ALD Deposited ZnO:Al Films on Mica for Flexible PDLC Devices
Abstract
:1. Introduction
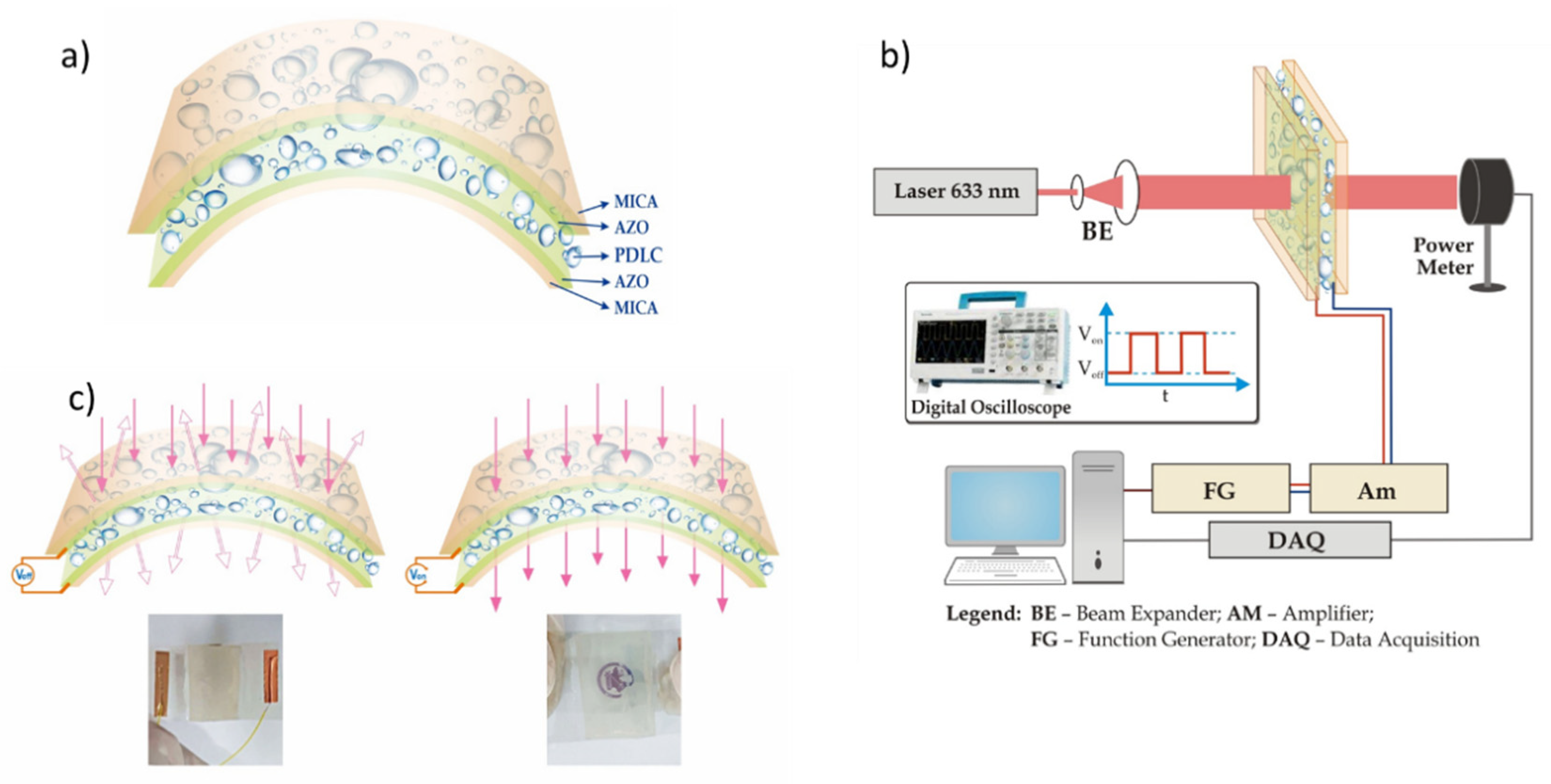
2. Materials and Methods
3. Results
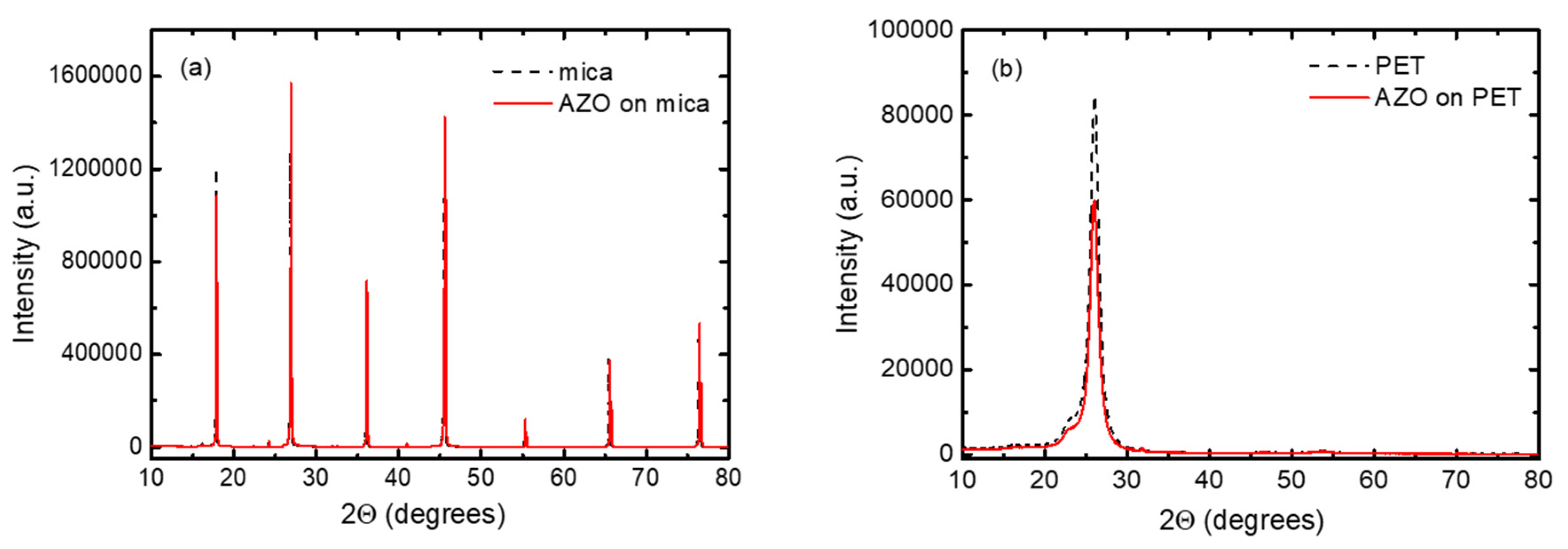
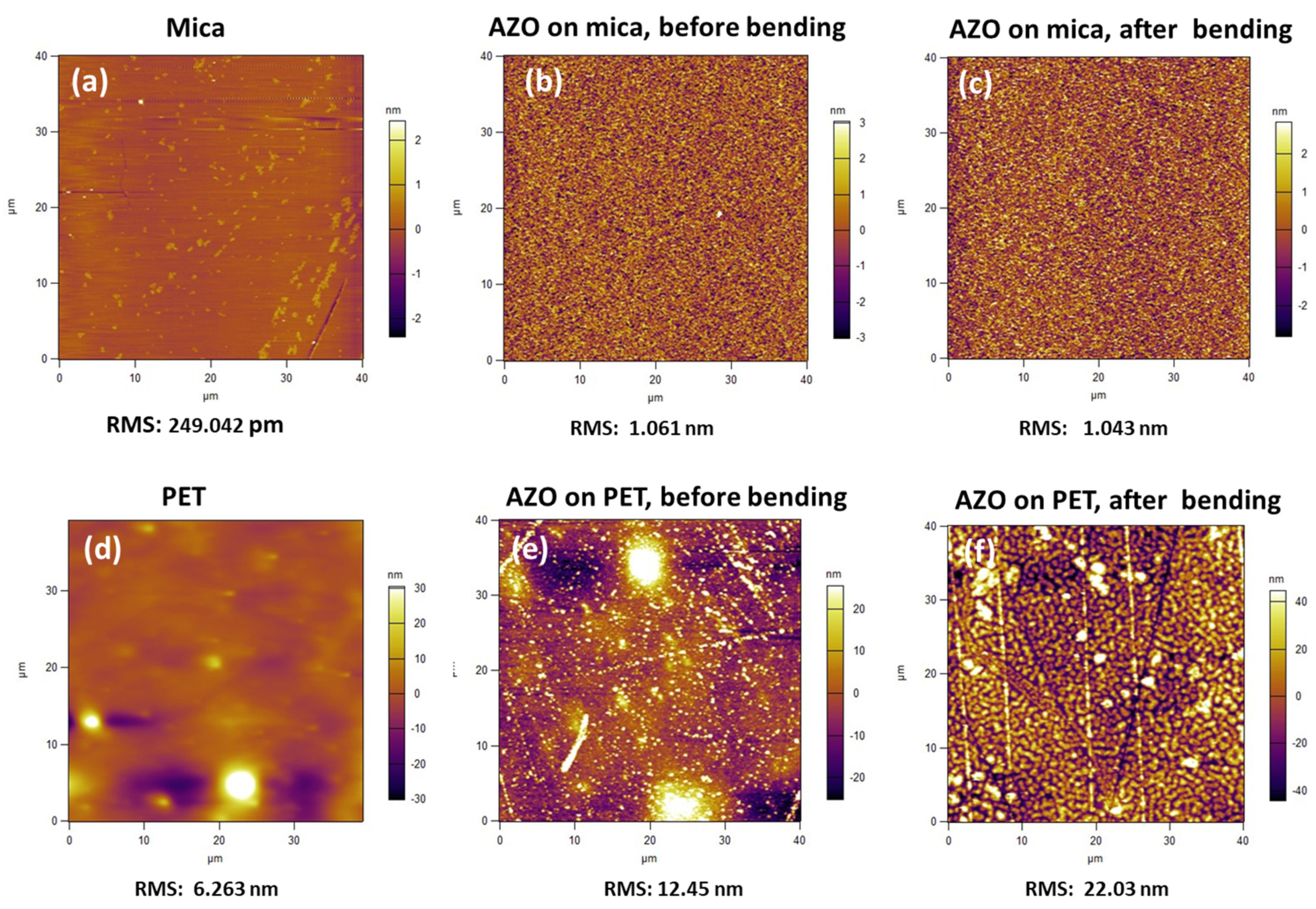
3.1. Structural and Morphological Properties
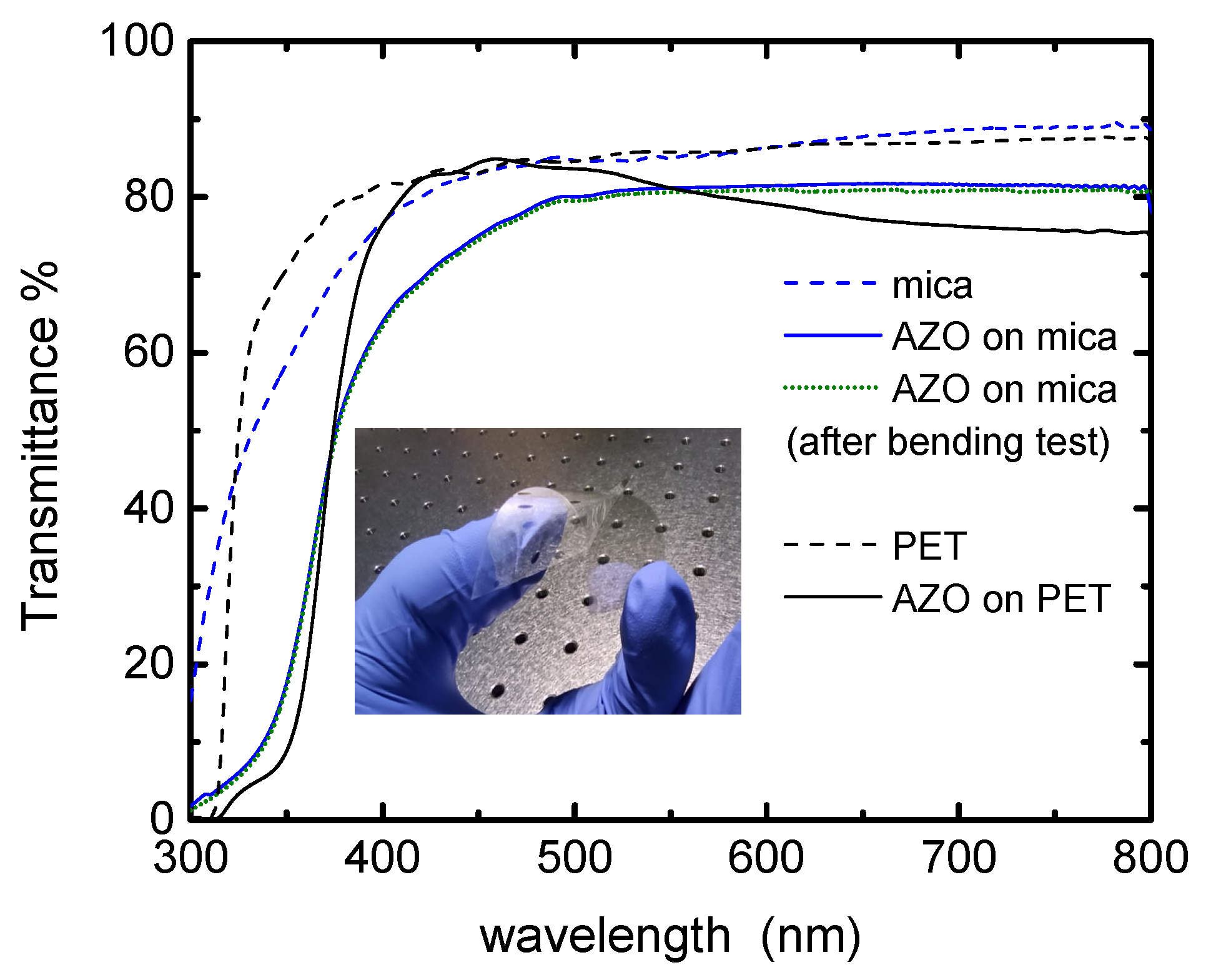
3.2. Optical Properties
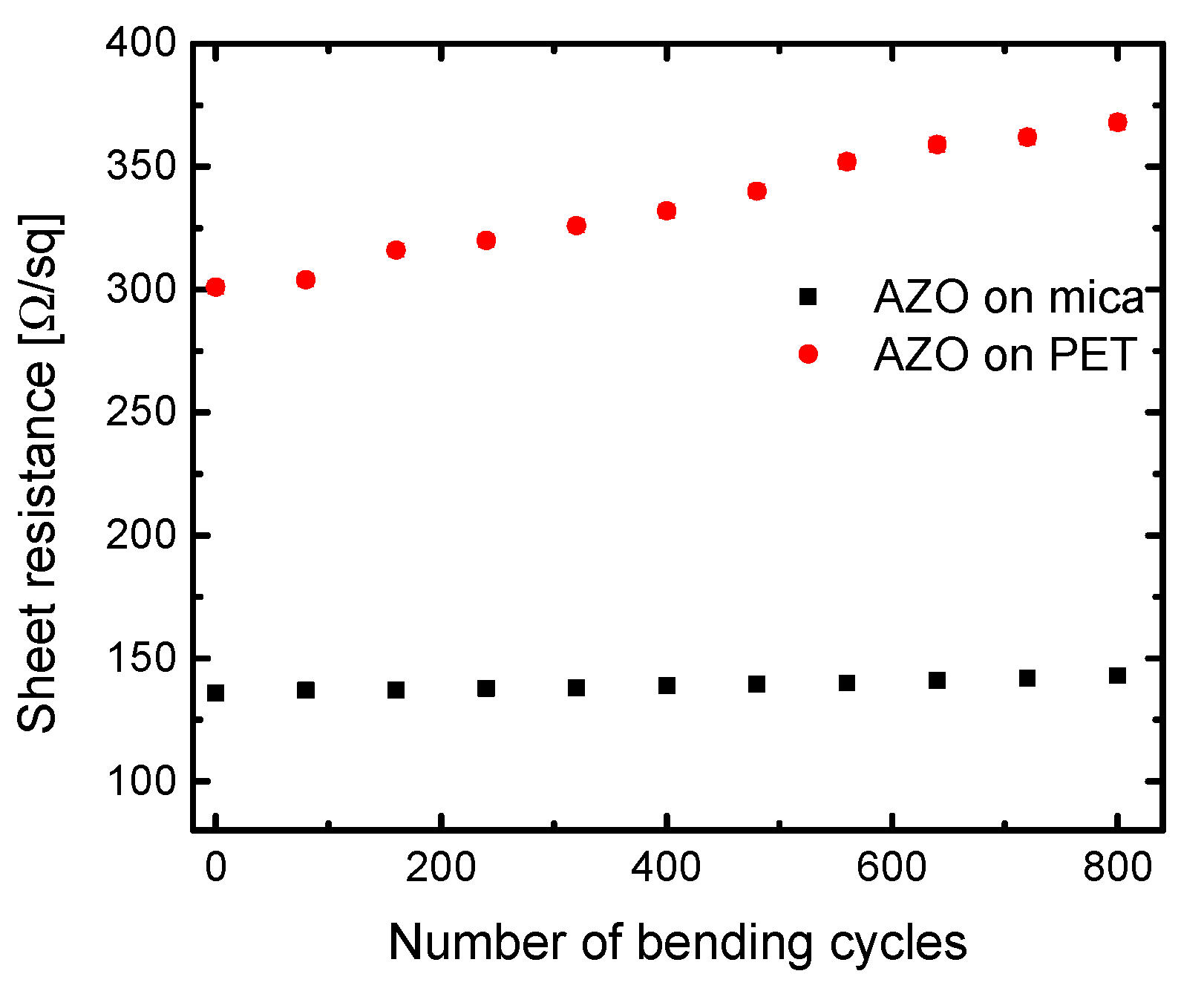
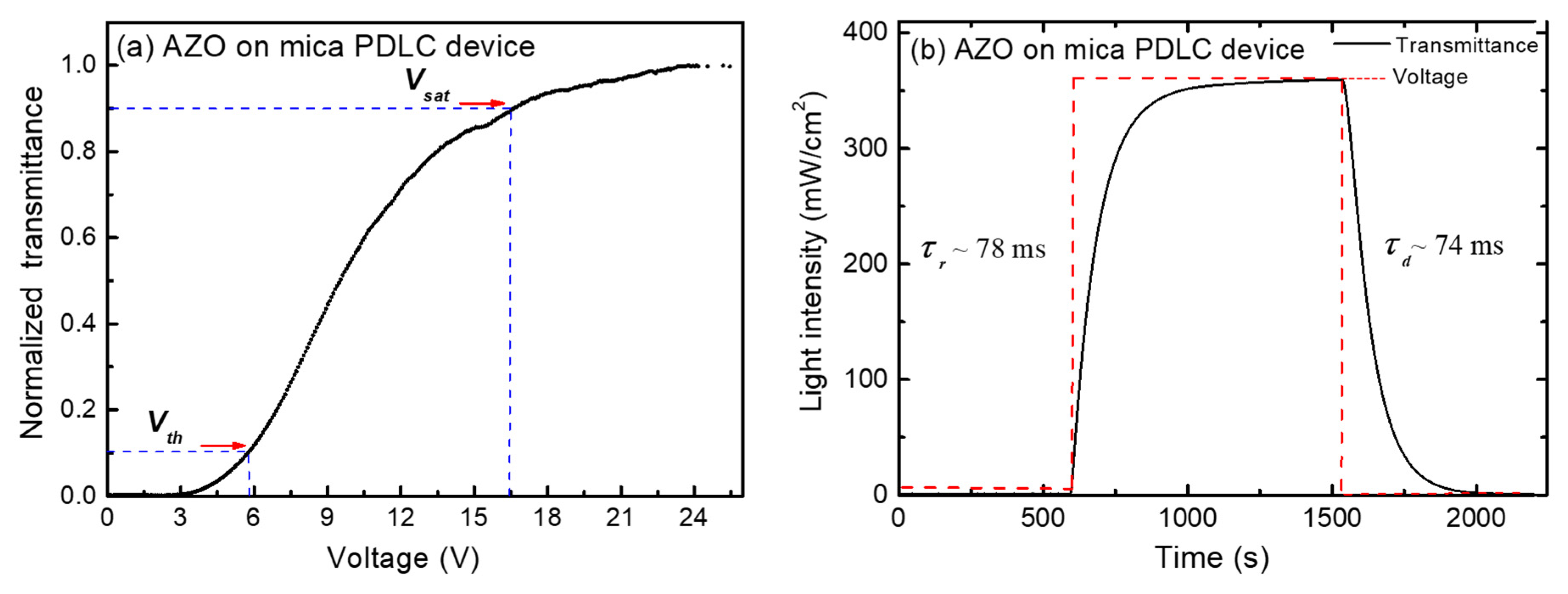
3.3. Electrical Properties and Bending Test
3.4. AZO on Mica for Assembling PDLC Devices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vidor, F.F.; Meyers, T.; Hilleringmann, U. Flexible Electronics: Integration processes for organic and inorganic semiconductor-based thin-film transistors electronics. Electronics 2015, 4, 480–506. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ma, C.; Lu, X.; Liu, M. Recent progress on flexible inorganic single-crystalline functional oxide films for advanced electronics. Mater. Horiz. 2019, 6, 911–930. [Google Scholar] [CrossRef]
- Du, Y.; Xu, J.; Paul, B.; Eklund, P. Flexible thermoelectric materials and devices. Appl. Mat. Today 2018, 12, 366–388. [Google Scholar] [CrossRef]
