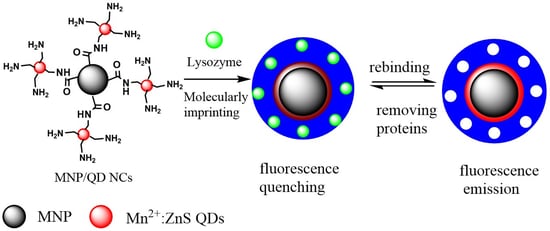
Molecularly Imprinted Magnetic Fluorescent Nanocomposite-Based Sensor for Selective Detection of Lysozyme
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Characterizations
2.3. Synthesis of MNP/QD
2.4. Fabrication of MNP/QD@MIPs Nanocomposites-Based Sensor
2.5. Selectivity Experiments
2.6. Application in Real Samples
3. Results and Discussion
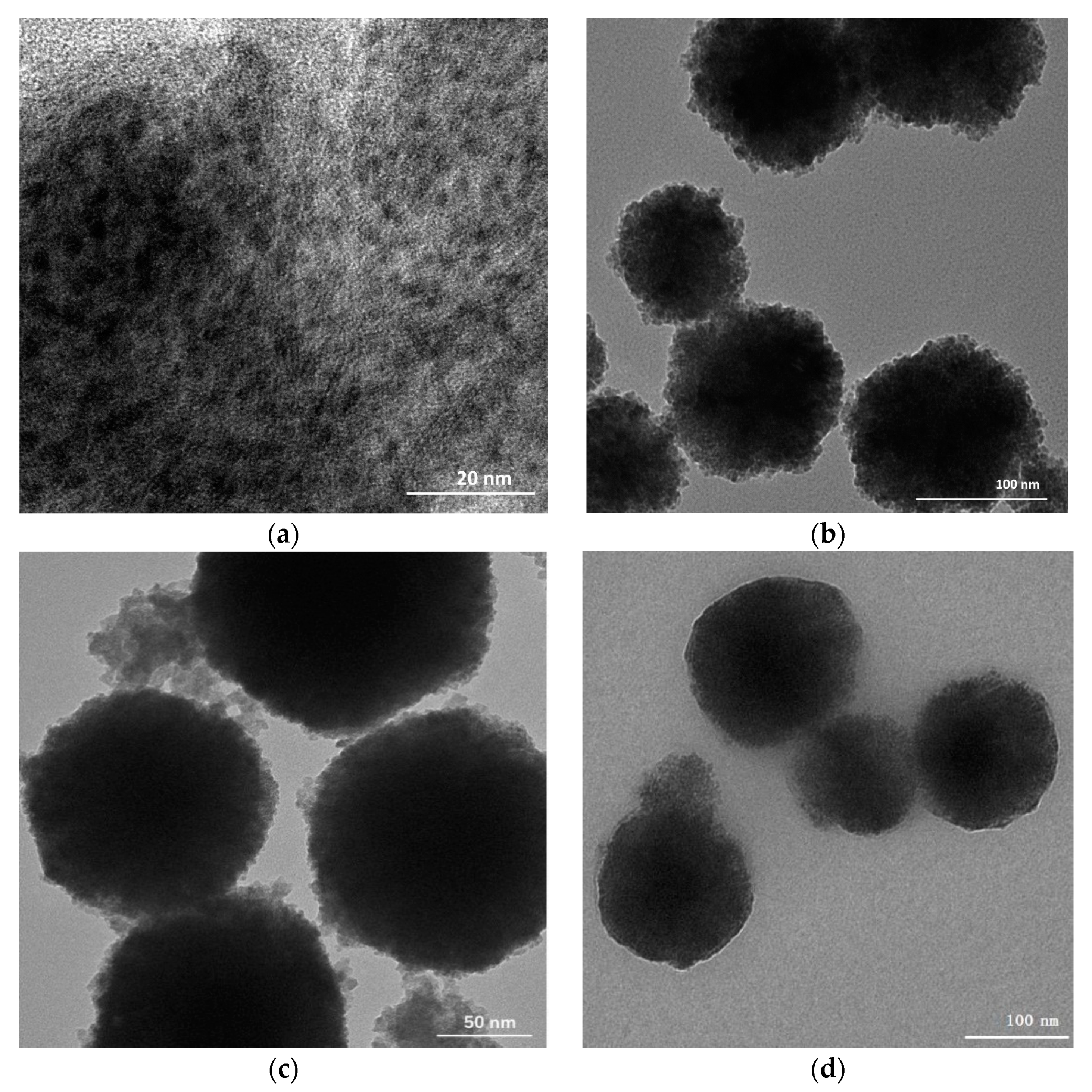
3.1. Characterization
3.2. Rebinding Experiments
3.2.1. Binding Kinetics of MNP/QD@MIPs
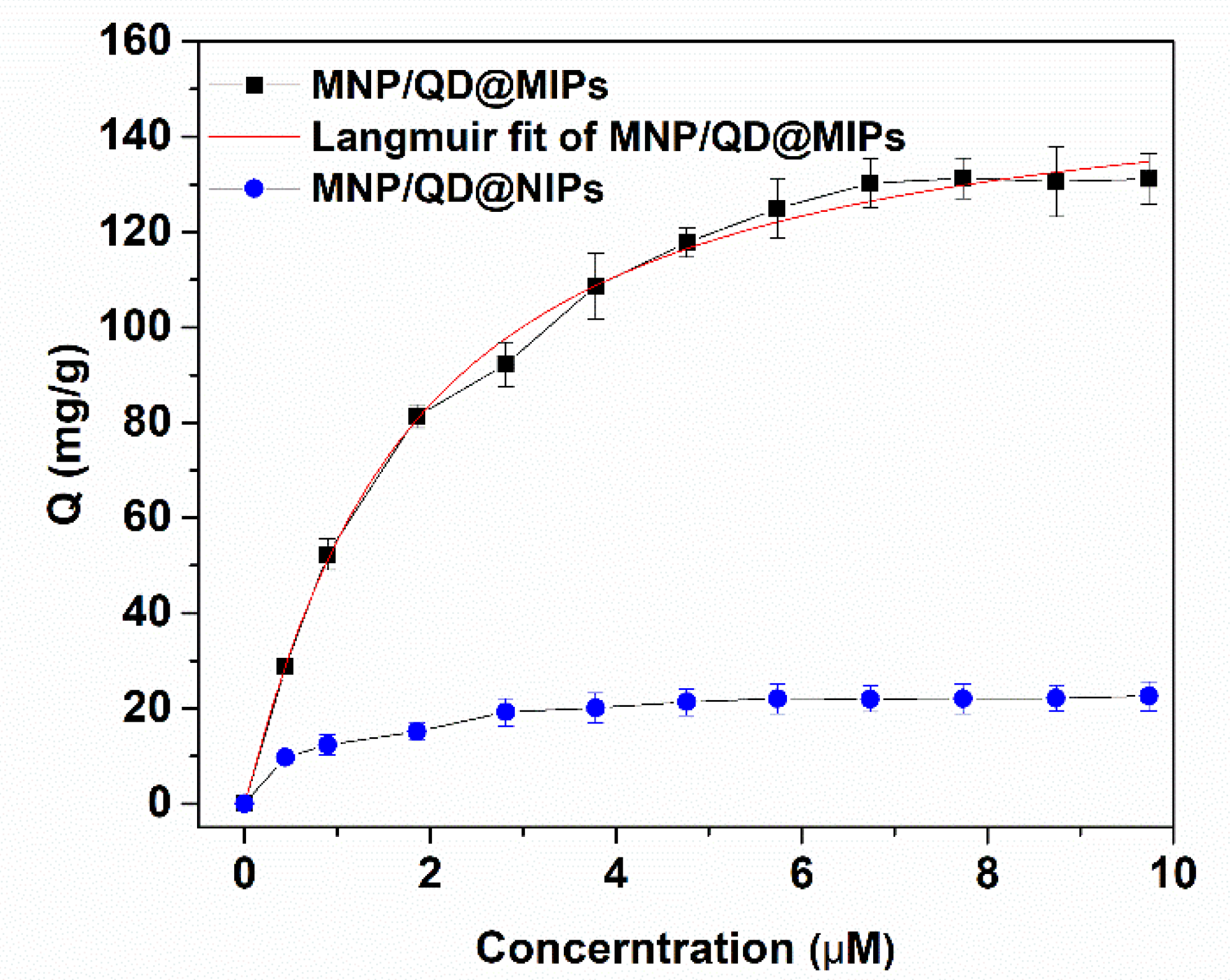
3.2.2. Adsorption Isotherm of MNP/QD@MIPs
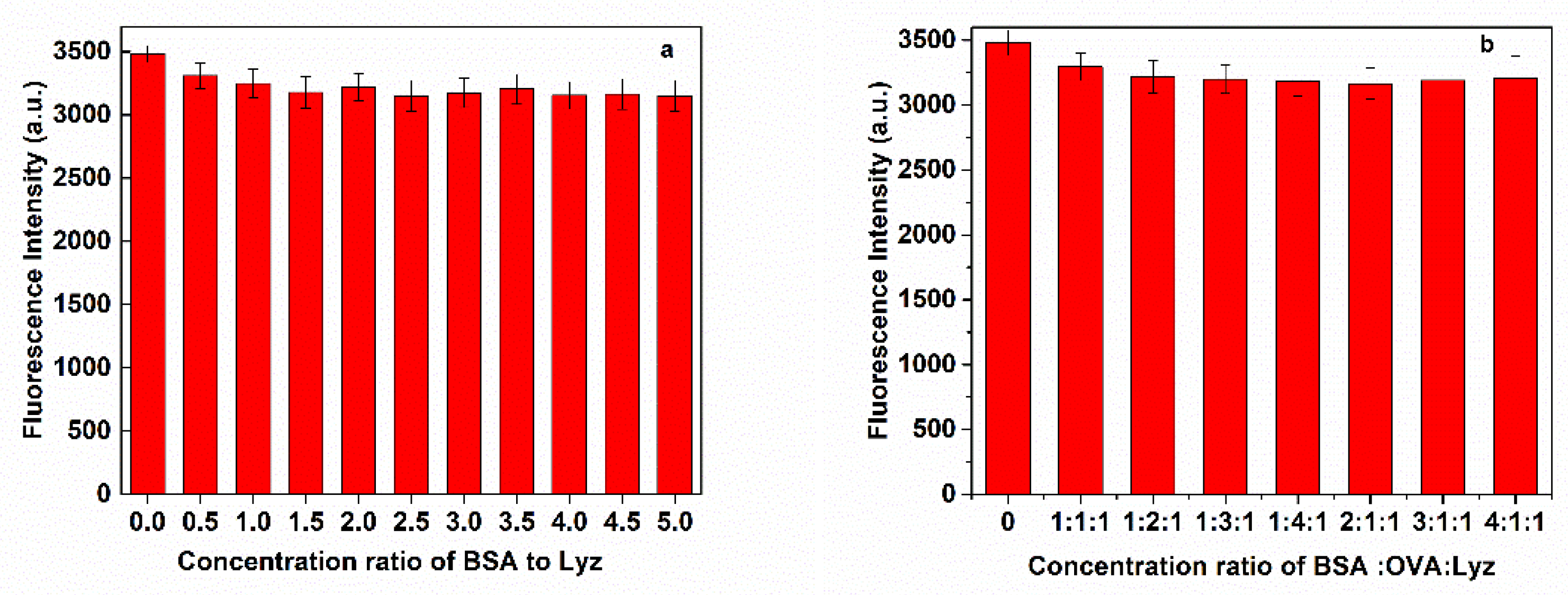
3.3. Selectivity of MNP/QD@MIPs
3.4. Fluorescence Sensing of Lyz Using MNP/QD@MIPs
3.5. Applications
3.6. Stability and Recyclability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Liang, X.; Ma, X.; Hu, Y.; Li, X.; Fan, J. Simple and greener synthesis of highly photoluminescence Mn2+-doped ZnS quantum dots and its surface passivation mechanism. Appl. Surf. Sci. 2014, 316, 54–61. [Google Scholar] [CrossRef]
- Jing, L.; Kershaw, S.V.; Li, Y.; Huang, X.; Li, Y.; Rogach, A.L.; Gao, M. Aqueous based semiconductor nanocrystals. Chem. Rev. 2016, 116, 10623–10730. [Google Scholar] [CrossRef] [PubMed]
- Aboulaich, A.; Geszke, M.; Balan, L.; Ghanbaja, J.; Medjahdi, G.; Schneider, R. Water based route to colloidal Mn-doped ZnSe and core/shell ZnSe/ZnS quantum dots. Inorg. Chem. 2010, 49, 10940–10948. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Pu, C.; Qin, H.; Liu, S.; Zhuan, X.; Peng, X. Temperature- and Mn2+ concentration-dependent emission properties of Mn2+-doped ZnSe nanocrystals. J. Am. Chem. Soc. 2019, 141, 2288–2298. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, S.; Hervé Rinnert, L.B.; Sébastien, B.; Jasniewski, J.; Medjahdi, G.; Chaabane, R.B.; Schneider, R. Aqueous synthesis of highly luminescent ternary alloyed mn-doped znses quantum dots capped with 2-mercaptopropionic acid. J. Alloys Compd. 2020, 858, 158315. [Google Scholar] [CrossRef]
