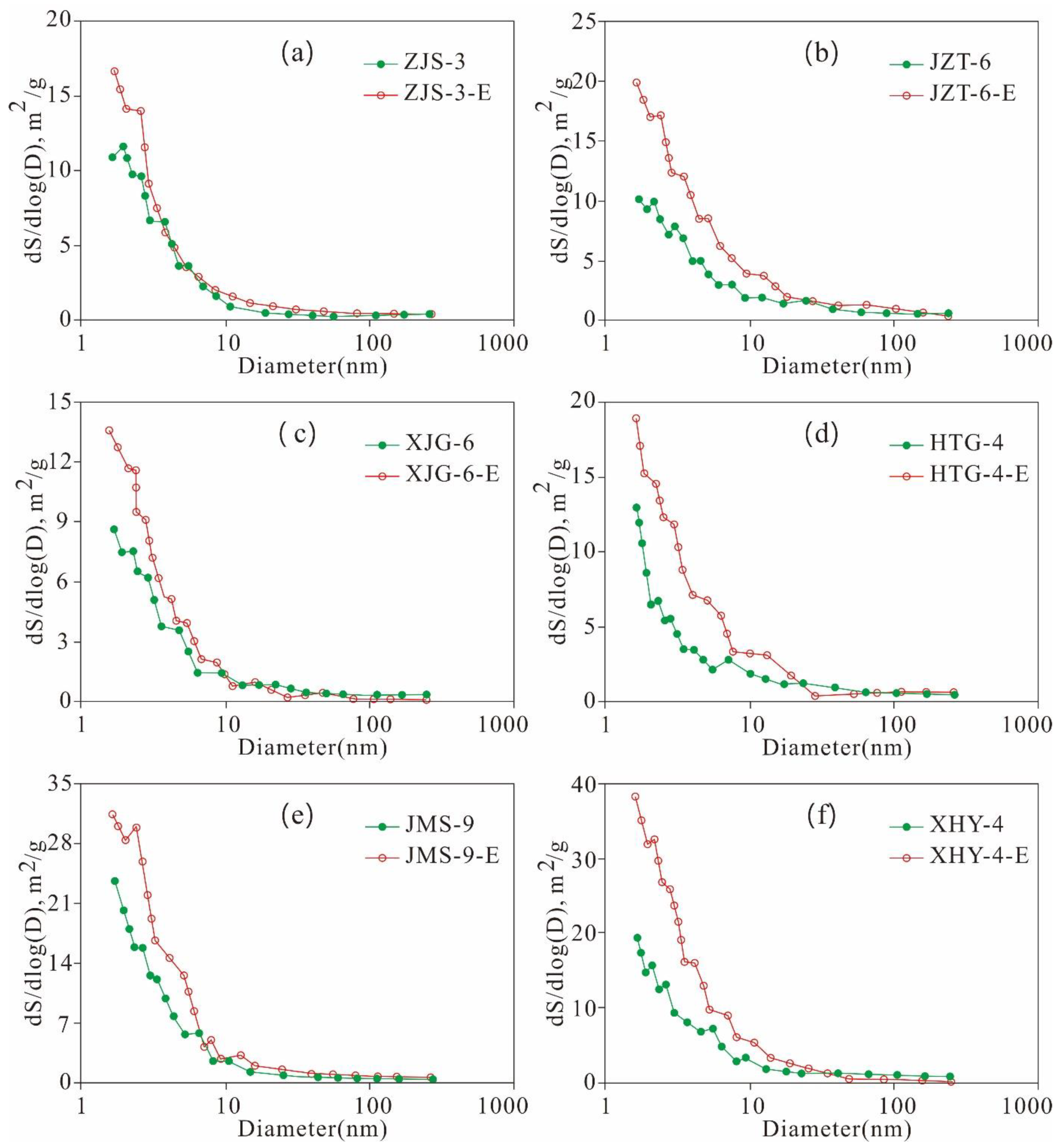
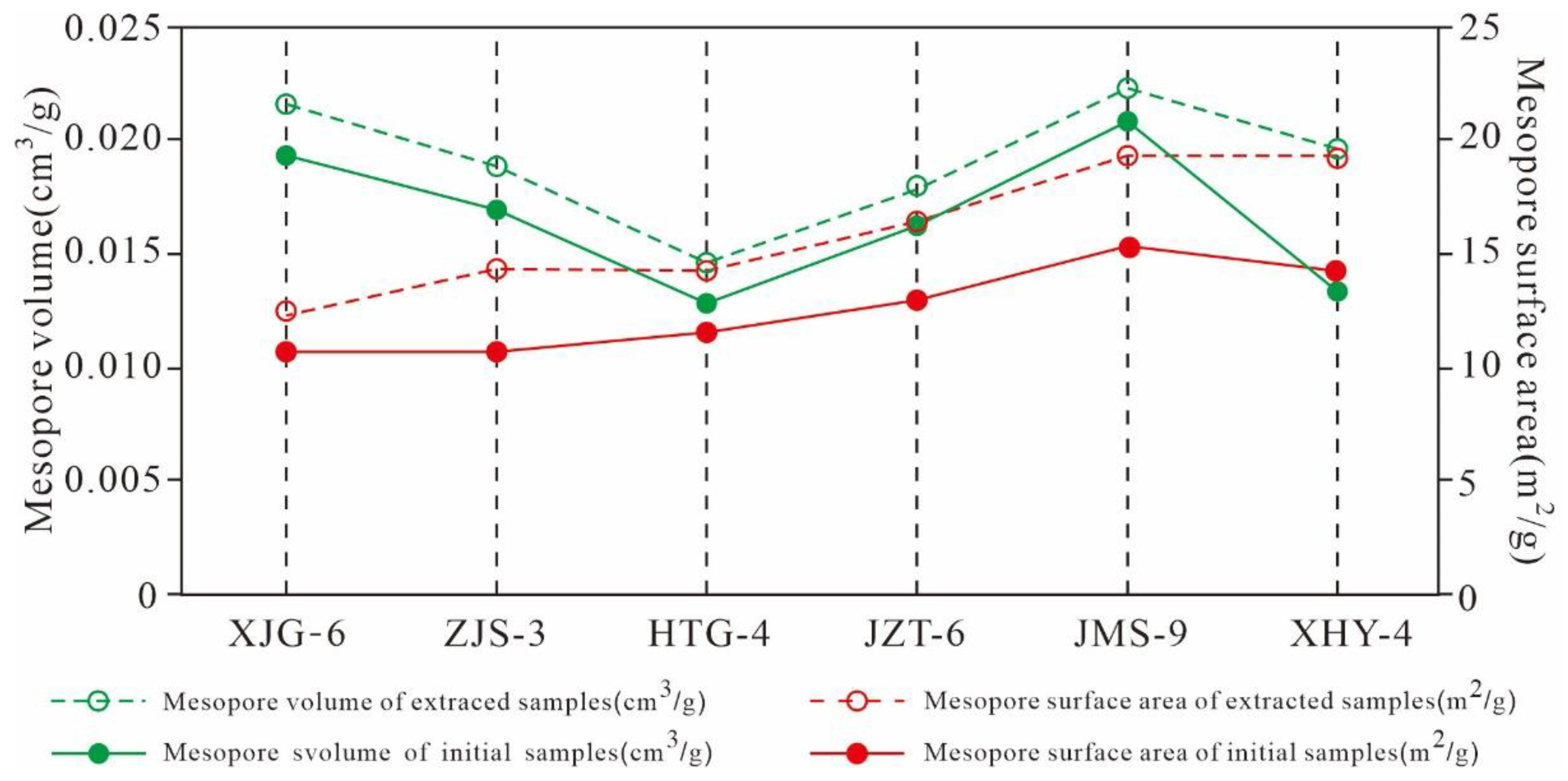
5.4.3. Variation Characteristics of the Mesopore and Macropore Structures
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ross, D.J.K.; Bustin, R.M. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Chalmers, G.R.L.; Bustin, R.M.; Power, I.M. Characterization of gas shale pore systems by porosimetry, pycnometry, surface area, and field emission scanning electron microscopy/transmission electron microscopy image analyses: Examples from the Barnett, Woodford, Haynesville, Marcellus, and Doig units. AAPG Bull. 2012, 96, 1099–1119. [Google Scholar]
- Li, J.; Zhou, S.X.; Li, Y.J.; Yang, Y.N.; Li, C.C. Effect of organic matter on pore structure of mature lacustrine organic-rich shale: A case study of the Triassic Yanchang shale, Ordos Basin, China. Fuel 2016, 185, 421–431. [Google Scholar] [CrossRef]
- Guo, H.J.; Jia, W.L.; Peng, P.A.; Lei, Y.H.; Luo, X.R.; Cheng, M.; Wang, X.Z.; Zhang, L.X.; Jiang, C.F. The composition and its impact on the methane sorption of lacustrine shales from the Upper Triassic Yanchang Formation, Ordos Basin, China. Mar. Pet. Geol. 2014, 57, 509–520. [Google Scholar] [CrossRef]
- Curtis, J.B. Fractured shale-gas systems. AAPG Bull. 2002, 11, 1921–1938. [Google Scholar]
- Clarkson, C.R.; Solano, N.; Bustin, R.M.; Chalmers, G.R.L.; Melnichenko, Y.B.; Radlinski, A.P. Pore structure characterization of North American shale gas reservoirs using USANS/SANS, gas adsorption, and mercury intrusion. Fuel 2013, 103, 606–616. [Google Scholar] [CrossRef]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Jarvie, D.M. Morphology, genesis, and distribution of nanometer-scale pores in siliceous mudstones of the Mississippian Barnett Shale. J. Sediment. Res. 2009, 79, 848–861. [Google Scholar] [CrossRef] [Green Version]
- Bernard, S.; Horsfield, B.; Schulz, H.M.; Wirth, R.; Schreiber, A.; Sherwood, N. Geochemical evolution of organic-rich shales with increasing maturity: A STXM and TEM study of the Posidonia Shale (Lower Toarcian, northern Germany). Mar. Pet. Geol. 2012, 31, 70–89. [Google Scholar] [CrossRef]
- Mastalerz, M.; He, L.; Melnichenko, Y.B.; Rupp, J.A. Porosity of coal and shale: Insights from gas adsorption and SANS/USANS techniques. Energy Fuels 2012, 8, 5109–5120. [Google Scholar] [CrossRef]
- Klaver, J.; Desbois, G.; Littke, R.; Urai, J.L. BIB-SEM characterization of pore space morphology and distribution in postmature to overmature samples from the Haynesville and Bossier Shales. Mar. Pet. Geol. 2015, 59, 451–466. [Google Scholar] [CrossRef]