- Tynell, T.; Karppinen, M. Atomic layer deposition of ZnO: A review. Semicond. Sci. Technol. 2014, 29, 043001. [Google Scholar] [CrossRef]
- Gao, Z.; Banerjee, P. Review Article: Atomic layer deposition of doped ZnO films. J. Vac. Sci. Technol. A 2019, 37, 050802. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Zhi, L. Graphene-Based Transparent Conductive Films: Material Systems. Prep. Appl. Small Methods 2019, 3, 1800199. [Google Scholar] [CrossRef] [Green Version]
- Stadler, A. Transparent conducting oxides-an up-to-date overview. Materials 2012, 5, 661–683. [Google Scholar] [CrossRef]
- Masis, M.M.; Wolf, S.D.; Robinson, R.W.; Ager, J.W.; Ballif, C. Transparent electrodes for efficient optoelectronics. Adv. Electron. Mater. 2017, 3, 1600529. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Shriwastava, S.; Kumar, S.; Bhatt, K.; Tripathi, C.C. Alternative transparent conducting electrode materials for flexible optoelectronic devices. Opto-Electr. Rev. 2018, 26, 223–235. [Google Scholar] [CrossRef]
- Minami, T. Transparent conducting oxide semiconductors for transparent electrodes. Semicond. Sci. Technol. 2005, 20, S35–S44. [Google Scholar] [CrossRef]
