Dual Plasmon Resonances and Tunable Electric Field in Structure-Adjustable Au Nanoflowers for Improved SERS and Photocatalysis
Abstract
:1. Introduction
2. Methods
2.1. Chemicals
2.2. Synthesis of Au/PbS Hybrids
2.3. Synthesis of Au Nanoflowers
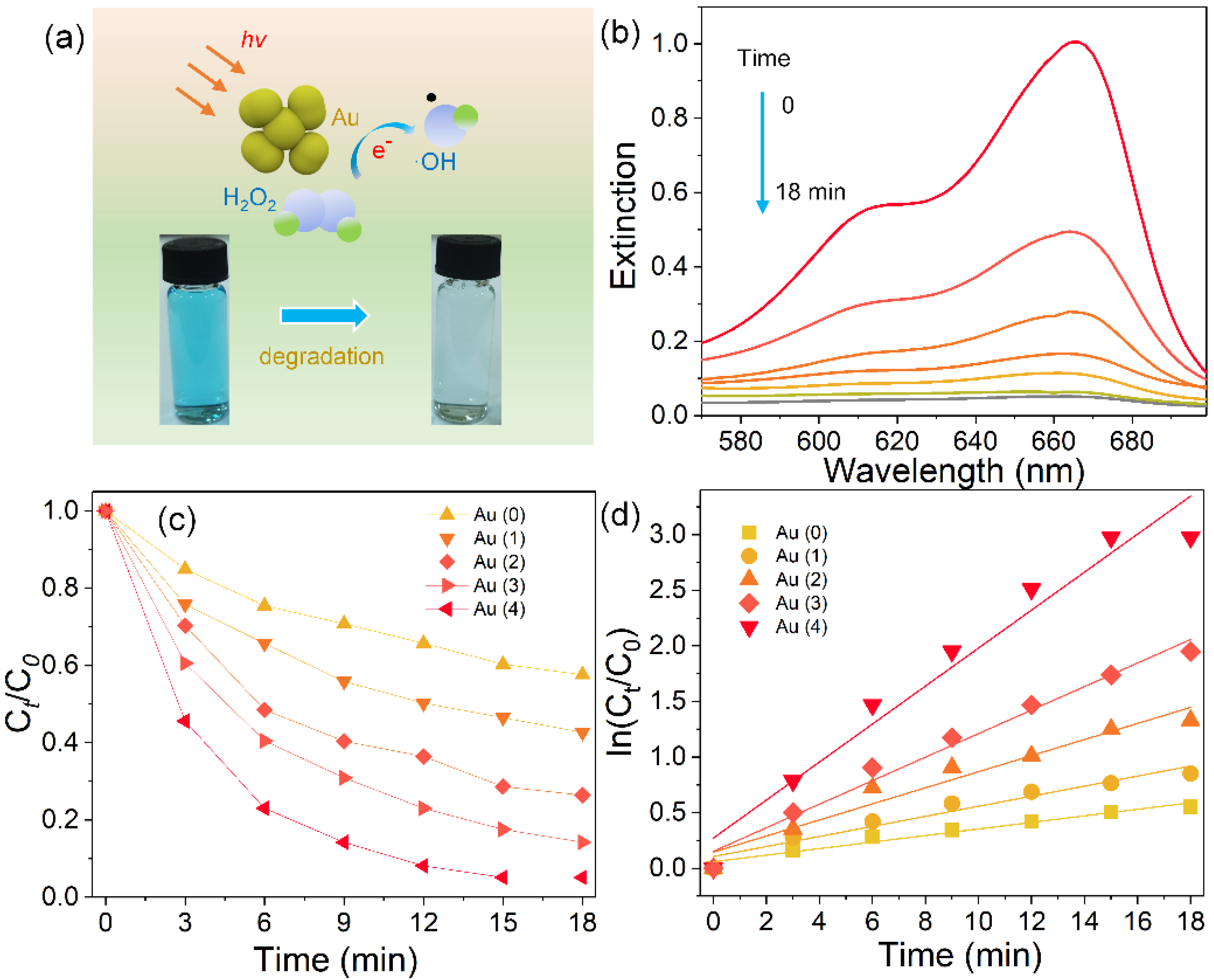
2.4. Photocatalytic Measurements
2.5. Numerical Simulation
2.6. Sample Characterization
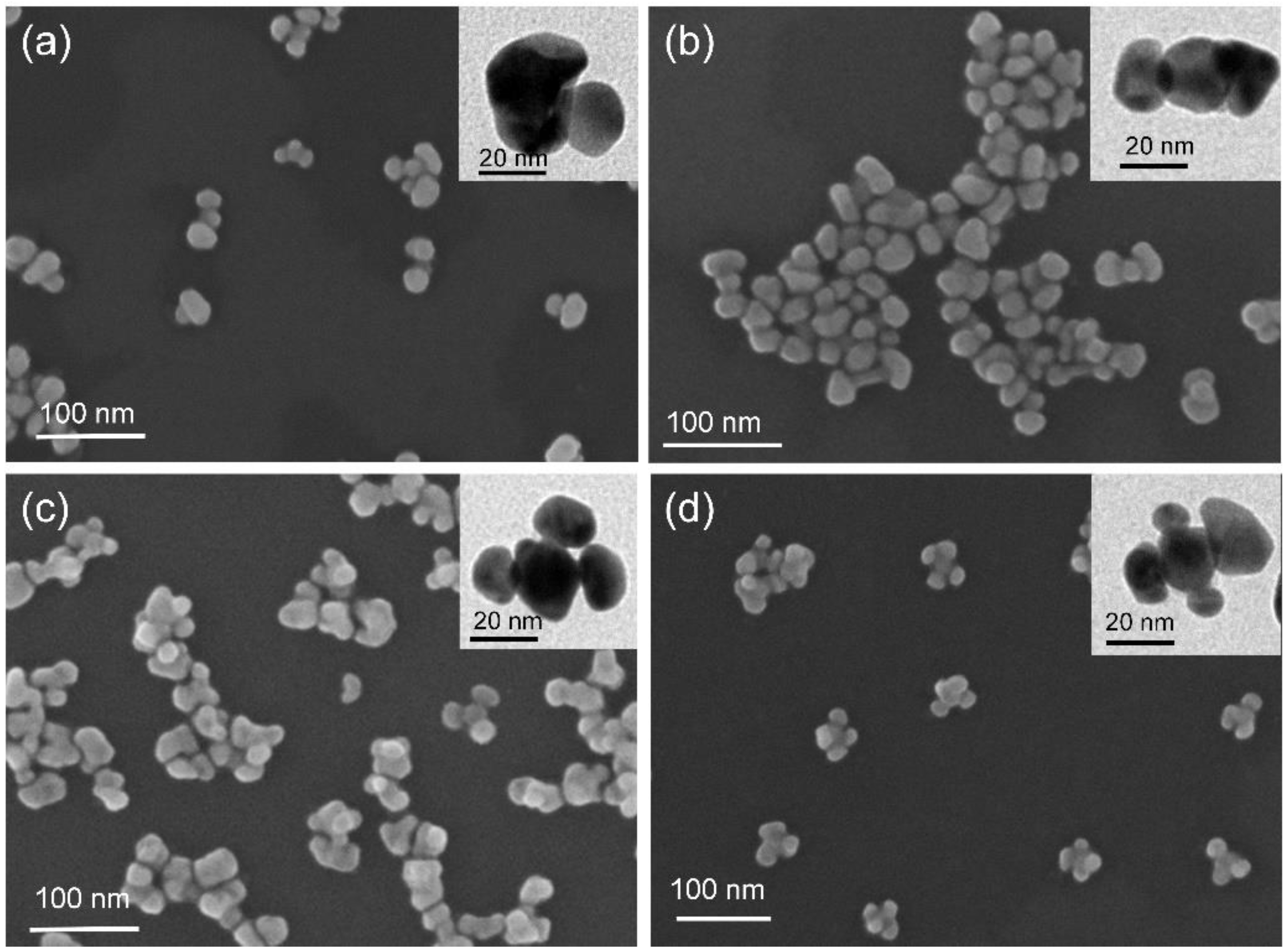
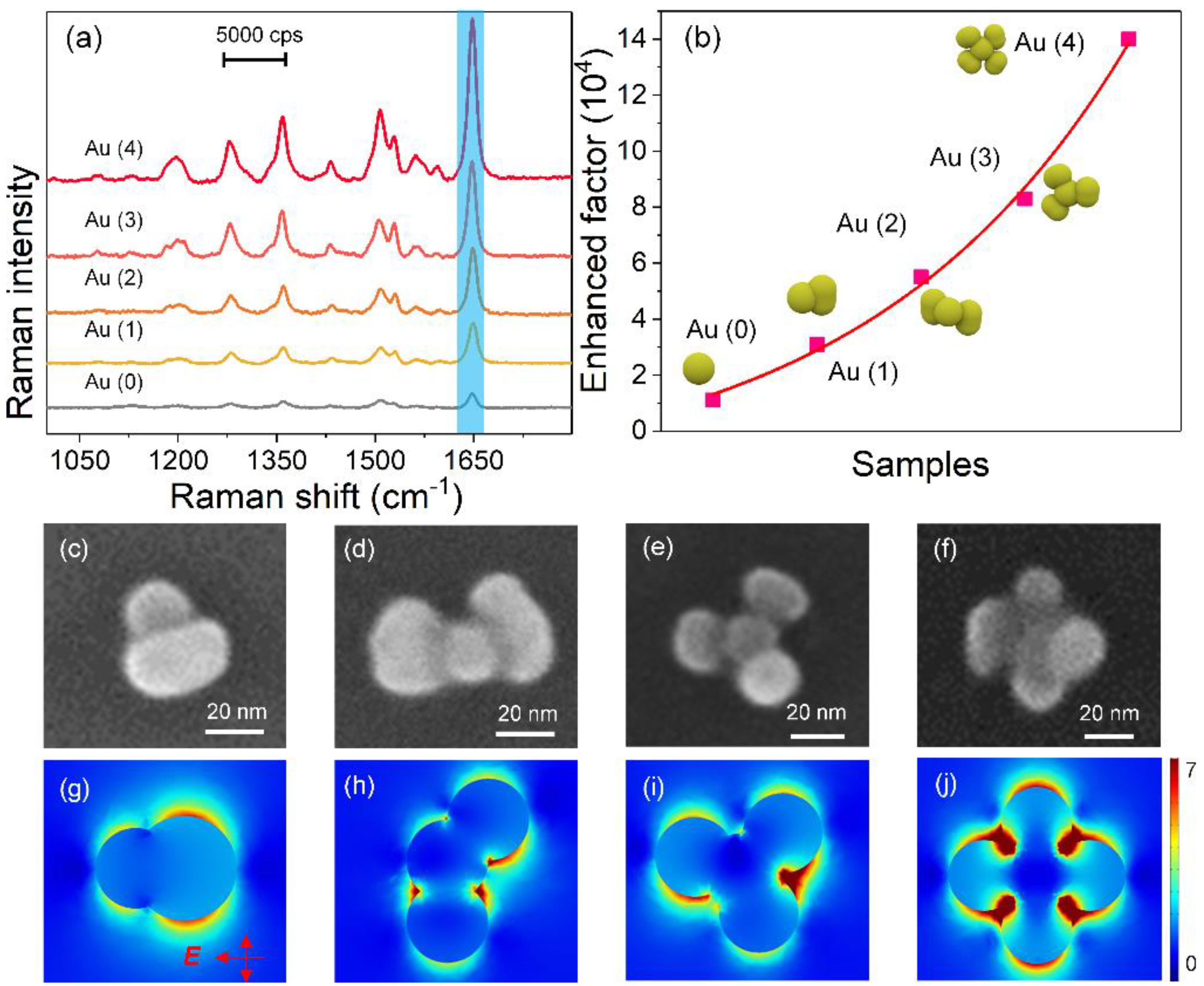
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; Ouyang, S.; Ye, J. Gold-Nanorod-Photosensitized Titanium Dioxide with Wide-Range Visible-Light Harvesting Based on Localized Surface Plasmon Resonance. Angew. Chem. Int. Ed. 2013, 125, 6821–6825. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, J.; Wu, Q.; Wang, Z.; Wang, Z.; Sun, J.; Liu, C.J. Plasmon Based Double-Layer Hydrogel Device for a Highly Efficient Solar Vapor Generation. Adv. Funct. Mater. 2019, 29, 1901312. [Google Scholar] [CrossRef]
- Dey, P.; Tabish, T.A.; Mosca, S.; Palombo, F.; Matousek, P.; Stone, N. Plasmonic Nanoassemblies: Tentacles Beat Satellites for Boosting Broadband NIR Plasmon Coupling Providing a Novel Candidate for SERS and Photothermal Therapy. Small 2020, 16, 1906780. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.J.; Zhang, H.; Yang, D.J.; Qiu, Y.H.; Nan, F.; Yang, Z.J.; Lin, H.Q. Magnetic Plasmon-Enhanced Second-Harmonic Generation on Colloidal Gold Nanocups. Nano Lett. 2019, 19, 2005–2011. [Google Scholar] [CrossRef]