- Gou, Q.Y.; Xu, S.; Hao, F.; Yang, F.; Zhang, B.Q.; Shu, Z.G.; Zhang, A.H.; Wang, Y.X.; Lu, Y.B.; Cheng, X.; et al. Full-scale pores and micro-fractures characterization using FE-SEM, gas adsorption, nano-CT and micro-CT: A case study of the Silurian Longmaxi Formation shale in the Fuling area, Sichuan Basin, China. Fuel 2019, 253, 167–179. [Google Scholar] [CrossRef]
- Wang, P.F.; Jiang, Z.X.; Ji, W.M.; Zhang, C.; Yuan, Y.; Chen, L.; Yin, L.S. Heterogeneity of intergranular, intraparticle and organic pores in Longmaxi shale in Sichuan Basin, South China: Evidence from SEM digital images and fractal and multifractal geometries. Mar. Pet. Geol. 2016, 72, 122–138. [Google Scholar] [CrossRef]
- Fu, C.Q.; Zhu, Y.M.; Chen, S.B.; Xiao, H.X. Structure and fractal characteristics of nano-micro pores in organic-rich Qiongzhusi formation shales in Eastern Yunnan Province. J. Nanosci. Nanotechnol. 2017, 17, 5996–6013. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.M.; Chen, S.B.; Li, W. Characteristics of the nanoscale pore structure in Northwestern Hunan Shale gas reservoirs using field emission scanning electron microscopy, high-pressure mercury intrusion, and gas adsorption. Energy Fuels 2014, 28, 945–955. [Google Scholar] [CrossRef]
- Yang, F.; Ning, Z.F.; Qiang, W.; Zhang, R.; Krooss, B.M. Pore structure characteristics of lower Silurian shales in the southern Sichuan Basin, China: Insights to pore development and gas storage mechanism. Int. J. Coal Geol. 2016, 156, 12–24. [Google Scholar] [CrossRef]
- Ma, Y.; Ardakani, O.H.; Zhong, N.N.; Liu, H.L.; Huang, H.P.; Larter, S.; Zhang, C. Possible pore structure deformation effects on the shale gas enrichment: An example from the Lower Cambrian shales of the Eastern Upper Yangtze Platform, South China. Int. J. Coal Geol. 2020, 217, 1–22. [Google Scholar] [CrossRef]
- Hao, F.; Zou, H.Y.; Lu, Y.C. Mechanisms of shale gas storage: Implications for shale gas exploration in China. AAPG Bull. 2013, 97, 1325–1346. [Google Scholar] [CrossRef]
- Liu, H.M.; Zhang, S.; Song, G.Q.; Wang, X.J.; Teng, J.B.; Wang, M.; Bao, Y.S.; Yao, S.P.; Wang, W.Q.; Zhang, S.P.; et al. Effect of shale diagenesis on pores and storage capacity in the Paleogene Shahejie Formation, Dongying Depression, Bohai Bay Basin, east China. Mar. Pet. Geol. 2019, 103, 738–752. [Google Scholar] [CrossRef]
- Milliken, K.L.; Rudnicki, M.; Awwiller, D.N.; Zhang, T.W. Organic matter-hosted pore system, Marcellus Formation (Devonian), Pennsylvania. AAPG Bull. 2013, 97, 177–200. [Google Scholar] [CrossRef]
- Jarvie, D.M.; Hill, R.J.; Ruble, T.E.; Pollastro, R.M. Unconventional shale-gas systems: The Mississippian Barnett Shale of north-central Texas as one model for thermogenic shale-gas assessment. AAPG Bull. 2007, 91, 475–499. [Google Scholar] [CrossRef]