- Minami, T. Substitution of transparent conducting oxide thin films for indium tin oxide transparent electrode applications. Thin. Solid Films 2008, 516, 1314–1321. [Google Scholar] [CrossRef]
- Gordon, P.G.; Balcic, G.; Lopinski, G.P.; Barry, S.T. Work function of doped zinc oxide films deposited by ALD. J. Mater. Res. 2020, 35, 756–761. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Ke, S.; Chen, C.; Fu, N.; Zhou, H.; Ye, M.; Lin, P.; Yuan, W.; Zeng, X.; Chen, L.; Huang, H. Transparent Indium Tin Oxide Electrodes on Muscovite Mica for High-Temperature-Processed Flexible Optoelectronic Devices. ACS Appl. Mater. Interfaces 2016, 8, 28406–28411. [Google Scholar] [CrossRef]
- Yen, M.; Bitla, Y.; Chu, Y.-H. Van der Waals heteroepitaxy on muscovite. Mater. Chem. Phys. 2019, 234, 185–195. [Google Scholar] [CrossRef]
- Bitla, Y.; Chu, Y.-H. MICAtronics: A new platform for flexible X-tronics. Flat Chem. 2017, 3, 26–42. [Google Scholar] [CrossRef]
- Bitla, Y.; Chu, Y.-H. van der Waals oxide heteroepitaxy for soft transparent electronics. Nanoscale 2020, 12, 18523–18544. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Khranovskyy, V.; Yakimova, R.; Eklund, P. Donor-doped ZnO thin films on mica for fully inorganic flexible thermoelectrics. Mater. Res. Lett. 2019, 7, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Bitla, Y.; Chen, C.; Lee, H.