Green Synthesis of Cu Nanoparticles in Modulating the Reactivity of Amine-Functionalized Composite Materials towards Cross-Coupling Reactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Leaf Extract Reducing Agent
2.2. Preparation of Amine-Functionalized Composite Materials
2.3. Preparation of Cu(0) Modified Composite Materials
2.4. Physico-Chemical Analytical Methods
2.5. General Procedure for Catalytic Ullman C-C/C-O/C-N Coupling Reaction
3. Results and Discussion
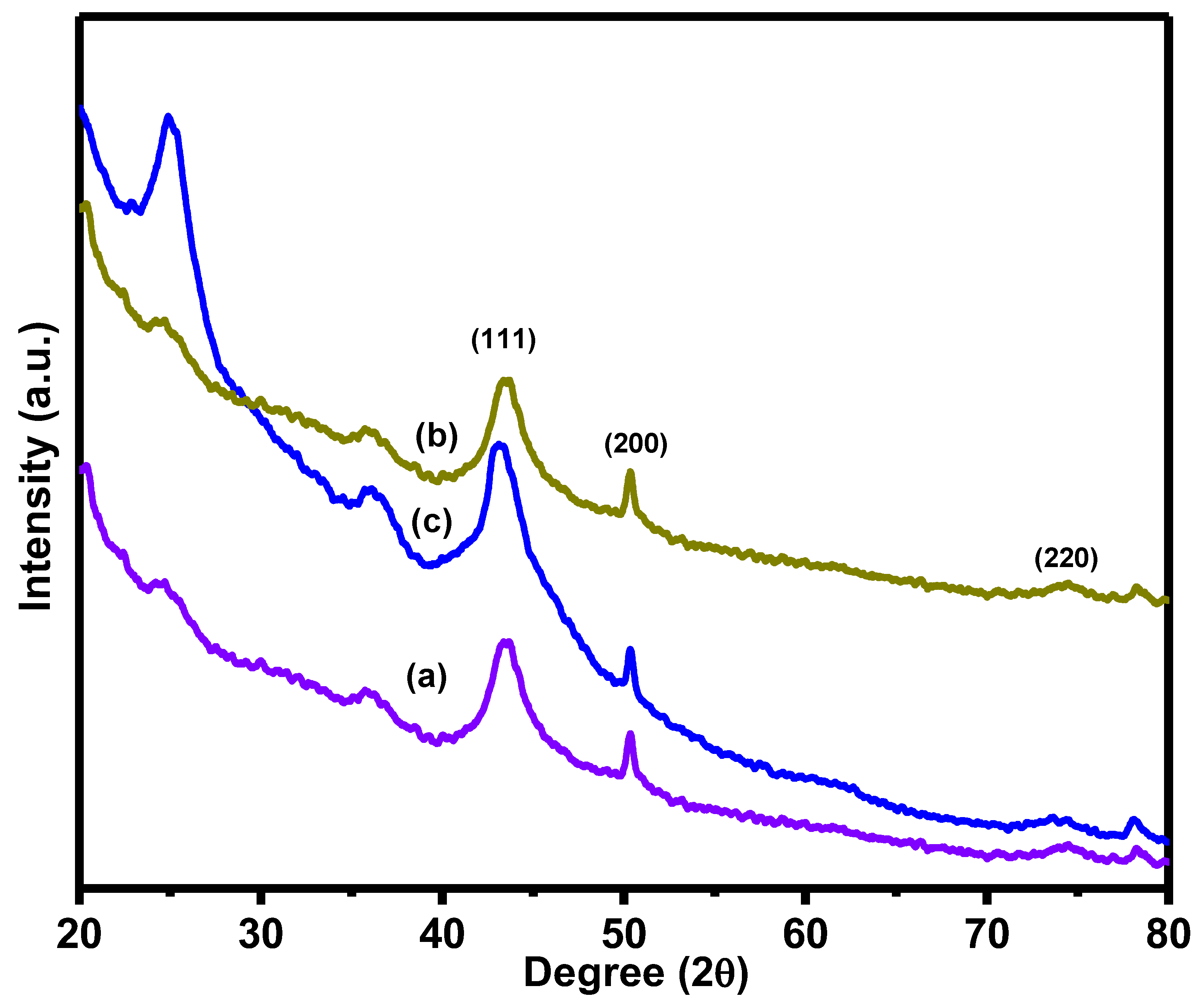
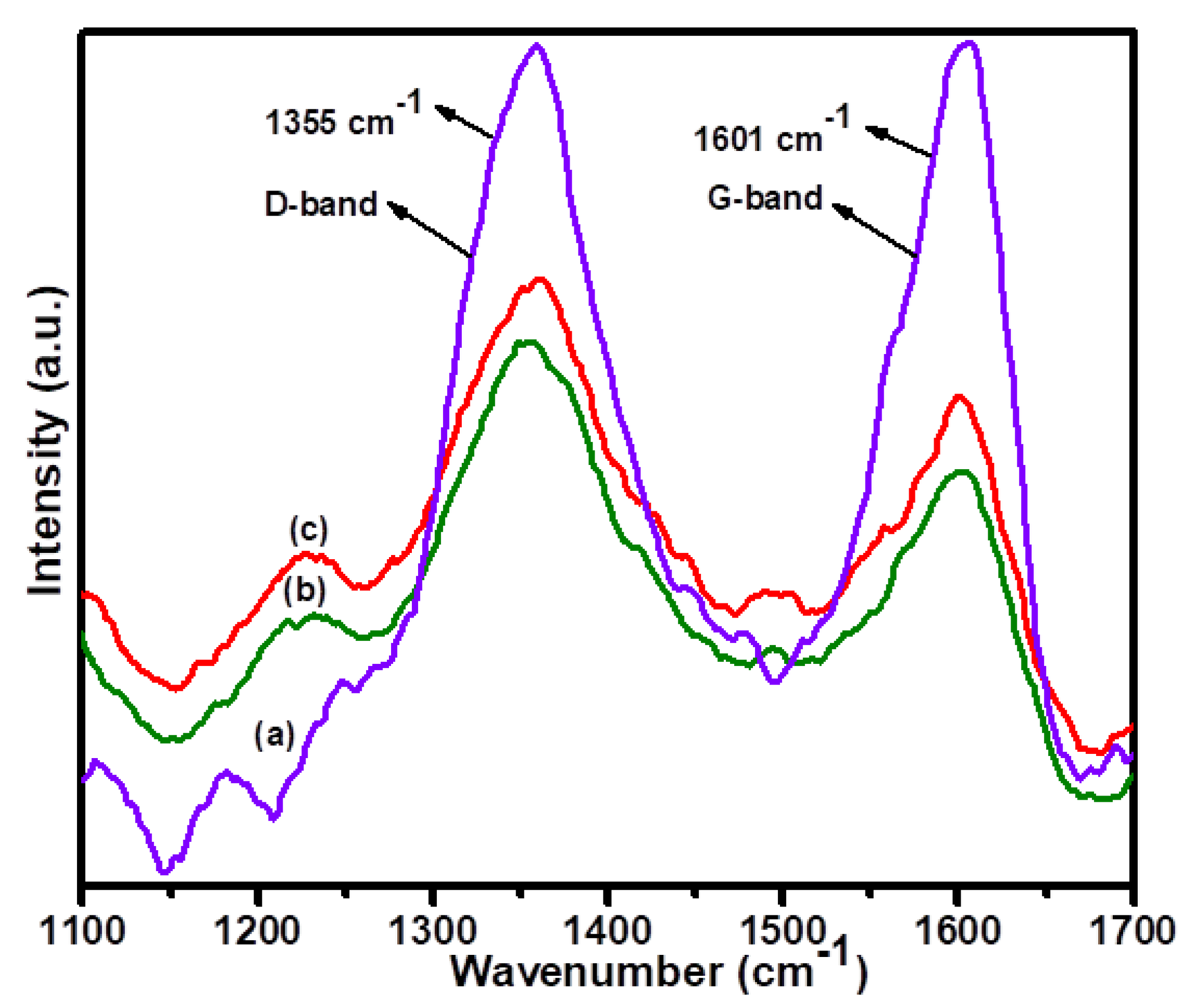