- Modica, C.J.; Lapierre, S.G. Estimation of kerogen porosity in source rocks as a function of thermal transformation: Example from the Mowry Shale in the Powder River Basin of Wyoming. AAPG Bull. 2012, 96, 87–108. [Google Scholar] [CrossRef]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C. Spectrum of poretypes and networks in mudrocks and a descriptive classification for matrix-related mudrock pores. AAPG Bull. 2012, 96, 1071–1098. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Jiang, Z.X.; Liu, K.Y.; Tan, J.Q.; Gao, F.L.; Wang, P.F. Pore structure characterization for organic-rich Lower Silurian shale in the Upper Yangtze Platform, South China: A possible mechanism for pore development. J. Nat. Gas Sci. Eng. 2017, 46, 1–15. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.F.; Zheng, S.S.; Luo, Z.H.; Sheng, Y.; Wang, X.M. Full-scale pore structure and its controlling factors of the Wufeng-Longmaxi shale, southern Sichuan Basin, China: Implications for pore evolution of highly overmature marine shale. J. Nat. Gas Sci. Eng. 2019, 67, 134–146. [Google Scholar] [CrossRef]
- Wang, P.F.; Jiang, Z.X.; Chen, L.; Yin, L.S.; Li, Z.; Zhang, C.; Tang, X.L.; Wang, G.Z. Pore structure characterization for the Longmaxi and Niutitang shales in the Upper Yangtze Platform, South China: Evidence from focused ion beam–He ion microscopy, nano-computerized tomography and gas adsorption analysis. Mar. Pet. Geol. 2016, 72, 1323–1337. [Google Scholar] [CrossRef]
- Bian, L.Z.; Zhang, S.C.; Zhang, B.M.; Wang, D.R. Red algal fossils discovered from the Neoproterozoic Xiamaling oil shales, Xiahuayuan town of Hebei province. Acta Micropalaeontol. Sin. 2005, 22, 209–216. [Google Scholar]
- Zhang, S.C.; Zhang, B.M.; Bian, L.Z.; Jin, Z.J.; Wang, D.R.; Chen, J.F. The Xiamaling oil shale generated through rhodophyal over 800 Ma ago. Sci. China Ser. D Earth Sci. 2007, 37, 636–643. [Google Scholar] [CrossRef]
- Gao, L.Z.; Zhang, C.H.; Shi, X.Y.; Zhou, H.R.; Wang, Z.Q. Zircon SHRIMP U-Pb dating of the tuffbed in the Ximaling formation of the Qinbaikou system in North China. Geol. Bull. China 2007, 26, 249–255. [Google Scholar]
- Liu, A.Q.; Tang, D.J.; Shi, X.Y.; Zhou, L.M.; Zhou, X.Q.; Shang, M.H.; Li, Y.; Song, H.Y. Growth mechanisms and environmental implications of carbonate concretions from the ~1.4 Ga Xiamaling Formation, North China. J. Palaeogeogr. 2019, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.M.; Wilkins, R.W.T.; Liu, D.H.; Liu, Z.F.; Fu, J.M. Investigation of thermal maturity of lower Palaeozoic hydrocarbon source rocks by means of vitrinite-like maceral reflectance—A Tarim Basin case study. Org. Geochem. 2000, 31, 1041–1052. [Google Scholar]