- Zong, C.; Xu, M.; Xu, L.J.; Wei, T.; Ma, X.; Zheng, X.S.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.I.; Lyu, D.; Lu, Z.; Liu, G.; Ren, B. Surface-Enhanced Raman Spectroscopy: Benefits, Trade-Offs and Future Developments. Chem. Sci. 2020, 11, 4563–4577. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Nie, W.; Zhu, Q.; Wang, X.; Wang, S.; Fan, F.; Li, C. The Polarization Effect in Surface-Plasmon-Induced Photocatalysis on Au/TiO2 Nanoparticles. Angew. Chem. Int. Ed. 2020, 132, 18375–18380. [Google Scholar] [CrossRef]
- Ding, S.Y.; You, E.M.; Tian, Z.Q.; Moskovits, M. Electromagnetic Theories of Surface-Enhanced Raman Spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef]
- Hu, Z.; Mi, Y.; Ji, Y.; Wang, R.; Zhou, W.; Qiu, X.; Wu, X. Multiplasmon Modes for Enhancing the Photocatalytic Activity of Au/Ag/Cu2O Core-Shell Nanorods. Nanoscale 2019, 11, 16445–16454. [Google Scholar] [CrossRef]
- Wang, X.; Ma, G.; Li, A.; Yu, J.; Yang, Z.; Lin, J.; Guo, L. Composition-Adjustable Ag-Au Substitutional Alloy Microcages Enabling Tunable Plasmon Resonance for Ultrasensitive SERS. Chem. Sci. 2018, 9, 4009–4015. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Wang, P.; Gao, Y.; Huang, H.; Tong, F.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; Dai, Y.; et al. Design and Synthesis of Porous M-ZnO/CeO2 Microspheres as Efficient Plasmonic Photocatalysts for Nonpolar Gaseous Molecules Oxidation: Insight into the Role of Oxygen Vacancy Defects and M = Ag, Au Nanoparticles. Appl. Catal. B Environ. 2020, 260, 118151. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Y.L.; Song, X.P.; Yang, D.J.; Li, H.X.; Ding, S.J.; Chen, X.B. Structure-Adjustable Gold Nanoingots with Strong Plasmon Coupling and Magnetic Resonance for Improved Photocatalytic Activity and SERS. ACS Appl. Mater. Interfaces 2020, 12, 38554–38562. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.M.; Alegret, J.; Käll, M.; Sutherland, D.S. Sensing Characteristics of NIR Localized Surface Plasmon Resonances in Gold Nanorings for Application as Ultrasensitive Biosensors. Nano Lett. 2007, 7, 1256–1263. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Y.L.; Yang, D.J.; Ding, S.J.; Xiong, L.; Qin, P.L.; Chen, X.B. Gap-Dependent Plasmon Coupling in Au/AgAu Hybrids for Improved SERS Performance. J. Phys. Chem. C 2020, 124, 25473–25479. [Google Scholar] [CrossRef]
- Yuan, H.; Fales, A.M.; Khoury, C.G.; Liu, J.; Vo-Dinh, T. Spectral Characterization and Intracellular Detection of Surface-Enhanced Raman Scattering (SERS)-Encoded Plasmonic Gold Nanostars. J. Raman Spectrosc. 2013, 44, 234–239. [Google Scholar] [CrossRef]
- Tian, F.; Conde, J.; Bao, C.; Chen, Y.; Curtin, J.; Cui, D. Gold Nanostars for Efficient in Vitro and in Vivo Real-Time SERS Detection and Drug Delivery via Plasmonic-Tunable Raman/FTIR Imaging. Biomaterials 2016, 106, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Schwenk, N.; Mizaikoff, B.; Cárdenas, S.; López-Lorente, Á.I. Gold-Nanostar-Based SERS Substrates for Studying Protein Aggregation Processes. Analyst 2018, 143, 5103–5111. [Google Scholar] [CrossRef]
- Belhout, S.A.; Baptista, F.R.; Devereux, S.J.; Parker, A.W.; Ward, A.D.; Quinn, S.J. Preparation of Polymer Gold Nanoparticle Composites with Tunable Plasmon Coupling and Their Application as SERS Substrates. Nanoscale 2019, 11, 19884–19894. [Google Scholar] [CrossRef]