- Montóna, H.; Medina-Sánchez, M.; Soler, J.A.; Chałupniak, A.; Nogués, C.; Merkoçi, A. Rapid on-chip apoptosis assay on human carcinoma cells based on annexin-V/quantum dot probes. Biosens. Bioelectron. 2017, 94, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Yu, M.; Xu, Y.; Wang, Q.; Hua, Y.; Fu, H.; She, Y. Nanoporphyrin/CdTe quantum dots: A robust tool for effective differentiation among DNA structures. Sens. Actuators B Chem. 2019, 281, 623–633. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.E.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Frey, N.A.; Peng, S.; Cheng, K.; Sun, S. Magnetic nanoparticles: Synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009, 38, 2532–2542. [Google Scholar] [CrossRef]
- Lan, J.; Chen, J.; Li, N.; Ji, X.; Yu, M.; He, Z. Microfluidic generation of magnetic-fluorescent Janus microparticles for biomolecular detection. Talanta 2016, 151, 126–131.–131. [Google Scholar] [CrossRef]
- Thamilselvan, A.; Manivel, P.; Rajagopal, V.; Nesakumar, N.; Suryanarayanan, V. Improved electrocatalytic activity of Au@ Fe3O4 magnetic nanoparticles for sensitive dopamine detection. Colloids Surf. B 2019, 180, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.N.; Miró, M.; Breadmore, M.C.; Macka, M. Trends in analytical separations of magnetic (nano)particles. TrAC Trend Anal. Chem. 2019, 114, 89–97. [Google Scholar] [CrossRef]
- Gloag, L.; Mehdipour, M.; Chen, D.; Tilley, R.D.; Gooding, J.J. Advances in the application of magnetic nanoparticles for sensing. Adv. Mater. 2019, 21, 1–26. [Google Scholar] [CrossRef]
- Rocha–Santos, T.A.P. Sensors and biosensors based on magnetic nanoparticles. TrAC Trend Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and bio-medical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, K.D.; Ruan, G.; Vieira, G.; Porter, T.; Chalmers, J.J.; Sooryakumar, R.; Winter, J.O. Biomolecular detection, tracking, and manipulation using a magnetic nanoparticle-quantum dot platform. J. Mater. Chem. B 2020, 48, 3534–3541. [Google Scholar] [CrossRef]