- Chalmers, G.R.L.; Bustin, R.M. Lower Cretaceous gas shales in northeastern British Columbia, Part II: Evaluation of regional potential gas resources. Bull. Can. Pet. Geol. 2008, 56, 22–61. [Google Scholar] [CrossRef]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Tian, H.; Pan, L.; Xiao, X.M.; Wilkins, R.W.T.; Meng, Z.P.; Huang, B.J. A preliminary study on the pore characterization of Lower Silurian black shales in the Chuandong Thrust Fold Belt, southwestern China using low pressure N2 adsorption and FE-SEM methods. Mar. Pet. Geol. 2013, 48, 8–19. [Google Scholar] [CrossRef]
- Mastalerz, M.; Schimmelmann, A.; Drobniak, A.; Chen, Y.Y. Porosity of Devonian and Mississippian New Albany Shale across a maturation gradient: Insights from organic petrology, gas adsorption, and mercury intrusion. AAPG Bull. 2013, 97, 1621–1643. [Google Scholar] [CrossRef]
- Chen, S.B.; Zhu, Y.M.; Li, W.; Wang, H. Influence of magma intrusion on gas outburst in a low rank coal mine. Int. J. Min. Sci. Technol. 2012, 22, 259–266. [Google Scholar] [CrossRef]
- Schmitt, M.; Fernandes, C.P.; Da Cunha Neto, J.A.B.; Wolf, F.G.; Santos, V.D. Characterization of pore systems in seal rocks using Nitrogen Gas Adsorption combined with Mercury Injection Capillary Pressure techniques. Mar. Pet. Geol. 2013, 39, 138–149. [Google Scholar] [CrossRef]
- Sing, K.; Everett, D.; Haul, R.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewsha, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 11, 603–619. [Google Scholar] [CrossRef]
- Chalmers, G.R.L.; Ross, D.J.K.; Bustin, R.M. Geological controls on matrix permeability of Devonian Gas Shales in the Horn River and Liard basins, northeastern British Columbia, Canada. Int. J. Coal Geol. 2012, 103, 120–131. [Google Scholar] [CrossRef]
- Pan, L.; Xiao, X.M.; Tian, H.; Zhou, Q.; Chen, J.; Li, T.F.; Wei, Q. A preliminary study on the characterization and controlling factors of porosity and pore structure of the Permian shales in Lower Yangtze region, Eastern China. Int. J. Coal Geol. 2015, 146, 68–78. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.M.; Wang, H.Y.; Feng, G.J. Nanoscale pore morphology and distribution of lacustrine shale reservoirs: Examples from the Upper Triassic Yanchang Formation, Ordos Basin. J. Energy Chem. 2015, 24, 512–519. [Google Scholar] [CrossRef]