- Carnegie, C.; Chikkaraddy, R.; Benz, F.; De Nijs, B.; Deacon, W.M.; Horton, M.; Horton, M.; Wang, W.; Readman, C.; Barrow, S.J.; et al. Mapping SERS in CB: Au Plasmonic Nanoaggregates. ACS Photon. 2017, 4, 2681–2686. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, C.; Lee, Y.; Lee, J.; Park, S.J.; Park, S.; Nam, J.M. Synthesis, Assembly, Optical Properties, and Sensing Applications of Plasmonic Gap Nanostructures. Adv. Mater. 2021, 2021, 2006966. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Shih, T.M.; Yang, W.; Hu, S.; Hu, X.; Li, J.; Ren, B.; Mao, B.; Yang, Z.; et al. Plasmon-Induced Magnetic Resonance Enhanced Raman Spectroscopy. Nano Lett. 2018, 18, 2209–2216. [Google Scholar] [CrossRef]
- Lee, D.; Yoon, S. Gold Nanocube-Nanosphere Dimers: Preparation, Plasmon Coupling, and Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2015, 119, 7873–7882. [Google Scholar] [CrossRef]
- Yoon, J.H.; Selbach, F.; Langolf, L.; Schlücker, S. Ideal Dimers of Gold Nanospheres for Precision Plasmonics: Synthesis and Characterization at the Single-Particle Level for Identification of Higher Order Modes. Small 2018, 14, 1702754. [Google Scholar] [CrossRef]
- Hu, H.; Ji, F.; Xu, Y.; Yu, J.; Liu, Q.; Chen, L.; Chen, Q.; Wen, P.; Lifshitz, Y.; Wang, Y.; et al. Reversible and Precise Self-Assembly of Janus Metal-Organosilica Nanoparticles through a Linker-Free Approach. ACS Nano 2016, 10, 7323–7330. [Google Scholar] [CrossRef]
- Huang, Z.; Meng, G.; Hu, X.; Pan, Q.; Huo, D.; Zhou, H.; Ke, Y.; Wu, N. Plasmon-Tunable Au@Ag Core-Shell Spiky Nanoparticles for Surface-Enhanced Raman Scattering. Nano Res. 2019, 12, 449–455. [Google Scholar] [CrossRef]
- Guo, P.; Sikdar, D.; Huang, X.; Si, K.J.; Xiong, W.; Gong, S.; Yap, L.W.; Premaratne, M.; Cheng, W. Plasmonic Core-Shell Nanoparticles for SERS Detection of the Pesticide Thiram: Size-and Shape-Dependent Raman Enhancement. Nanoscale 2015, 7, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Song, L.; Huang, Y.; Zhang, L.; Lu, X.; Zhang, J.; Chen, T. Bimetallic Au/Ag Core-Shell Superstructures with Tunable Surface Plasmon Resonance in the Near-Infrared Region and High-Performance Surface-Enhanced Raman Scattering. Langmuir 2017, 33, 5378–5384. [Google Scholar] [CrossRef]
- Lee, J.; Hua, B.; Park, S.; Ha, M.; Lee, Y.; Fan, Z.; Ko, H. Tailoring Surface Plasmons of High-Density Gold Nanostar Assemblies on Metal Films for Surface-Enhanced Raman Spectroscopy. Nanoscale 2014, 6, 616–623. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Huang, Y. Electromagnetic Field Redistribution in Hybridized Plasmonic Particle-Film System. Appl. Phys. Lett. 2013, 102, 153108. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Zhang, Q.; Park, S.; Choe, A.; Fan, Z.; Ko, H. Particle-Film Plasmons on Periodic Silver Film over Nanosphere (AgFON): A Hybrid Plasmonic Nanoarchitecture for Surface-Enhanced Raman Spectroscopy. ACS Appl. Mater. Interfaces 2016, 8, 634–642. [Google Scholar] [CrossRef]
- Xiong, M.; Jin, X.; Ye, J. Strong Plasmon Coupling in Self-Assembled Superparamagnetic Nanoshell Chains. Nanoscale 2016, 8, 4991–4999. [Google Scholar] [CrossRef]
- Klinkova, A.; Choueiri, R.M.; Kumacheva, E. Self-Assembled Plasmonic Nanostructures. Chem. Soc. Rev. 2014, 43, 3976–3991. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hung, L.; Lang, G.S.; Cornett, J.E.; Mayergoyz, I.D.; Rabin, O. Dispersion in the SERS Enhancement with Silver Nanocube Dimers. ACS Nano 2010, 4, 5763–5772. [Google Scholar] [CrossRef]