- Shi, D.; Ni, M.; Zeng, J.; Ye, J.; Ni, P.; Liu, X.; Chen, M. Simultaneous detection and removal of metal ions based on a chemosensor composed of a rhodamine derivative and cyclodextrin-modified magnetic nanoparticles. J. Mater. Sci. 2015, 50, 168–175. [Google Scholar] [CrossRef]
- Arvand, M.; Hemmati, S. Magnetic nanoparticles embedded with graphene quantum dots and multiwalled carbon nanotubes as a sensing platform for electrochemical detection of progesterone. Sens. Actuators B Chem. 2017, 238, 346–356. [Google Scholar] [CrossRef]
- Leng, Y.; Wu, W.; Li, L.; Lin, K.; Sun, K.; Chen, X.; Li, W. Magnetic/Fluorescent barcodes based on cadmium-free near-infrared-emitting quantum dots for multiplexed detection. Adv. Funct. Mater. 2016, 26, 7581–7589. [Google Scholar] [CrossRef]
- Li, Z.; Wang, G.; Shen, Y.; Guo, N.; Ma, N. DNA-templated magnetic nanoparticle-quantum dot polymers for ultrasensitive capture and detection of circulating tumor cells. Adv. Funct. Mater. 2018, 28, 1707152. [Google Scholar] [CrossRef]
- Lorenz, S.; Erickson, C.S.; Riesner, M.; Gamelin, D.R.; Fainblat, R.; Bacher, G. Directed exciton magnetic polaron formation in a single colloidal Mn2+:CdSe/CdS quantum dot. Nano Lett. 2020, 20, 1896–1906. [Google Scholar] [CrossRef]
- Pathan, S.; Jalal, M.; Prasad, S.; Suryasarathi, B. Aggregation-induced enhanced photoluminescence in magnetic graphene oxide quantum dots as a fluorescence probe for As(III) sensing. J. Mater. Chem. A 2019, 14, 8510–8520. [Google Scholar] [CrossRef]
- Haupt, K.; Linares, A.V.; Bompart, M.; Bui, B.T.S. Molecular Imprinting; Springer: New York, NY, USA, 2012; pp. 1–28. [Google Scholar]
- Xie, X.; Hu, Q.; Ke, R.; Zhen, X.; Bu, Y.; Wang, S. Facile preparation of photonic and magnetic dual responsive protein imprinted nanomaterial for specific recognition of bovine hemoglobin. Chem. Eng. J. 2019, 371, 130–137. [Google Scholar] [CrossRef]