-C.; Do, T.H.; Ma, C.-H.; Qui, L.V.; Huang, C.-W.; Wu, W.-W.; Chang, L.; Chiu, P.-W.; et al. Oxide Heteroepitaxy for Flexible Optoelectronics. ACS Appl. Mater. Interfaces 2016, 8, 32401–32407. [Google Scholar] [CrossRef]
- Chu, Y.H. Van der Waals oxide heteroepitaxy. NPJ Quantum Mater. 2017, 2, 67. [Google Scholar] [CrossRef] [Green Version]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Properties, requirements, and possibilities of smart windows for dynamic daylight and solar energy control in buildings: State-of-the-art. Sol. Energy Mater. Sol. Cells 2010, 94, 8–105. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.H.; Noh, H.Y. Polymer-dispersed liquid crystal devices with graphene electrodes. Opt. Express 2015, 23, 32149. [Google Scholar] [CrossRef]
- Luka, G.; Witkowski, B.S.; Wachnicki, L.; Jakiela, R.; Virt, I.S.; Andrzejczuk, M.; Lewandowska, M.; Godlewski, M. Electrical and mechanical stability of aluminum-doped ZnO films grown on flexible substrates by atomic layer deposition. Mater. Sci. Eng. B 2014, 186, 15–20. [Google Scholar] [CrossRef]
