Specific Absorption Rate Dependency on the Co2+ Distribution and Magnetic Properties in CoxMn1-xFe2O4 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CoxMn1-xFe2O4 (x = 0, 0.2, 0.4, 0.6, 0.8, and 1.0) Nanoparticles
2.2. Characterization of the Nanoparticles
2.3. Magneto Thermal Measurements
3. Results and Discussions
3.1. XRD of CoxMn1-xFe2O4 (x = 0, 0.2, 0.4, 0.6, 0.8, and 1.0) Nanoparticles
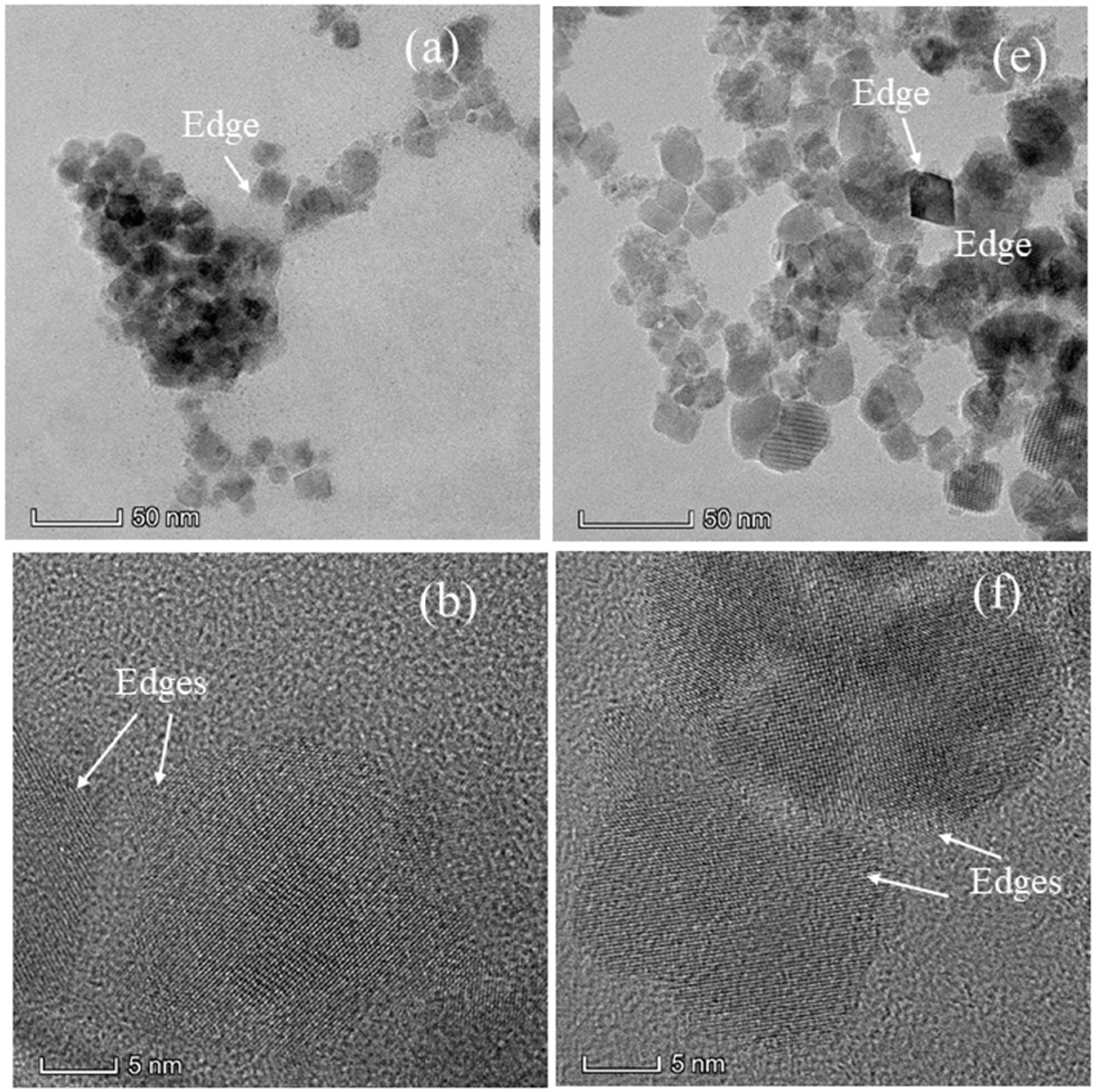
3.2. TEM Images and SAED Pattern of CoxMn1-xFe2O4 (x = 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0) Nanoparticles
3.3. Raman Spectra of CoxMn1-xFe2O4 (x = 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0) Nanoparticles
3.4. Mössbauer Spectrum of Co0.2Mn0.8Fe2O4 Nanoparticles
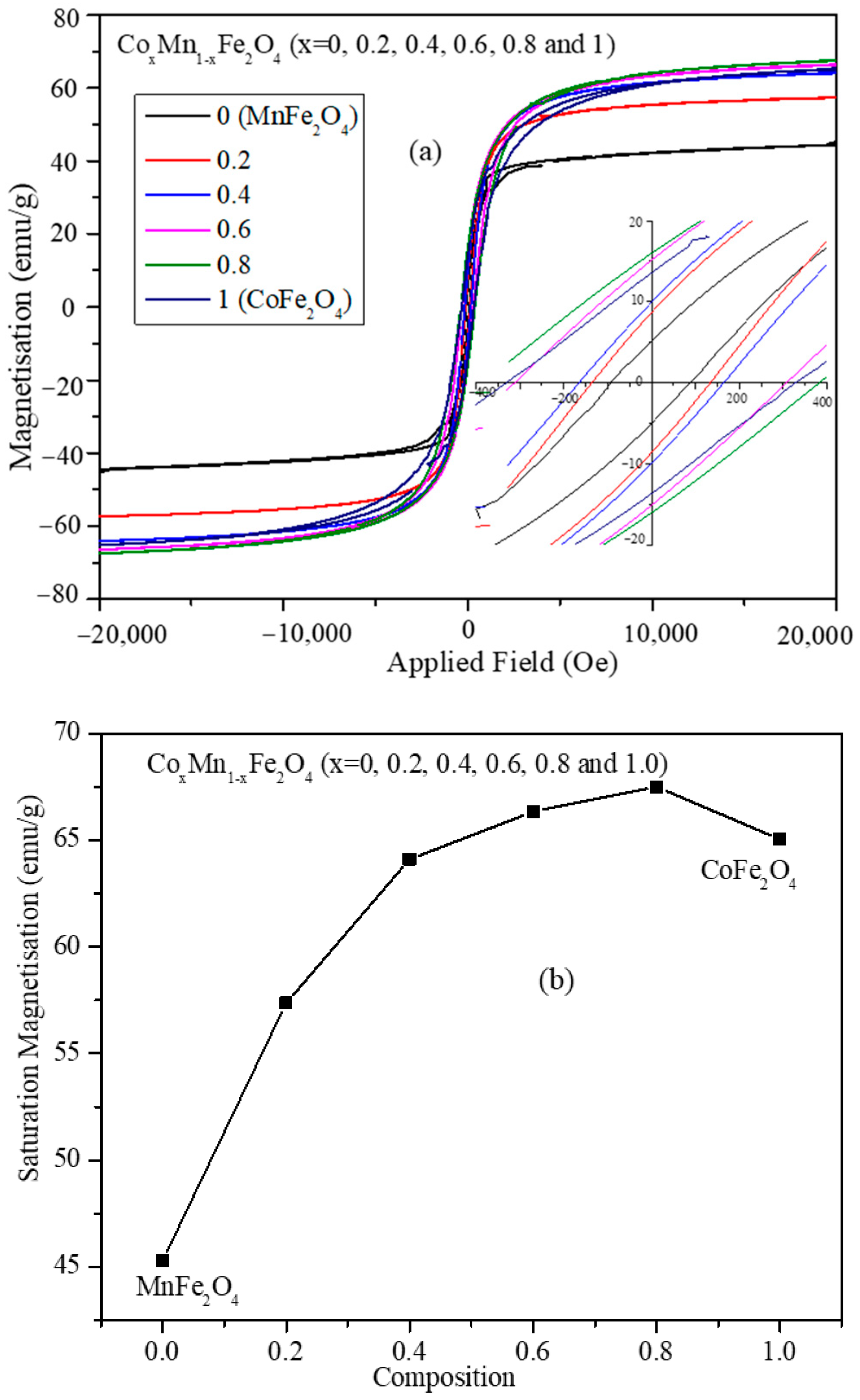
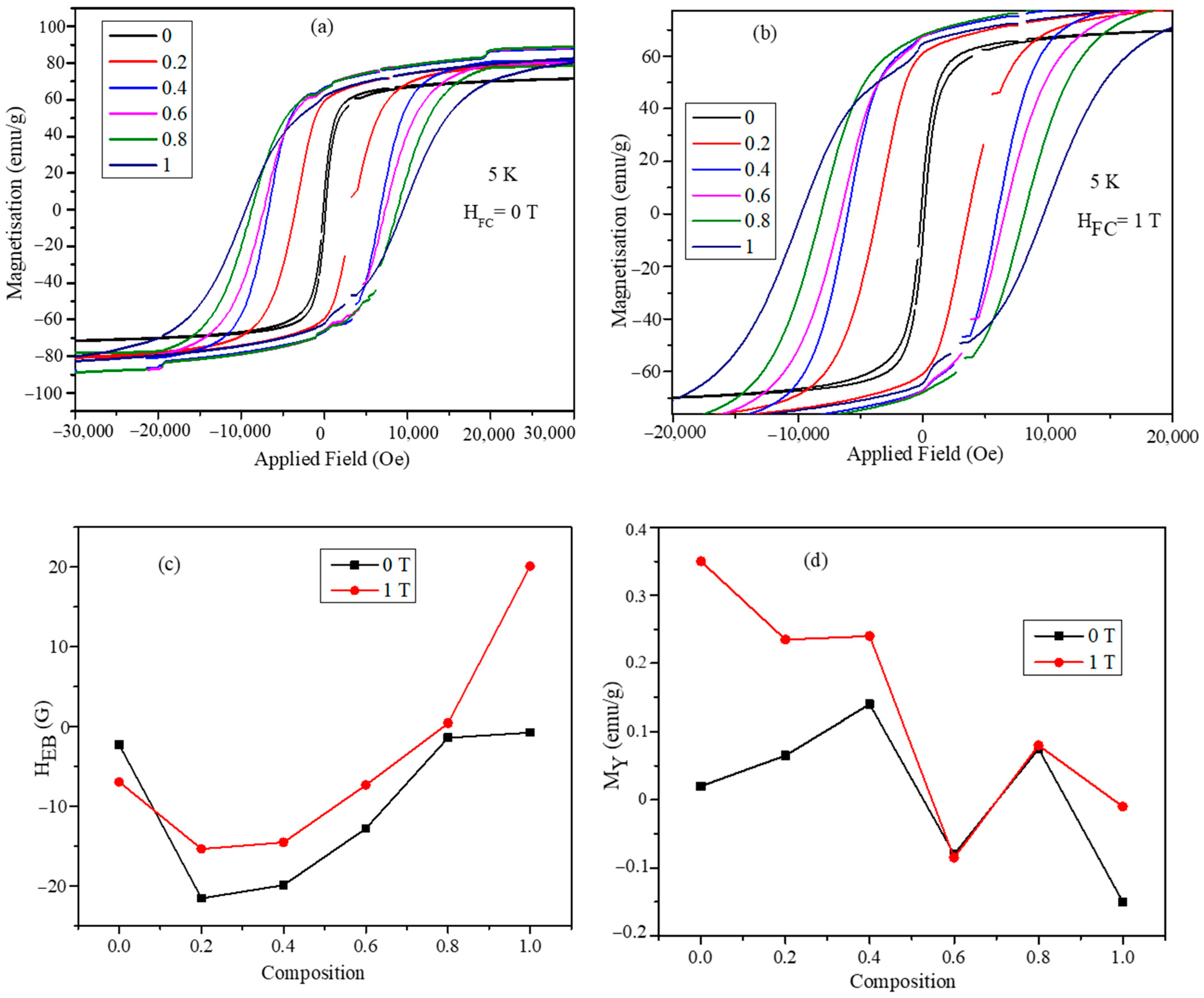
3.5. Magnetic Characterization of CoxMn1-xFe2O4 (x = 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0) Nanoparticles
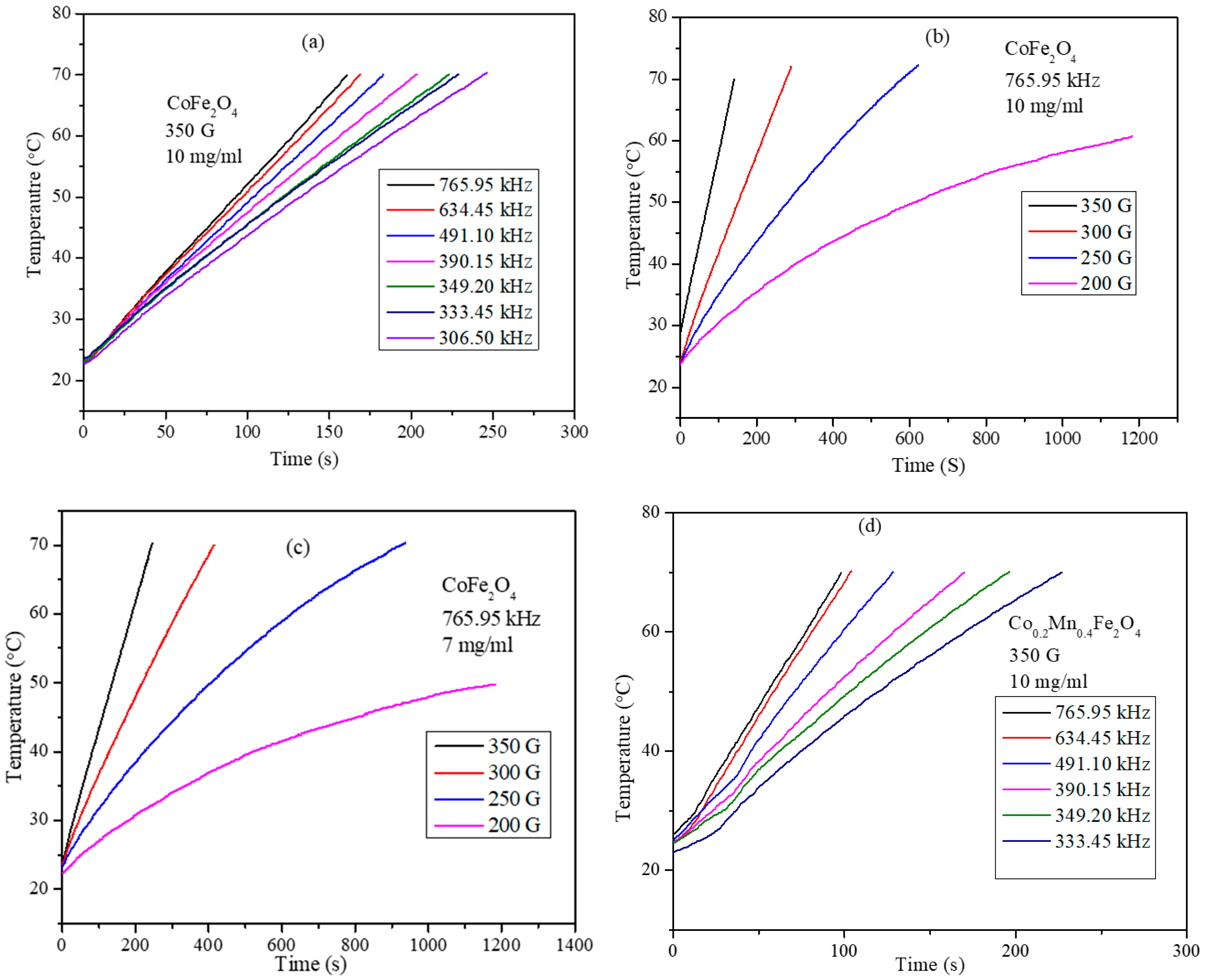
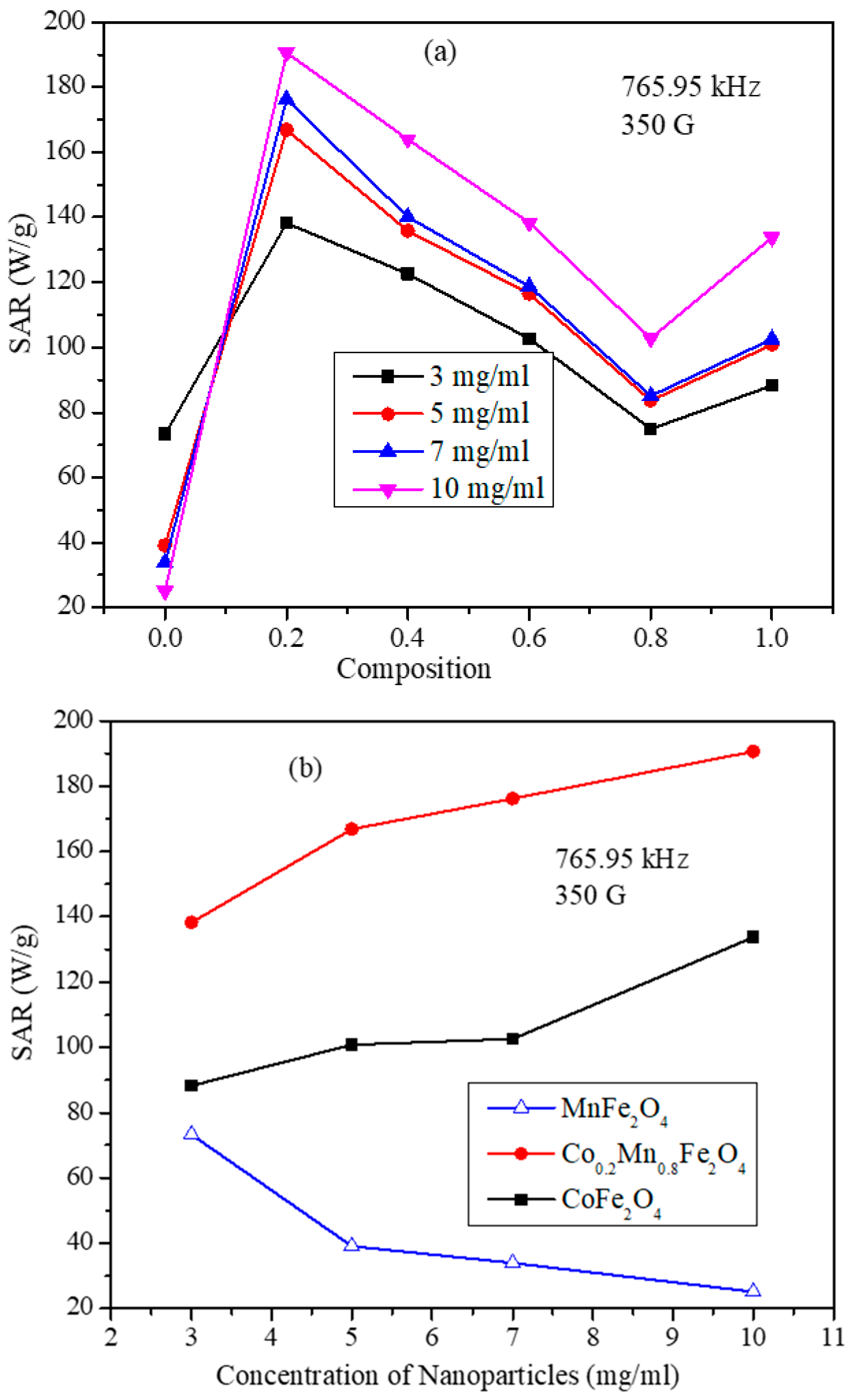
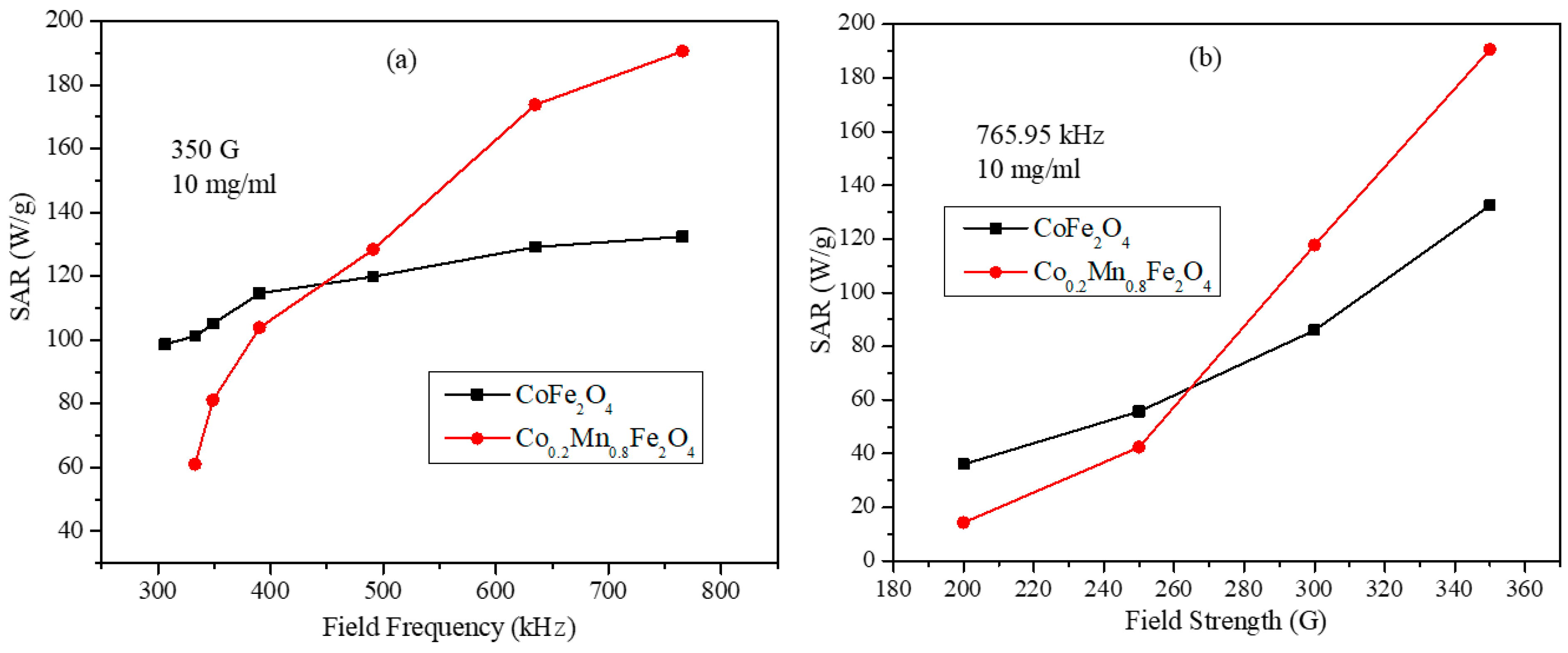
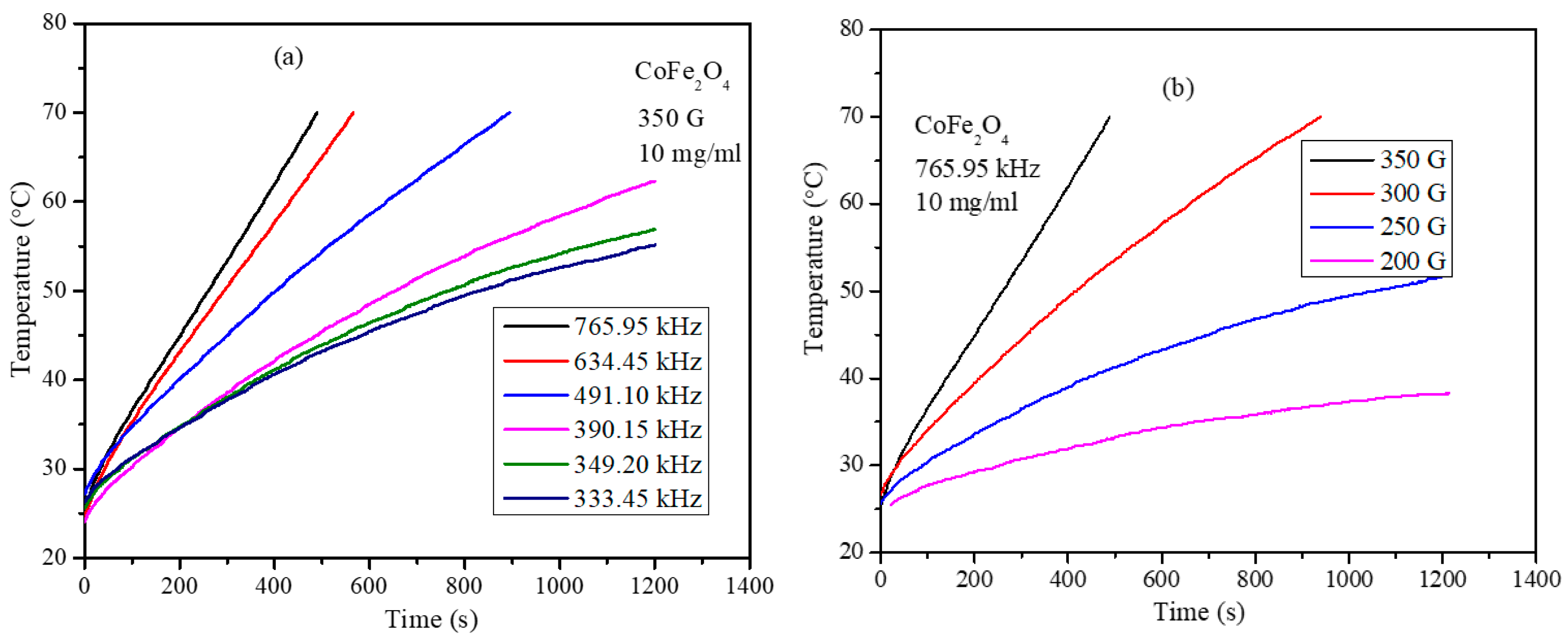
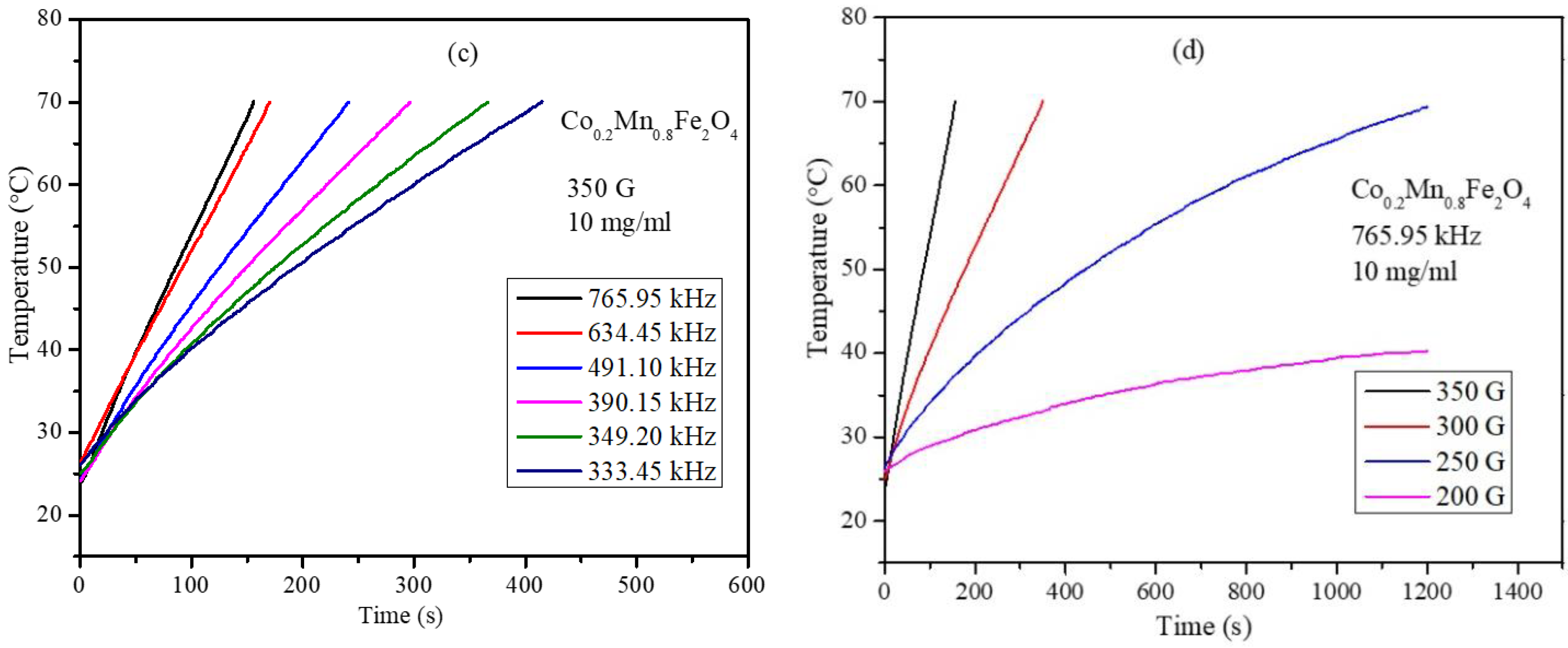
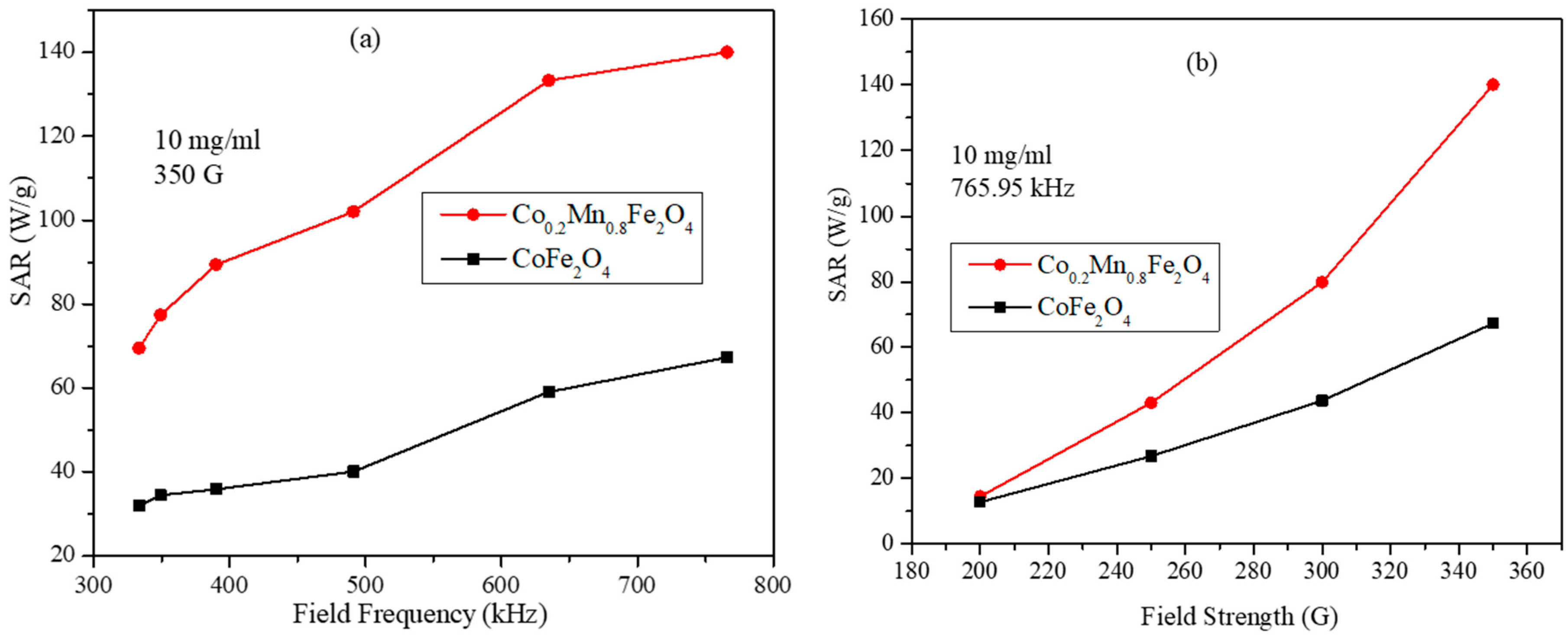
3.6. Magnetic Hyperthermia Studies of the CoxMn1-xFe2O4 (x = 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0) Nanoparticle Water Dispersions
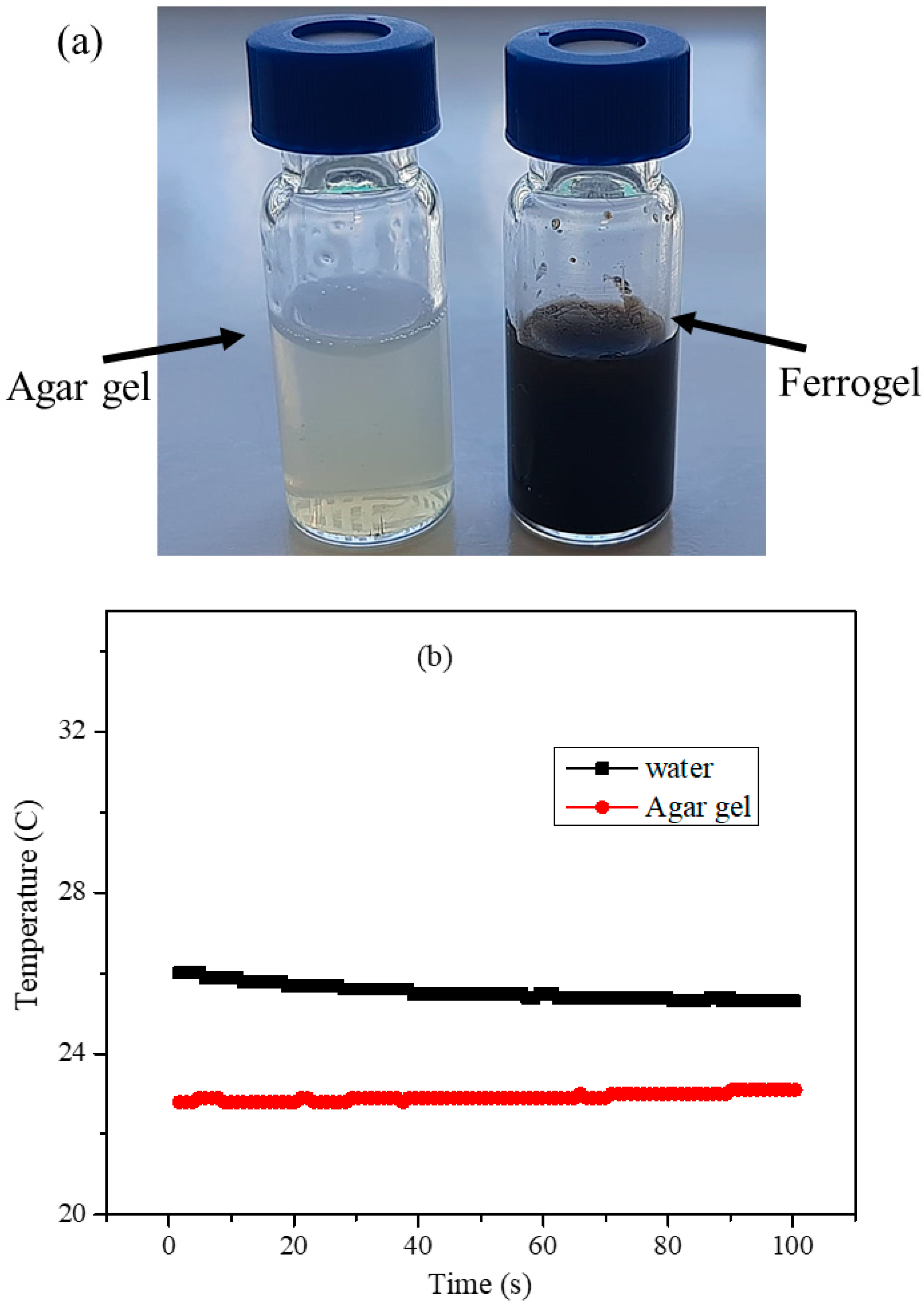