- Grothe, R.A.; Lobato, A.; Mounssef, B.; Tasi, N.; Gonalves, L.M. Electroanalytical profiling of cocaine samples by means of an electropolymerized molecularly imprinted polymer using benzocaine as the template molecule. Analyst 2021, 146, 1747–1759. [Google Scholar] [CrossRef]
- Cheubong, C.; Takano, E.; Kitayama, Y.; Sunayama, H.; Takeuchi, T. Molecularly imprinted polymer nanogel-based fluorescence sensing of pork contamination in halal meat extracts. Biosens. Bioelectron. 2021, 172, 112775. [Google Scholar] [CrossRef]
- Yang, Q.; Li, J.; Wang, X.; Xiong, H.; Chen, L. Ternary Emission of a Blue-, Green-, and Red-based Molecular Imprinting Fluorescence Sensor for the Multiplexed and Visual Detection of Bovine Hemoglobin. Anal. Chem. 2019, 10, 6561–6568. [Google Scholar] [CrossRef]
- Amiripour, F.; Shahram, G.; Azizi, S.N. Design of turn-on luminescent sensor based on nanostructured molecularly imprinted polymer-coated zirconium metal-organic framework for selective detection of chloramphenicol residues in milk and honey. Food Chem. 2021, 347, 129034. [Google Scholar] [CrossRef]
- Ge, Y.; Turner, A.P. Too large to fit? Recent developments in macromolecular imprinting. Trends Biotechnol. 2008, 26, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Sun, J.; Chen, H.; Yang, J.; Li, Y.; Dan, L.; Yang, M.; Zhang, S. Immobilization of BSA on ionic liquid functionalized magnetic Fe3O4 nanoparticles for use in surface imprinting strategy. Talanta 2017, 168, 174–182. [Google Scholar] [CrossRef]
- Tan, J.; Guo, M.; Tan, L.; Geng, Y.; Huang, S.; Tang, Y.; Su, C.; Lin, C.C.; Liang, Y. Highly efficient fluorescent QDs sensor for specific detection of protein through double recognition of hybrid aptamer-molecular imprinted polymers. Sens. Actuators B Chem. 2018, 274, 627–635. [Google Scholar] [CrossRef]
- Wang, J.; Liang, R.; Qin, W. Molecularly imprinted polymer-based potentiometric sensors. TrAC Trend Anal. Chem. 2020, 130, 115980. [Google Scholar] [CrossRef]