- Dhakal, T.; Nandur, A.; Christian, R.; Vanhart, D.; Vasekar, P.; Westgate, C.R. Growth of AZO on flexible substrate using ALD system as a transparent conducting oxide for solar cells. Mater. Res. Soc. Symp. Proc. 2011, 1327. [Google Scholar] [CrossRef]
- Lee, G.-B.; Song, S.H.; Lee, M.-W.; Kim, Y.-J.; Choi, B.-H. Characterization of physical and mechanical properties of Al2O3-doped ZnO (AZO) thin films deposited on transparent polyimide supports with various ALD process parameters. Appl. Surf. Sci. 2021, 535, 147731. [Google Scholar] [CrossRef]
- Marques, A.C.; Faria, J.; Perdigão, P.; Faustino, B.M.M.; Ritasalo, R.; Costabello, K.; da Silva, R.C.; Ferreira, I. Stability under humidity, UV-light and bending of AZO films deposited by ALD on Kapton. Sci. Rep. 2019, 9, 17919. [Google Scholar] [CrossRef]
- Blagoev, B.S.; Aleksandrova, M.; Terziyska, P.; Tzvetkov, P.; Kovacheva, D.; Kolev, G.; Mehandzhiev, V.; Denishev, K.; Dimitrov, D. Investigation of the structural, optical and piezoe-lectric properties of ALD ZnO films on PEN substrates. J. Phys. Conf. Ser. 2018, 992, 012027. [Google Scholar] [CrossRef] [Green Version]