- Lee, H.; Kim, G.H.; Lee, J.H.; Kim, N.H.; Nam, J.M.; Suh, Y.D. Quantitative Plasmon Mode and Surface-Enhanced Raman Scattering Analyses of Strongly Coupled Plasmonic Nanotrimers with Diverse Geometries. Nano Lett. 2015, 15, 4628–4636. [Google Scholar] [CrossRef]
- Lee, J.H.; You, M.H.; Kim, G.H.; Nam, J.M. Plasmonic Nanosnowmen with a Conductive Junction as Highly Tunable Nanoantenna Structures and Sensitive, Quantitative and Multiplexable Surface-Enhanced Raman Scattering Probes. Nano Lett. 2014, 14, 6217–6225. [Google Scholar] [CrossRef]
- Lim, D.K.; Jeon, K.S.; Kim, H.M.; Nam, J.M.; Suh, Y.D. Nanogap-Engineerable Raman-Active Nanodumbbells for Single-Molecule Detection. Nat. Mater. 2010, 9, 60–67. [Google Scholar] [CrossRef]
- Kariuki, V.M.; Hoffmeier, J.C.; Yazgan, I.; Sadik, O.A. Seedless Synthesis and SERS Characterization of Multi-Branched Gold Nanoflowers Using Water Soluble Polymers. Nanoscale 2017, 9, 8330–8340. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Li, H.; Luo, Y.; Yu, R.; Zhang, L.; Song, Q. Au Nanoflower-Ag Nanoparticle Assembled SERS-Active Substrates for Sensitive MC-LR Detection. Chem. Commun. 2015, 51, 16908–16911. [Google Scholar] [CrossRef]
- Song, C.Y.; Yang, B.Y.; Chen, W.Q.; Dou, Y.X.; Yang, Y.J.; Zhou, N.; Wang, L.H. Gold Nanoflowers with Tunable Sheet-Like Petals: Facile Synthesis, SERS Performances and Cell Imaging. J. Mater. Chem. B 2016, 4, 7112–7118. [Google Scholar] [CrossRef]
- Ye, S.; Benz, F.; Wheeler, M.C.; Oram, J.; Baumberg, J.J.; Cespedes, O.; Evans, S.D. One-Step Fabrication of Hollow-Channel Gold Nanoflowers with Excellent Catalytic Performance and Large Single-Particle SERS Activity. Nanoscale 2016, 8, 14932–14942. [Google Scholar] [CrossRef] [Green Version]