- Tian, H.; Pan, L.; Zhang, T.W.; Xiao, X.M.; Meng, Z.P.; Huang, B.J. Pore characterization of organic-rich Lower Cambrian shales in Qiannan Depression of Guizhou Province, Southwestern China. Mar. Pet. Geol. 2015, 62, 28–43. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.C.; Tang, X.; Ding, J.H.; Zhao, Q.R.; Dang, W.; Chen, H.Y.; Su, Y.; Li, B.W.; Lu, D.F. Nanoscale pore structure and fractal characteristics of marine-continental transitional shale: A case study from the Lower Permian Shanxi Shale in the southeastern Ordos Basin, China. Mar. Pet. Geol. 2017, 88, 54–68. [Google Scholar] [CrossRef]
- Zeng, W.T.; Zhang, J.C.; Ding, W.L.; Wang, X.Z.; Zhu, D.W.; Liu, Z.J. The gas content of continental Yanchang shale and its main controlling factores: A case study of Liuping-171 well in Ordos Basin. Nat. Gas Geosci. 2014, 25, 291–301. [Google Scholar]
- Ji, L.M.; Zhang, T.W.; Milliken, K.L.; Qu, J.L.; Zhang, X.L. Experimental investigation of main controls to methane adsorption in clay-rich rocks. Appl. Geochem. 2012, 27, 2533–2545. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.C.; Tang, X.; Ding, J.H.; Zhao, Q.R.; Dang, W.; Chen, H.Y.; Su, Y.; Li, B.W.; Lu, D.F. Comparative study on micro-pore structure of marine, terrestrial, and transitional shales in key areas, China. Int. J. Coal Geol. 2017, 171, 76–92. [Google Scholar] [CrossRef]
- Shao, X.H.; Pang, X.Q.; Li, Q.W.; Wang, P.W.; Chen, D.; Shen, W.B.; Zhao, Z.F. Pore structure and fractal characteristics of organic-rich shales: A case study of the lower Silurian Longmaxi shales in the Sichuan Basin, SW China. Mar. Pet. Geol. 2017, 80, 192–202. [Google Scholar] [CrossRef]
- Topór, T.; Derkowski, A.; Ziemiański, P.; Xzczurowski, J.; McCarty, D.K. The effect of organic matter maturation and porosity evolution on methane storage potential in the Baltic Basin (Poland) shale-gas reservoir. Int. J. Coal Geol. 2017, 180, 46–56. [Google Scholar] [CrossRef]
- Pommer, M.; Milliken, K. Pore types and pore-size distributions across thermal maturity, Eagle Ford Formation, southern Texas. AAPG Bull. 2015, 99, 1713–1744. [Google Scholar] [CrossRef]
- Liu, W.P.; Liu, J.; Cai, M.L.; Luo, C.; Shi, X.W.; Zhang, J. Pore evolution characteristic of shale in the Longmaxi Formation, Sichuan Basin. Pet. Res. 2017, 2, 291–300. [Google Scholar] [CrossRef]
- Xu, L.W.; Wang, Y.; Liu, L.F.; Chen, L.; Chen, J. Evolution characteristics and model of nanopore structure and adsorption capacity in organic-rich shale during artificial thermal maturation: A pyrolysis study of the Mesoproterozoic Xiamaling marine shale with type II kerogen from Zhangjiakou, Hebei, China. Energy Explor. Exploit. 2019, 37, 493–518. [Google Scholar]
- Guo, S.B.; Mao, W.J. Division of diagenesis and pore evolution of a Permian Shanxi shale in the Ordos Basin, China. J. Pet. Sci. Eng. 2019, 182, 1–9. [Google Scholar] [CrossRef]