3.7. Agar Hydrogel Phantom for the Hyperthermia Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McNamara, K.; Tofail, S.A.M. Nanoparticles in Biomedical Applications. Adv. Phys. X 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Laurent, S.; Elst, L.V.; Muller, R.N. Superparamagnetic Iron Oxide Nanoparticles for MRI. In The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2013; pp. 427–447. ISBN 978-1-118-50365-2. [Google Scholar]
- Price, P.M.; Mahmoud, W.E.; Al-Ghamdi, A.A.; Bronstein, L.M. Magnetic Drug Delivery: Where the Field Is Going. Front. Chem. 2018, 6, 619. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.C.; Zhang, Y.; Steinberg, G.; Qu, H.; Huang, S.; Cheng, M.; Bliss, T.; Du, F.; Rao, J.; Song, G.; et al. A Review of Magnetic Particle Imaging and Perspectives on Neuroimaging. Am. J. Neuroradiol. 2019, 40, 206–212. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic Particle Hyperthermia: Nanoparticle Magnetism and Materials Development for Cancer Therapy. J. Phys. Condens. Matter 2006, 18, S2919–S2934. [Google Scholar] [CrossRef]
- Bañobre-López, M.; Teijeiro, A.; Rivas, J. Magnetic Nanoparticle-Based Hyperthermia for Cancer Treatment. Rep. Pract. Oncol. Radiother. 2013, 18, 397–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of Iron Oxide-Based Nanoparticles for MRI and Magnetic Hyperthermia. Nanomedicine 2016, 11, 1889–1910. [Google Scholar] [CrossRef] [PubMed]
- Venkatesha, N.; Pudakalakatti, S.M.; Qurishi, Y.; Atreya, H.S.; Srivastava, C. MnFe2O4–Fe3O4 Core–Shell Nanoparticles as a Potential Contrast Agent for Magnetic Resonance Imaging. RSC Adv. 2015, 5, 97807–97815. [Google Scholar] [CrossRef]