- He, H.; Muhammad, P.; Guo, Z.; Peng, Q.; Lu, H.; Liu, Z. Controllably prepared molecularly imprinted core-shell plasmonic nanostructure for plasmon-enhanced fluorescence assay. Biosens. Bioelectron. 2019, 146, 111733. [Google Scholar] [CrossRef]
- Yang, Q.; Li, C.; Li, J.; Wang, X.; Arabi, M.; Peng, H.; Xiong, H.; Chen, L. Rational construction of a triple emission molecular imprinting sensor for accurate naked-eye detection of folic acid. Nanoscale 2020, 12, 6529–6536. [Google Scholar] [CrossRef]
- Mori, K.; Hitsuhiro, M.; Morishige, T.; Takano, E.; Sunayama, H.; Kitayama, Y.; Inubushi, S.; Sasaki, R.; Yashiro, M.; Takeuchi, T. A Pretreatment-Free, Polymer-Based Platform Prepared by molecular imprinting and post-imprinting modifications for sensing intact exosomes. Angew. Chem. 2019, 131, 1626–1629. [Google Scholar] [CrossRef]
- Duong, H.D.; Reddy, C.V.G.; Rhee, J.I.; Vo-Dinh, T. Amplification of fluorescence emission of CdSe/ZnS QDs entrapped in a sol-gel matrix, a new approach for detection of trace level of PAHs. Sens. Actuators B Chem. 2011, 157, 139–145. [Google Scholar] [CrossRef]
- Dan, L.; Wang, H.F. Mn-doped ZnS quantum dot imbedded two-fragment imprinting silica for enhanced room temperature phosphorescence probing of domoic acid. Anal. Chem. 2013, 85, 4844–4848. [Google Scholar] [CrossRef]
- Harrison, J.F.; Lunt, G.S.; Scott, P.; Blainey, J.D. Urinary lysozyme, ribonuclease, and low-molecular-weight protein in renal disease. Lancet 1968, 1, 371–375. [Google Scholar] [CrossRef]
- Levinson, S.S.; Elin, R.J.; Yam, L. Light chain proteinuria and lysozymeuria in a patient with acute monocytic leukemia. Clin. Chem. 2002, 48, 1131–1132. [Google Scholar] [CrossRef]
- Carstens, C.; Deckwart, M.; Webber-Witt, M.; Schäfer, V.; Eichhorn, L.; Brockow, K.; Fischer, M.; Christmann, M.; Paschke-Kratzin, A. Evaluation of the efficiency of enological procedures on lysozyme depletion in wine by an indirect ELISA method. J. Agric. Food Chem. 2014, 62, 6247–6253. [Google Scholar] [CrossRef]