- Guo, H.J.; Jia, W.L.; Peng, P.A.; Lei, Y.H.; Luo, X.R.; Cheng, M.; Wang, X.Z.; Zhang, L.X.; Jiang, C.F. Evolution of organic matter and nanometer-scale pores in an artificially matured shale undergoing two distinct types of pyrolysis: A study of the Yanchang Shale with Type II kerogen. Org. Geochem. 2017, 105, 56–66. [Google Scholar] [CrossRef]
- Hu, H.Y.; Hao, F.; Liu, J.F.; Lu, Y.C.; Ma, Y.Q.; Li, Q. Organic matter-hosted pore system in the Wufeng-Longmaxi (O3W-S1L) shale, Jiaoshiba, Eastern Sichuan Basin, China. Int. J. Coal Geol. 2017, 173, 40–50. [Google Scholar] [CrossRef]
- Kuila, U.; McCarty, D.K.; Derkowski, A.; Fischer, T.B.; Topór, T. Nano-scale texture and porosity of organic matter and clay minerals in organic-rich mudrocks. Fuel 2014, 135, 359–373. [Google Scholar] [CrossRef] [Green Version]
- Curtis, M.E.; Cardott, B.J.; Sondergeld, C.H.; Chandra, S.R. Development of organic porosity in the Woodford Shale with increasing thermal maturity. Int. J. Coal Geol. 2012, 103, 26–31. [Google Scholar] [CrossRef]
- Valenza, J.J., II; Drenzek, N.; Marques, J.M.; Pagels, M.; Mastalerz, M. Geochemical controls on shale microstructure. Geology 2013, 41, 611–614. [Google Scholar] [CrossRef]
- Xu, L.W.; Liu, L.F.; Jiang, Z.X.; Chen, L.; Wang, Y. Methane adsorption in the low-middle-matured Neoproterozoic Xiamaling marine shale in Zhangjiakou, Hebei. Aust. J. Earth Sci. 2018, 65, 691–710. [Google Scholar] [CrossRef]
- Löhr, S.C.; Baruch, E.T.; Hall, P.A.; Kennedy, M.J. Is organic pore development in gas shales influenced by the primary porosity and structure of thermally immature organic matter? Org. Geochem. 2015, 87, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.; Zhong, N.N. The role of residual bitumen on the pore structure of organic-rich shales from low to over mature Insight from shale and coal samples after the hydrous pyrolysis. Int. J. Coal Geol. 2020, 226, 92–116. [Google Scholar] [CrossRef]
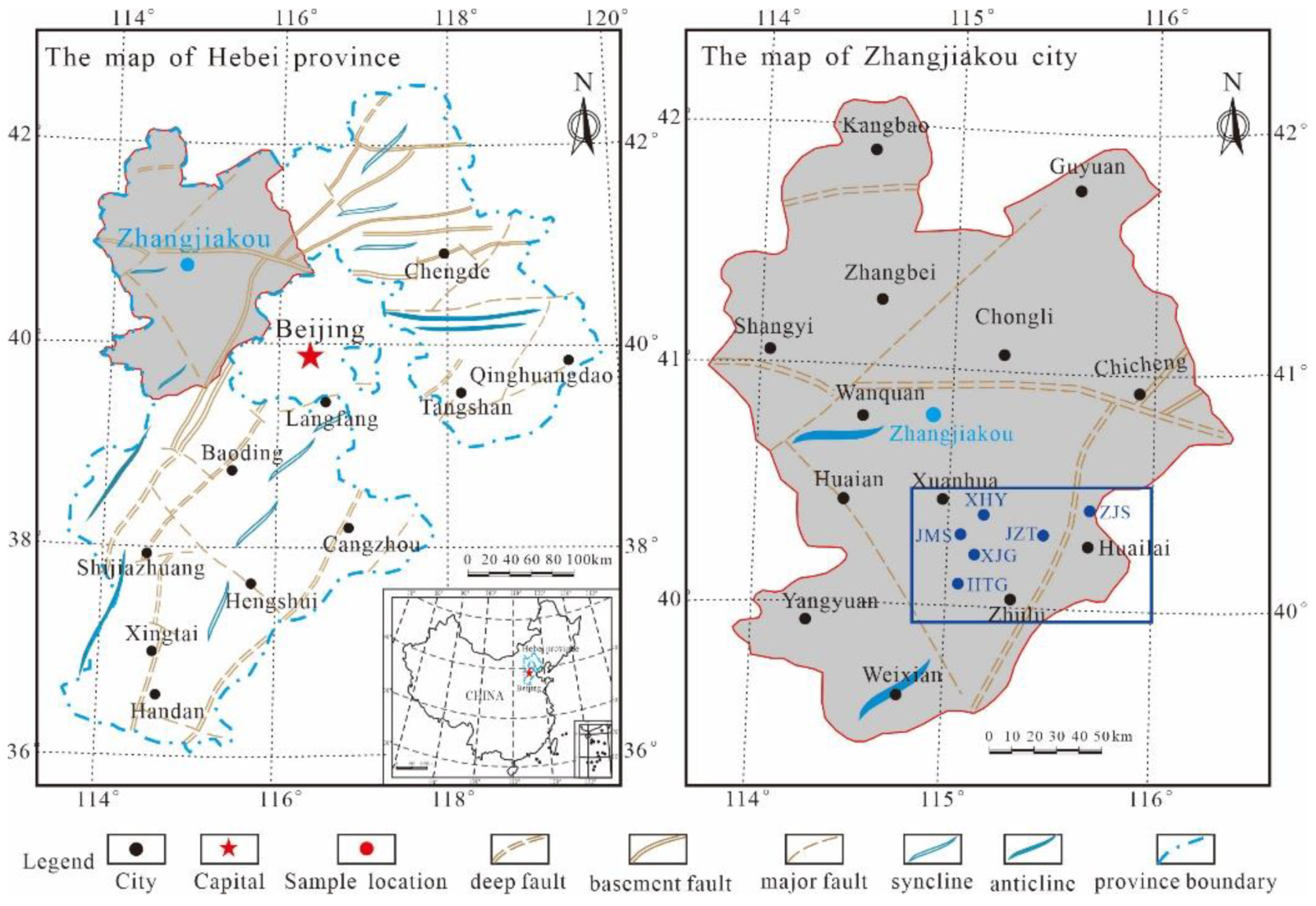





| Sample ID | Formation | TOC (%) | EqRo (%) | Mineralogical Composition Relative Percent (%) | EOM (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quartz | Calcite | Feldspar | Pyrite | Clays | Smectite | I/S | Illite | Chlorite | |||||
| ZJS-1 | Qnx | 2.23 | 0.89 | 31.5 | 10.2 | 3.1 | 2.4 | 52.8 | 6.7 | 15.2 | 27.4 | 3.5 | – |
| ZJS-3 | Qnx | 4.48 | 0.77 | 27.8 | 19.6 | 2.3 | 2.2 | 48.1 | 8.2 | 15.8 | 20.1 | 4.0 | 0.86 |
| ZJS-6 | Qnx | 2.58 | 0.81 | 35.2 | 8.1 | 4.7 | 3.6 | 48.4 | 4.0 | 40.0 | 4.4 | 0 | – |
| JZT-1 | Qnx | 3.41 | 0.83 | 37.8 | 19.1 | 4.0 | 5.1 | 34.0 | 4.9 | 20.1 | 8.1 | 0.9 | – |