- Chung, H.; Bae, H.; Kim, C.; Rhee, I. Cube-Shaped Triethylene Glycol-Coated Ni−Mn Ferrite Nanoparticles for Use as T2 Contrast Agents in Magnetic Resonance Imaging. J. Korean Phys. Soc. 2019, 74, 48–52. [Google Scholar] [CrossRef]
- Yang, G.; Phua, S.Z.F.; Bindra, A.K.; Zhao, Y. Degradability and Clearance of Inorganic Nanoparticles for Biomedical Applications. Adv. Mater. 2019, 31, 1805730. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Narayanaswamy, V.; Alaabed, S.; Sambasivam, S.; Muralee Gopi, C.V.V. Principles of Magnetic Hyperthermia: A Focus on Using Multifunctional Hybrid Magnetic Nanoparticles. Magnetochemistry 2019, 5, 67. [Google Scholar] [CrossRef] [Green Version]
- Kafrouni, L.; Savadogo, O. Recent Progress on Magnetic Nanoparticles for Magnetic Hyperthermia. Prog. Biomater. 2016, 5, 147–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abenojar, E.C.; Wickramasinghe, S.; Bas-Concepcion, J.; Samia, A.C.S. Structural Effects on the Magnetic Hyperthermia Properties of Iron Oxide Nanoparticles. Prog. Nat. Sci. Mater. Int. 2016, 26, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Obaidat, I.M.; Issa, B.; Haik, Y. The Role of Aggregation of Ferrite Nanoparticles on Their Magnetic Properties. J. Nanosci. Nanotechnol. 2011, 11, 3882–3888. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Kameli, P.; Salamati, H.; Concas, G.; Salvador Fernandez, M.; Talone, A.; Muscas, G.; Peddis, D. Co-Doped MnFe2O4 Nanoparticles: Magnetic Anisotropy and Interparticle Interactions. Beilstein J. Nanotechnol. 