- Blagoev, B.S.; Dimitrov, D.Z.; Mehandzhiev, V.B.; Kovacheva, D.; Terziyska, P.; Pavlic, J.; Lovchinov, K.; Mateev, E.; Leclercq, J.; Sveshtarov, P. Electron transport in Al-doped ZnO nanolayers obtained by atomic layer deposition. J. Phys. Conf. Ser. 2016, 700, 012040. [Google Scholar] [CrossRef]
- Blagoev, B.S.; Vlakhov, E.; Videkov, V.; Tzaneva, B.; Łuka, G.; Witkowski, B.S.; Terziyska, P.; Leclercq, J.; Krajewski, T.A.; Guziewicz, E.; et al. Atomic layer deposition of ZnO: Al on PAA substrates. J. Phys. Conf. Ser. 2016, 764, 012004. [Google Scholar] [CrossRef]
- Kwon, S.J. Effect of precursor-pulse on properties of Al-doped ZnO films grown by Atomic Layer Deposition. Jpn. J. Appl. Phys. 2005, 44, 1062. [Google Scholar] [CrossRef]
- Banerjee, P.; Lee, W.-J.; Bae, K.-R.; Lee, S.B.; Rubloff, G.W. Structural, electrical, and optical properties of atomic layer deposition Al-doped ZnO films. J. Appl. Phys. 2010, 108, 043504. [Google Scholar] [CrossRef] [Green Version]
- Ke, S.; Xie, J.; Chen, C.; Lin, P.; Zeng, X.; Shu, L.; Fei, L.; Wang, Y.; Ye, M.; Wang, D. van der Waals epitaxy of Al-doped ZnO film on mica as a flexible transparent heater with ultrafast thermal response. Appl. Phys. Lett. 2018, 112, 031905. [Google Scholar] [CrossRef]