| JZT-6 | Qnx | 4.69 | 0.85 | 46.1 | 15.8 | 8.2 | 4.9 | 25.0 | 5.1 | 12.3 | 6.0 | 1.6 | 1.02 |
| XJG-3 | Qnx | 1.31 | 0.93 | 32.2 | 10.1 | 4.9 | 1.9 | 50.9 | 12.3 | 21.8 | 9.9 | 6.9 | – |
| XJG-6 | Qnx | 2.45 | 0.98 | 36.1 | 5.2 | 3.5 | 1.4 | 53.8 | 7.3 | 27.6 | 17.4 | 1.5 | 0.79 |
| XJG-9 | Qnx | 1.47 | 1.01 | 32.6 | 15.7 | 6.7 | 4.1 | 40.9 | 14.6 | 20.8 | 5.5 | 0 | – |
| HTG-2 | Qnx | 4.12 | 1.46 | 27.2 | 28.6 | 3.7 | 1.3 | 39.2 | 8.4 | 14.4 | 16.4 | 0 | – |
| HTG-3 | Qnx | 3.67 | 1.18 | 27.6 | 32.4 | 1.7 | 1.7 | 36.6 | 7.7 | 19.8 | 7.5 | 1.6 | – |
| HTG-4 | Qnx | 4.66 | 1.43 | 48.4 | 24.3 | 1.9 | 3.1 | 22.3 | 7.8 | 8.5 | 5.1 | 0.9 | 0.94 |
| HTG-5 | Qnx | 3.28 | 1.88 | 28.5 | 29.6 | 8.6 | 1.1 | 32.2 | 3.7 | 13.1 | 13.3 | 2.1 | – |
| JMS-4 | Qnx | 0.55 | 0.52 | 28.3 | 5.8 | 9.4 | 7.2 | 49.3 | 12.7 | 25.6 | 6.3 | 4.7 | – |
| JMS-5 | Qnx | 0.97 | 0.62 | 21.8 | 9.6 | 11.3 | 4.4 | 52.9 | 17.9 | 26.7 | 8.3 | 0 | – |
| JMS-9 | Qnx | 6.80 | 1.82 | 42.3 | 22.5 | 11.4 | 0 | 23.8 | 5.6 | 12.1 | 3.8 | 2.3 | 1.42 |
| XHY-3 | Qnx | 0.82 | 0.57 | 26.7 | 12.3 | 10.7 | 3.1 | 47.2 | 13.2 | 25.1 | 5.7 | 3.2 | – |
| XHY-4 | Qnx | 7.60 | 1.62 | 45.8 | 24.3 | 5.1 | 3.3 | 21.5 | 3.6 | 12.8 | 4.1 | 1.0 | 1.93 |
| XHY-7 | Qnx | 0.74 | 1.74 | 40.7 | 5.4 | 2.5 | 0 | 51.4 | 15.4 | 25.8 | 6.2 | 4.0 | – |
| Sample ID | Macropore PV (cm3/g) | Macropore SA (m2/g) | Bulk Density (g/cm3) | Skeletal Density (g/cm3) | Porosity (%) |
|---|---|---|---|---|---|
| ZJS-1 | 0.0091 | 0.2414 | 2.2201 | 2.2614 | 1.84 |
| ZJS-3 | 0.0127 | 0.2909 | 2.1961 | 2.2345 | 1.72 |
| ZJS-6 | 0.0103 | 1.0617 | 2.3139 | 2.3456 | 1.35 |
| JZT-1 | 0.0062 | 2.6703 | 2.2421 | 2.2644 | 0.99 |
| JZT-6 | 0.0091 | 0.2122 | 2.2802 | 2.3332 | 2.27 |
| XJG-3 | 0.0043 | 0.3128 | 2.2146 | 2.2345 | 0.89 |
| XJG-6 | 0.0081 | 0.2139 | 2.3062 | 2.3511 | 1.91 |
| XJG-9 | 0.0042 | 1.5341 | 2.1791 | 2.2163 | 1.68 |
| HTG-2 | 0.0112 | 0.3629 | 2.3577 | 2.3712 | 0.57 |
| HTG-3 | 0.0081 | 0.5828 | 2.2523 | 2.2654 | 0.58 |
| HTG-4 | 0.0043 | 0.2227 | 2.1987 | 2.2625 | 2.82 |
| HTG-5 | 0.0056 | 1.0839 | 2.3513 | 2.3743 | 0.97 |
| JMS-4 | 0.0016 | 0.5642 | 2.3389 | 2.3683 | 1.24 |
| JMS-5 | 0.0041 | 1.2618 | 2.3581 | 2.3825 | 1.03 |
| JMS-9 | 0.0111 | 0.3628 | 2.3079 | 2.3847 | 3.22 |
| XHY-3 | 0.0032 | 0.5509 | 2.6341 | 2.6641 | 1.13 |
| XHY-4 | 0.0136 | 0.2642 | 2.2755 | 2.3454 | 2.98 |
| XHY-7 | 0.0026 | 1.3133 | 2.3903 | 2.4314 | 1.69 |
| Sample ID | N2 Adsorption | CO2 Adsorption | ||||
|---|---|---|---|---|---|---|
| VBJH (cm3/g) | SBET (m2/g) | APD (nm) | VDFT (cm3/g) | SDFT (m2/g) | APD (nm) | |
| ZJS-1 | 0.01701 | 11.7 | 22.69 | 0.00907 | 14.4 | 0.885 |
| ZJS-3 | 0.01699 | 10.7 | 12.34 | 0.01882 | 16.4 | 0.845 |