2019, 10, 856–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerroum, M.A.A.; Iacovita, C.; Baaziz, W.; Ihiawakrim, D.; Rogez, G.; Benaissa, M.; Lucaciu, C.M.; Ersen, O. Quantitative Analysis of the Specific Absorption Rate Dependence on the Magnetic Field Strength in ZnxFe3−xO4 Nanoparticles. Int. J. Mol. Sci. 2020, 21, 7775. [Google Scholar] [CrossRef] [PubMed]
- Ondeck, C.L.; Habib, A.H.; Ohodnicki, P.; Miller, K.; Sawyer, C.A.; Chaudhary, P.; McHenry, M.E. Theory of Magnetic Fluid Heating with an Alternating Magnetic Field with Temperature Dependent Materials Properties for Self-Regulated Heating. J. Appl. Phys. 2009, 105, 07B324. [Google Scholar] [CrossRef]
- Glöckl, G.; Hergt, R.; Zeisberger, M.; Dutz, S.; Nagel, S.; Weitschies, W. The Effect of Field Parameters, Nanoparticle Properties and Immobilization on the Specific Heating Power in Magnetic Particle Hyperthermia. J. Phys. Condens. Matter 2006, 18, S2935–S2949. [Google Scholar] [CrossRef]
- Deatsch, A.E.; Evans, B.A. Heating Efficiency in Magnetic Nanoparticle Hyperthermia. J. Magn. Magn. Mater. 2014, 354, 163–172. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Issa, B.; Haik, Y. Magnetic Properties of Magnetic Nanoparticles for Efficient Hyperthermia. Nanomaterials 2015, 5, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuncser, A.; Iacob, N.; Kuncser, V.E. On the Relaxation Time of Interacting Superparamagnetic Nanoparticles and Implications for Magnetic Fluid Hyperthermia. Beilstein J. Nanotechnol. 2019, 10, 1280–1289. [Google Scholar] [CrossRef]