- Dong, B.-Z.; Hu, H.; Fang, G.-J.; Zhao, X.-Z.; Zheng, D.-Y.; Sun, Y.-P. Comprehensive investigation of structural, electrical, and optical properties for ZnO: Al films deposited at different substrate temperature and oxygen ambient. Appl. Phys. 2008, 103, 073711. [Google Scholar] [CrossRef]
- Coates, D. Polymer-dispersed liquid crystals. J. Mater. Chem. 1995, 5, 2063–2072. [Google Scholar] [CrossRef]
- Pozhidaev, E.P.; Kaznacheev, A.V.; Torgova, S.I.; Kesaev, V.V.; Barbashov, V.A. Polymer dispersed liquid crystals with electrically controlled light scattering in the visible and near-infrared ranges. Opt. Mater. Express 2020, 10, 3030–3040. [Google Scholar] [CrossRef]
- Dimitrov, D.; Marinova, V.; Petrov, S.; Petrova, D.; Napoleonov, B.; Blagoev, B.; Strijkova, V.; Hsu, K.Y.; Lin, S.H. Atomic layer deposited Al-doped ZnO thin films for display applications. Coatings 2020, 10, 539. [Google Scholar] [CrossRef]
- Chiou, C.C.; Hsu, F.H.; Petrov, S.; Marinova, V.; Dikov, H.; Vitanov, P.; Dimitrov, D.; Hsu, K.Y.; Lin, Y.H.; Lin, S.H. Flexible light valves using polymer dispersed liquid crystals and TiO2/Ag/TiO2 multilayers. Opt. Express 2019, 27, 16911–16921. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.C.; Marinova, V.; Petrov, S.; Fidanova, C.; Angelova, I.; Petrova, D.; Dimitrov, D.Z.; Lin, S.H. Flexible and stretchable optoelectronic devices using graphene. In Proceedings of the SPIE, 20th International Conference and School on Quantum Electronics: Laser Physics and Applications, Nessebar, Bulgaria, 17–21 September 2019; p. 110471H. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrov, D.Z.; Chen, Z.F.; Marinova, V.; Petrova, D.; Ho, C.Y.; Napoleonov, B.; Blagoev, B.; Strijkova, V.; Hsu, K.Y.; Lin, S.H.; et al. ALD Deposited ZnO:Al Films on Mica for Flexible PDLC Devices. Nanomaterials 2021, 11, 1011. https://doi.org/10.3390/nano11041011
Dimitrov DZ, Chen ZF, Marinova V, Petrova D, Ho CY, Napoleonov B, Blagoev B, Strijkova V, Hsu KY, Lin SH, et al. ALD Deposited ZnO:Al Films on Mica for Flexible PDLC Devices. Nanomaterials. 2021; 11(4):1011. https://doi.org/10.3390/nano11041011
Chicago/Turabian StyleDimitrov, Dimitre Z., Zih Fan Chen, Vera Marinova, Dimitrina Petrova, Chih Yao Ho, Blagovest Napoleonov, Blagoy Blagoev, Velichka Strijkova, Ken Yuh Hsu, Shiuan Huei Lin, and et al. 2021. "ALD Deposited ZnO:Al Films on Mica for Flexible PDLC Devices" Nanomaterials 11, no. 4: 1011. https://doi.org/10.3390/nano11041011