| ZJS-3-E | 0.01881 | 14.6 | 16.87 | 0.02449 | 18.7 | 1.236 |
| ZJS-6 | 0.01667 | 16.9 | 4.11 | 0.01026 | 19.7 | 0.554 |
| JZT-1 | 0.01715 | 11.4 | 16.23 | 0.00942 | 19.7 | 0.886 |
| JZT-6 | 0.01626 | 12.8 | 11.63 | 0.01931 | 19.8 | 0.995 |
| JZT-6-E | 0.01784 | 16.4 | 14.32 | 0.02148 | 22.5 | 1.689 |
| XJG-3 | 0.01075 | 4.8 | 10.48 | 0.00601 | 14.9 | 0.596 |
| XJG-6 | 0.01935 | 10.7 | 4.45 | 0.01137 | 14.3 | 0.972 |
| XJG-6-E | 0.02154 | 12.2 | 6.89 | 0.01639 | 16.6 | 1.554 |
| XJG-9 | 0.01234 | 8.1 | 4.22 | 0.00584 | 8.2 | 1.511 |
| HTG-2 | 0.01664 | 14.1 | 7.84 | 0.01666 | 23.8 | 0.885 |
| HTG-3 | 0.01293 | 13.5 | 9.36 | 0.00833 | 23.0 | 1.287 |
| HTG-4 | 0.01286 | 11.4 | 8.04 | 0.01063 | 18.7 | 0.844 |
| HTG-4-E | 0.01456 | 14.2 | 12.89 | 0.01945 | 22.3 | 1.248 |
| HTG-5 | 0.01271 | 8.8 | 9.66 | 0.00803 | 20.4 | 1.567 |
| JMS-4 | 0.00459 | 5.4 | 3.98 | 0.00211 | 7.4 | 1.264 |
| JMS-5 | 0.00925 | 4.2 | 5.54 | 0.00428 | 8.7 | 1.563 |
| JMS-9 | 0.02081 | 15.4 | 8.53 | 0.01002 | 29.3 | 1.267 |
| JMS-9-E | 0.02221 | 19.3 | 13.05 | 0.02086 | 34.3 | 1.994 |
| XHY-3 | 0.00512 | 8.3 | 3.66 | 0.00422 | 10.1 | 0.875 |
| XHY-4 | 0.01324 | 14.3 | 4.09 | 0.01262 | 36.6 | 0.866 |
| XHY-4-E | 0.01963 | 19.3 | 9.29 | 0.02093 | 41.3 | 1.632 |
| XHY-7 | 0.00589 | 5.1 | 3.54 | 0.00359 | 8.6 | 0.894 |
| Sample ID | CO 2 Adsorption | N2 Adsorption | ||
|---|---|---|---|---|
| Gap of VDFT (cm3/g) | Gap of SDFT (m2/g) | Gap of VBJH (cm3/g) | Gap of SBET (m2/g) | |
| ZJS-3 | 0.006 | 2.309 | 0.002 | 3.890 |
| JZT-6 | 0.010 | 2.620 | 0.002 | 3.551 |
| XJG-6 | 0.005 | 2.311 | 0.002 | 1.579 |
| HTG-4 | 0.009 | 3.625 | 0.002 | 2.807 |
| JMS-9 | 0.011 | 5.060 | 0.001 | 3.967 |
| XHY-4 | 0.001 | 4.676 | 0.006 | 4.992 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Yang, K.; Wei, H.; Liu, L.; Li, X.; Chen, L.; Xu, T.; Wang, X. Full-Scale Pore Structure Characteristics and the Main Controlling Factors of Mesoproterozoic Xiamaling Shale in Zhangjiakou, Hebei, China. Nanomaterials 2021, 11, 527. https://doi.org/10.3390/nano11020527
Xu L, Yang K, Wei H, Liu L, Li X, Chen L, Xu T, Wang X. Full-Scale Pore Structure Characteristics and the Main Controlling Factors of Mesoproterozoic Xiamaling Shale in Zhangjiakou, Hebei, China. Nanomaterials. 2021; 11(2):527. https://doi.org/10.3390/nano11020527
Chicago/Turabian StyleXu, Liangwei, Keji Yang, Hao Wei, Luofu Liu, Xiao Li, Lei Chen, Tong Xu, and Ximeng Wang. 2021. "Full-Scale Pore Structure Characteristics and the Main Controlling Factors of Mesoproterozoic Xiamaling Shale in Zhangjiakou, Hebei, China" Nanomaterials 11, no. 2: 527. https://doi.org/10.3390/nano11020527