- Soetaert, F.; Kandala, S.K.; Bakuzis, A.; Ivkov, R. Experimental Estimation and Analysis of Variance of the Measured Loss Power of Magnetic Nanoparticles. Sci. Rep. 2017, 7, 6661. [Google Scholar] [CrossRef] [Green Version]
- Obaidat, I.M.; Mohite, V.; Issa, B.; Tit, N.; Haik, Y. Predicting a Major Role of Surface Spins in the Magnetic Properties of Ferrite Nanoparticles. Cryst. Res. Technol. 2009, 44, 489–494. [Google Scholar] [CrossRef]
- Ramana, C.V.; Kolekar, Y.D.; Kamala Bharathi, K.; Sinha, B.; Ghosh, K. Correlation between Structural, Magnetic, and Dielectric Properties of Manganese Substituted Cobalt Ferrite. J. Appl. Phys. 2013, 114, 183907. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef] [PubMed]
- Babić-Stojić, B.; Jokanović, V.; Milivojevic, D.; Jagličić, Z.; Makovec, D.; Jović Orsini, N.; Marinović-Cincović, M. Magnetic and Structural Studies of CoFe2O4 Nanoparticles Suspended in an Organic Liquid. J. Nanomater. 2013, 2013, 1036. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Ding, Z.; Zhao, X.; Wu, S.; Li, F.; Yue, M.; Liu, J.P. Microstructure and Magnetic Properties of MFe2O4 (M = Co, Ni, and Mn) Ferrite Nanocrystals Prepared Using Colloid Mill and Hydrothermal Method. J. Appl. Phys. 2015, 117, 17A328. [Google Scholar] [CrossRef] [Green Version]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Salgueiriño, V. Raman Spectroscopy to Unravel the Magnetic Properties of Iron Oxide Nanocrystals for Bio-Related Applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef] [Green Version]