- Tong, J.; Xu, Z.; Bian, Y.; Niu, Y.; Zhang, Y.; Wang, Z. Flexible and Smart Fibers Decorated with Ag Nanoflowers for Highly Active Surface-Enhanced Raman Scattering Detection. J. Raman Spectrosc. 2019, 50, 1468–1476. [Google Scholar] [CrossRef]
- Song, C.Y.; Zhou, N.; Yang, B.Y.; Yang, Y.J.; Wang, L.H. Facile Synthesis of Hydrangea Flower-Like Hierarchical Gold Nanostructures with Tunable Surface Topographies for Single-Particle Surface-Enhanced Raman Scattering. Nanoscale 2015, 7, 17004–17011. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Xue, B.; Peng, Z.; Cao, Z.; Mu, Q.; Xuan, L. Highly Effective and Chemically Stable Surface Enhanced Raman Scattering Substrates with Flower-Like 3D Ag-Au Hetero-Nanostructures. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B Condens. Matter Mater. Phys. 1972, 6, 4370. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.-X.; Kang, H.-S.; Zhao, W.-Q.; Chen, Y.-L.; Ma, L.; Ding, S.-J.; Chen, X.-B.; Wang, Q.-Q. Dual Plasmon Resonances and Tunable Electric Field in Structure-Adjustable Au Nanoflowers for Improved SERS and Photocatalysis. Nanomaterials 2021, 11, 2176. https://doi.org/10.3390/nano11092176
Zhao Y-X, Kang H-S, Zhao W-Q, Chen Y-L, Ma L, Ding S-J, Chen X-B, Wang Q-Q. Dual Plasmon Resonances and Tunable Electric Field in Structure-Adjustable Au Nanoflowers for Improved SERS and Photocatalysis. Nanomaterials. 2021; 11(9):2176. https://doi.org/10.3390/nano11092176
Chicago/Turabian StyleZhao, Yi-Xin, Hao-Sen Kang, Wen-Qin Zhao, You-Long Chen, Liang Ma, Si-Jing Ding, Xiang-Bai Chen, and Qu-Quan Wang. 2021. "Dual Plasmon Resonances and Tunable Electric Field in Structure-Adjustable Au Nanoflowers for Improved SERS and Photocatalysis" Nanomaterials 11, no. 9: 2176. https://doi.org/10.3390/nano11092176