- Mihai, I.; Vezeanu, A.; Polonschii, C.; Albu, C.; Radu, G.L.; Vasilescu, A. Label-free detection of lysozyme in wines using an aptamer based biosensor and SPR detection. Sens. Actuators B Chem. 2015, 206, 198–204. [Google Scholar] [CrossRef]
- Kondekova, M.; Maier, V.; Ginterova, P.; Marak, J.; Sevcik, J. Analysis of lysozyme in cheese samples by on-line combination of capillary zone electrophoresis and mass spectrometry. Food Chem. 2014, 153, 398–440. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Jiang, R.; Sun, L.Q.; Pang, S.P.; Luo, A.Q. Fluorescent molecularly imprinted membranes as biosensor for the detection of target protein. Sens. Actuators B Chem. 2018, 254, 1078–1086. [Google Scholar] [CrossRef]
- Giulio, T.D.; Mazzotta, E.; Malitesa, C. Molecularly imprinted polyscopoletin for the electrochemical detection of the chronic disease marker lysozyme. Biosensors 2021, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Sathe, T.R.; Agrawal, A.; Nie, S.M. Mesoporous silica beads embedded with semiconductor quantum dots and iron oxide nanocrystals: Dual-function microcarriers for optical encoding and magnetic separation. Anal. Chem. 2006, 78, 5627–5632. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Berti, L.; Medintz, I.L. Materials for fluorescence resonanceenergy transfer analysis: Beyond traditional donor-acceptor combinations. Angew. Chem. Int. Ed. Engl. 2006, 45, 4562–4589. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Kang, C.; Xu, S.; Tang, Y. Selective room temperature phosphorescencesensing of target protein using Mn-doped ZnS QDs-embedded molecularlyimprinted polymer. Biosens. Bioelectron. 2013, 48, 216–223. [Google Scholar] [CrossRef]
- Lim, G.W.; Lim, J.K.; Ahmad, A.L.; Chan, D.J.C. Fluorescent molecularly imprinted polymer based on Navicula sp. frustules for optical detection of lysozyme. Anal. Bioanal. Chem. 2016, 408, 2083–2093. [Google Scholar] [CrossRef]