- Mansour, H.; Letifi, H.; Bargougui, R.; De Almeida-Didry, S.; Negulescu, B.; Autret, C.; Gadri, A.; Ammar, S. Structural, Optical, Magnetic and Electrical Properties of Hematite (α-Fe2O3) Nanoparticles Synthesized by Two Methods: Polyol and Precipitation. Appl. Phys. A 2017, 123, 787. [Google Scholar] [CrossRef]
- Gil, A.F.; Benavides, O.; Vargas, S.M.; May, L.D.L.C.; Carachure, C.P. Synthesis and Characterization of Cobalt Ferrite CoxFe3-xO4 Nanoparticles by Raman Spectroscopy and X-ray Diffraction. Int. J. Metall. Met. Phys. 2020, 5, 47. [Google Scholar]
- Noh, K.M.; Lee, Y.B.; Kwon, W.H.; Kang, J.Y.; Choi, W.O.; Chae, K.P. Crystallographic and Magnetic Properties of Co, Zn, Ni-Zn Substituted Nano-Size Manganese Ferrites Synthesized by Sol-Gel Method. J. Magn. 2016, 21, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Obaidat, I.M.; Nayek, C.; Manna, K.; Bhattacharjee, G.; Al-Omari, I.A.; Gismelseed, A. Investigating Exchange Bias and Coercivity in Fe3O4–γ-Fe2O3 Core–Shell Nanoparticles of Fixed Core Diameter and Variable Shell Thicknesses. Nanomaterials 2017, 7, 415. [Google Scholar] [CrossRef] [Green Version]
- Golovin, Y.I.; Klyachko, N.L.; Majouga, A.G.; Sokolsky, M.; Kabanov, A.V. Theranostic Multimodal Potential of Magnetic Nanoparticles Actuated by Non-Heating Low Frequency Magnetic Field in the New-Generation Nanomedicine. J. Nanopart. Res. 2017, 19, 63. [Google Scholar] [CrossRef]
- De Biasi, R.S.; Cardoso, L.H.G. A Simple Model for the Magnetocrystalline Anisotropy in Mixed Ferrite Nanoparticles. Phys. B Condens. Matter 2012, 407, 3893–3896. [Google Scholar] [CrossRef] [Green Version]
- Portakal, Z.G.; Shermer, S.; Jenkins, C.; Spezi, E.; Perrett, T.; Tuncel, N.; Phillips, J. Design and Characterization of Tissue-Mimicking Gel Phantoms for Diffusion Kurtosis Imaging. Med. Phys. 2018, 45, 2476–2485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blechinger, J.C.; Madsen, E.L.; Frank, G.R. Tissue-Mimicking Gelatin-Agar Gels for Use in Magnetic Resonance Imaging Phantoms. Med. Phys. 1988, 15, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, K.; Mrówczyński, R.; Hornowski, T.; Bielas, R.; Józefczak, A. The Effect of Tissue-Mimicking Phantom Compressibility on Magnetic Hyperthermia. Nanomaterials 2019, 9, 803. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Composition | Average Sizes (nm) | Lattice Constant (Å) |
|---|---|---|
| 0 (MnFe2O4) | 34.9 ± 0.6 | 8.4889 |
| 0.2 | 18.6 ± 0.5 | 8.4824 |
| 0.4 | 17.2 ± 0.3 | 8.4473 |
| 0.6 | 16.8 ± 0.4 | 8.4183 |
| 0.8 | 16.6 ± 0.4 | 8.4054 |
| 1(CoFe2O4) | 15.0 ± 0.3 | 8.3891 |
| Composition | A1g(1) | A1g(2) | T2g(1) | Eg | T2g(3) | IA1g(1)/IA1g(2) |
|---|---|---|---|---|---|---|
| 0 | 622 | 480 | 324 | 166 | ||
| 0.2 | 619 | 471 | 327 | 166 | ||
| 0.4 | 664 | 606 | 473 | 318 | 167 | 0.75 |
| 0.6 | 669 | 604 | 471 | 308 | 176 | 0.84 |
| 0.8 | 680 | 625 | 471 | 328 | 177 | 0.98 |
| 1 | 683 | 633 | 472 | 327 | 180 | 1.05 |
| Composition | Coercive Field (Oe) | Remnant Magnetization (Emu/g) |
|---|---|---|