- Lv, Y.; Qin, Y.; Svec, F.; Tan, T. Molecularly imprinted plasmonic nanosensor for selective SERS detection of protein biomarkers. Biosens. Bioelectron. 2016, 80, 433–441. [Google Scholar] [CrossRef]
- Fang, M.; Zhuo, K.; Chen, Y.; Zhao, Y.; Bai, G.; Wang, J. Fluorescent probe based on carbon dots/silica/molecularly imprinted polymer for lysozyme detection and cell imaging. Anal. Bioanal. Chem. 2019, 411, 5799–5807. [Google Scholar] [CrossRef]





| Sample | Spiked Lysozyme (μM) | Measured (μM) | Recovery (%) | RSD |
|---|---|---|---|---|
| Egg white 1 | 0.50 | 0.51 ± 0.03 | 101.78 | 5.29 |
| Egg white 2 | 1.50 | 1.51 ± 0.05 | 97.51 | 3.15 |
| Urine 1 | 0.50 | 0.50 ± 0.02 | 103.33 | 3.72 |
| Urine 2 | 1.50 | 1.49 ± 0.06 | 95.40 | 4.25 |
| Recognition Element | Detection Technique | Linearity (μM) | LOD (μM) | References |
|---|---|---|---|---|
| MIPs | Electrochemical | 0.15–20 | 0.14 | [44] |
| MIP based on Naviculasp. frustule | Fluorescence spectrum | 0–1.74 | 0.10 | [48] |
| MIP@GNR | SERS | 5.6 × 10−2–2.10 | 10−2 | [49] |
| CDs/SiO2/MIP | Fluorescence spectrum | 6.9 × 10−2–0.69 | 3.84 × 10−2 | [50] |
| QDs embedded MIMs | Fluorescence spectrum | 0.1–1.0 | 1.02 × 10−2 | [43] |
| MIP@MNP/QDs | Fluorescence spectrum | 0.2–2.0 | 4.53 × 10−3 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Tang, B.; Li, Y.; Liu, C.; Jiao, P.; Wei, Y. Molecularly Imprinted Magnetic Fluorescent Nanocomposite-Based Sensor for Selective Detection of Lysozyme. Nanomaterials 2021, 11, 1575. https://doi.org/10.3390/nano11061575
Zhang X, Tang B, Li Y, Liu C, Jiao P, Wei Y. Molecularly Imprinted Magnetic Fluorescent Nanocomposite-Based Sensor for Selective Detection of Lysozyme. Nanomaterials. 2021; 11(6):1575. https://doi.org/10.3390/nano11061575
Chicago/Turabian StyleZhang, Xin, Bo Tang, Yansong Li, Chengbin Liu, Pengfei Jiao, and Yuping Wei. 2021. "Molecularly Imprinted Magnetic Fluorescent Nanocomposite-Based Sensor for Selective Detection of Lysozyme" Nanomaterials 11, no. 6: 1575. https://doi.org/10.3390/nano11061575