| 0 (MnFe2O4) | 329.00 | 13.66 |
| 0.2 | 382.60 | 16.05 |
| 0.4 | 308.80 | 15.11 |
| 0.6 | 165.10 | 12.49 |
| 0.8 | 135.45 | 8.62 |
| 1 (CoFe2O4) | 90.00 | 5.07 |
| Composition | Coercive Field (G) | |
|---|---|---|
| Zero Field Cooled | 1 T Field Cooled | |
| 0 (MnFe2O4) | 142.68 | 138.45 |
| 0.2 | 3471.55 | 3661.55 |
| 0.4 | 6715.60 | 6028.35 |
| 0.6 | 7258.10 | 6566.45 |
| 0.8 | 8748.60 | 8147.20 |
| 1(CoFe2O4) | 9716.80 | 9461.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanaswamy, V.; Al-Omari, I.A.; Kamzin, A.S.; Issa, B.; Tekin, H.O.; Khourshid, H.; Kumar, H.; Mallya, A.; Sambasivam, S.; Obaidat, I.M. Specific Absorption Rate Dependency on the Co2+ Distribution and Magnetic Properties in CoxMn1-xFe2O4 Nanoparticles. Nanomaterials 2021, 11, 1231. https://doi.org/10.3390/nano11051231
Narayanaswamy V, Al-Omari IA, Kamzin AS, Issa B, Tekin HO, Khourshid H, Kumar H, Mallya A, Sambasivam S, Obaidat IM. Specific Absorption Rate Dependency on the Co2+ Distribution and Magnetic Properties in CoxMn1-xFe2O4 Nanoparticles. Nanomaterials. 2021; 11(5):1231. https://doi.org/10.3390/nano11051231
Chicago/Turabian StyleNarayanaswamy, Venkatesha, Imaddin A. Al-Omari, Aleksandr S. Kamzin, Bashar Issa, Huseyin O. Tekin, Hafsa Khourshid, Hemant Kumar, Ambresh Mallya, Sangaraju Sambasivam, and Ihab M. Obaidat. 2021. "Specific Absorption Rate Dependency on the Co2+ Distribution and Magnetic Properties in CoxMn1-xFe2O4 Nanoparticles" Nanomaterials 11, no. 5: 1231. https://doi.org/10.3390/nano11051231