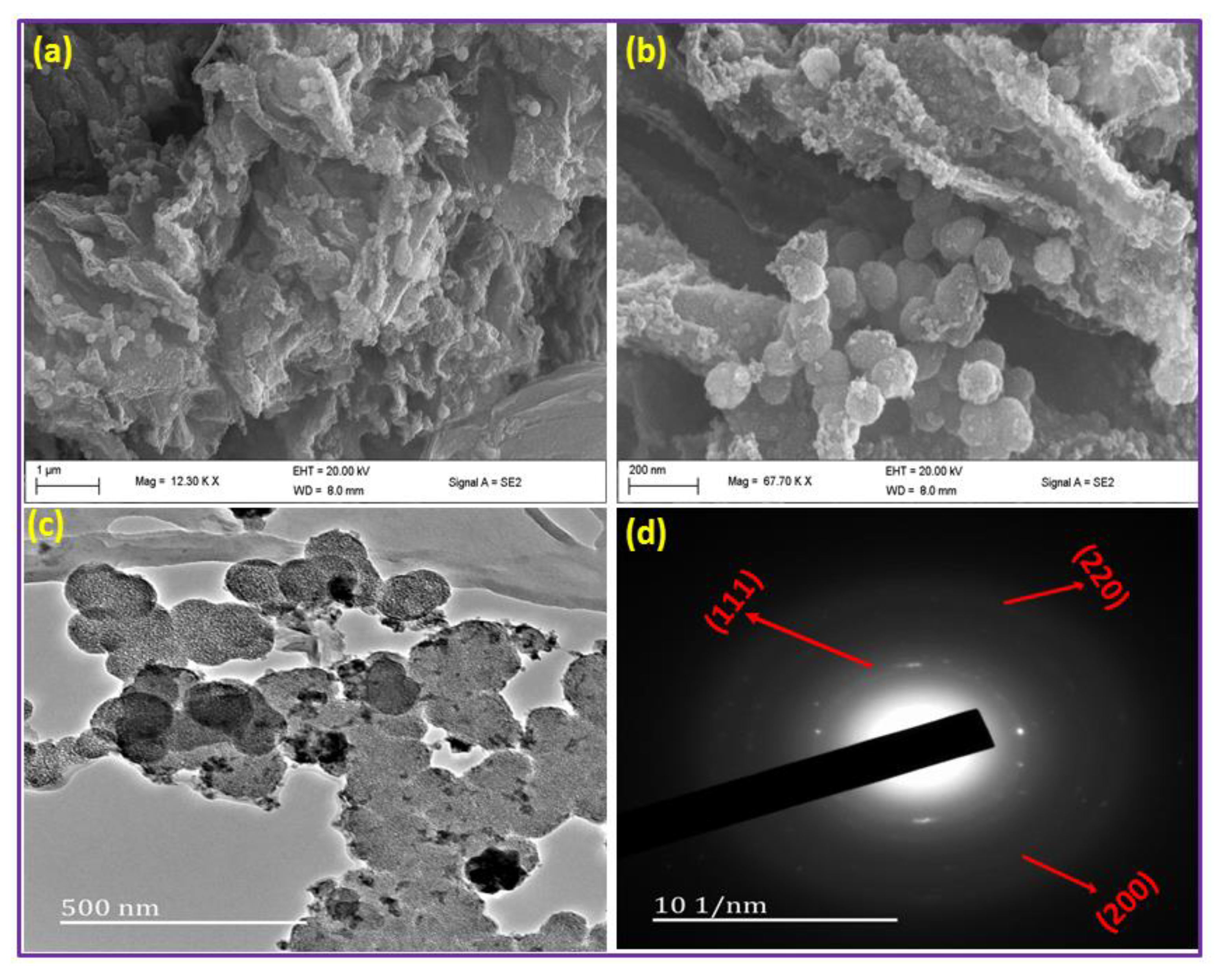
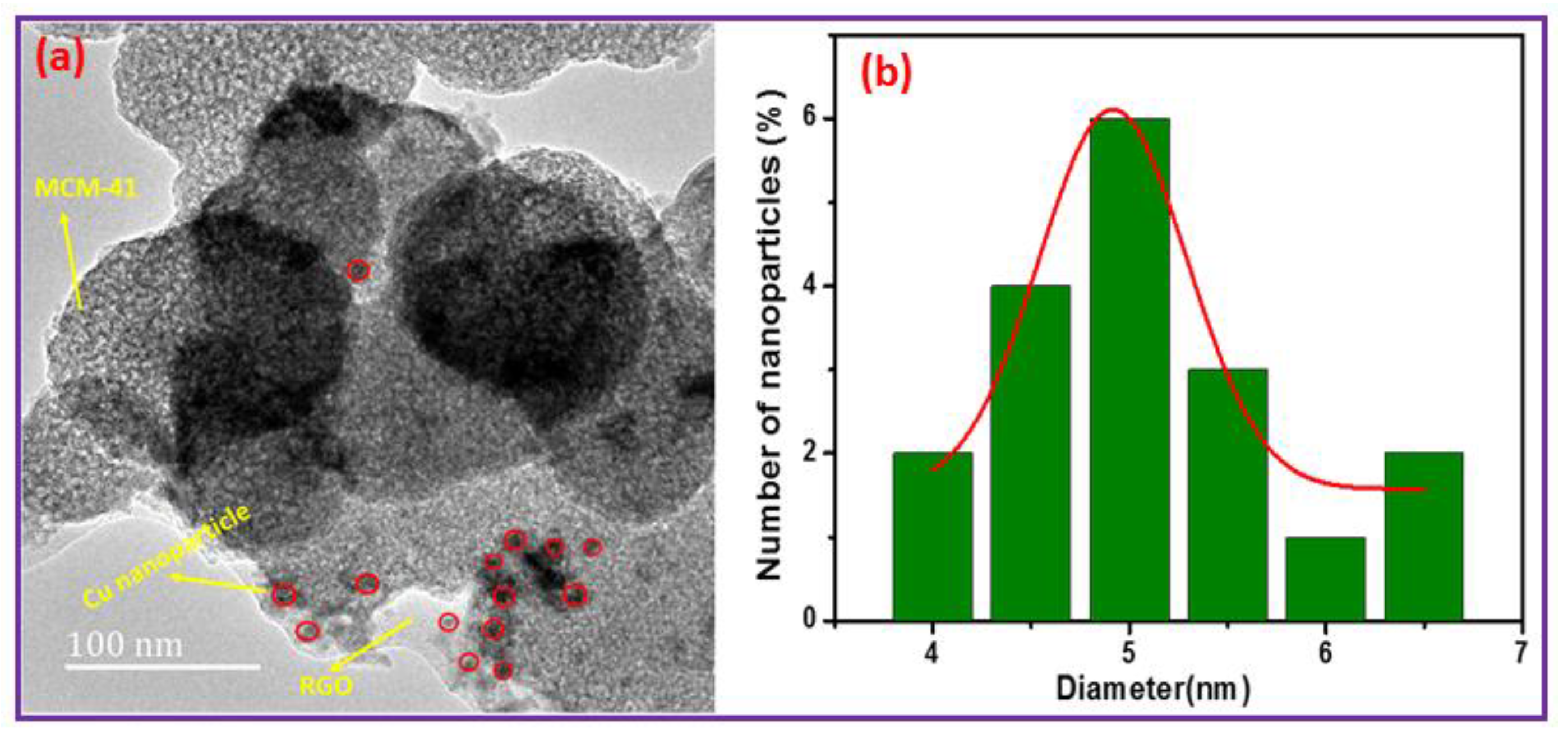
3.1. Surface Characterization
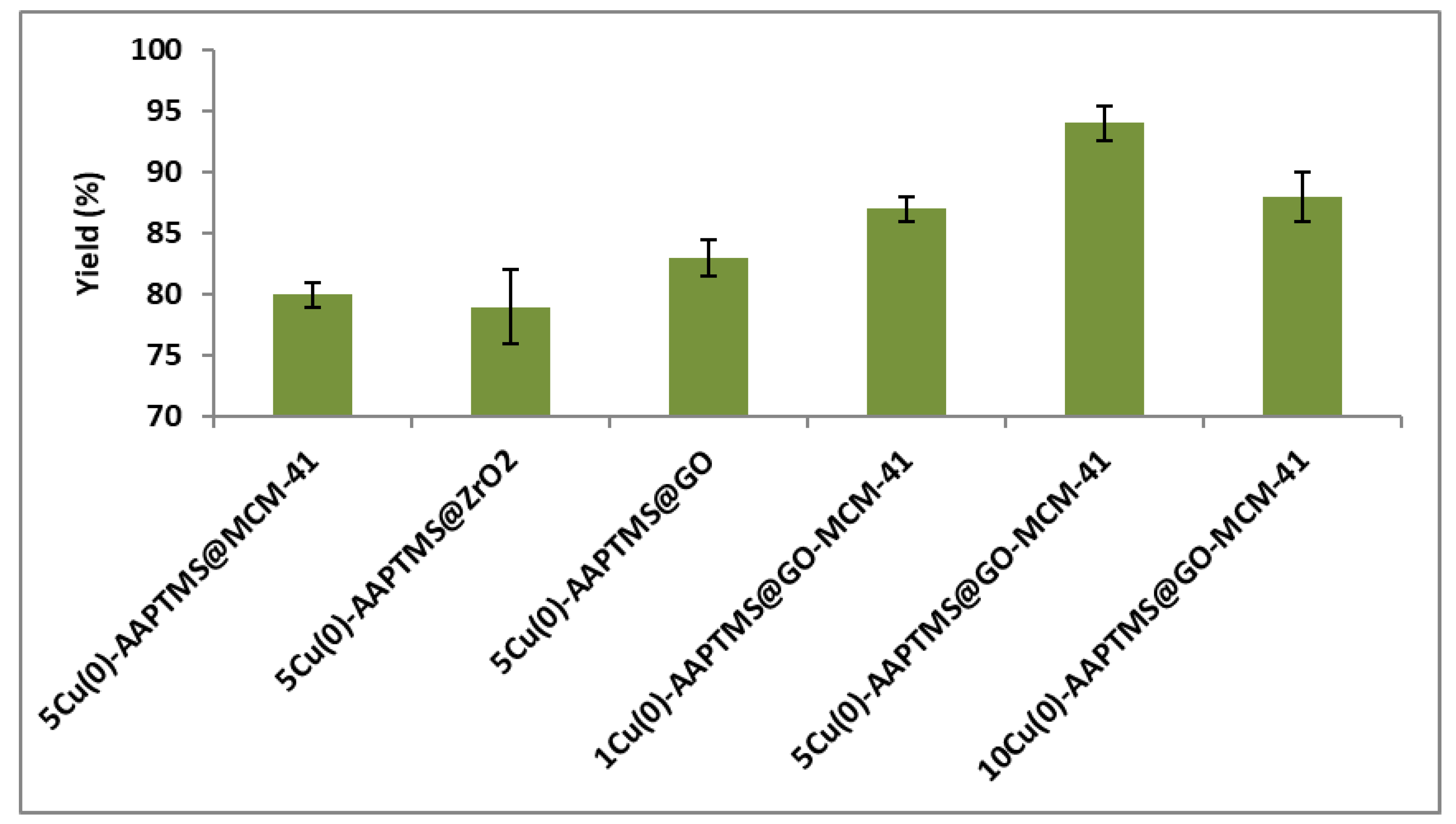
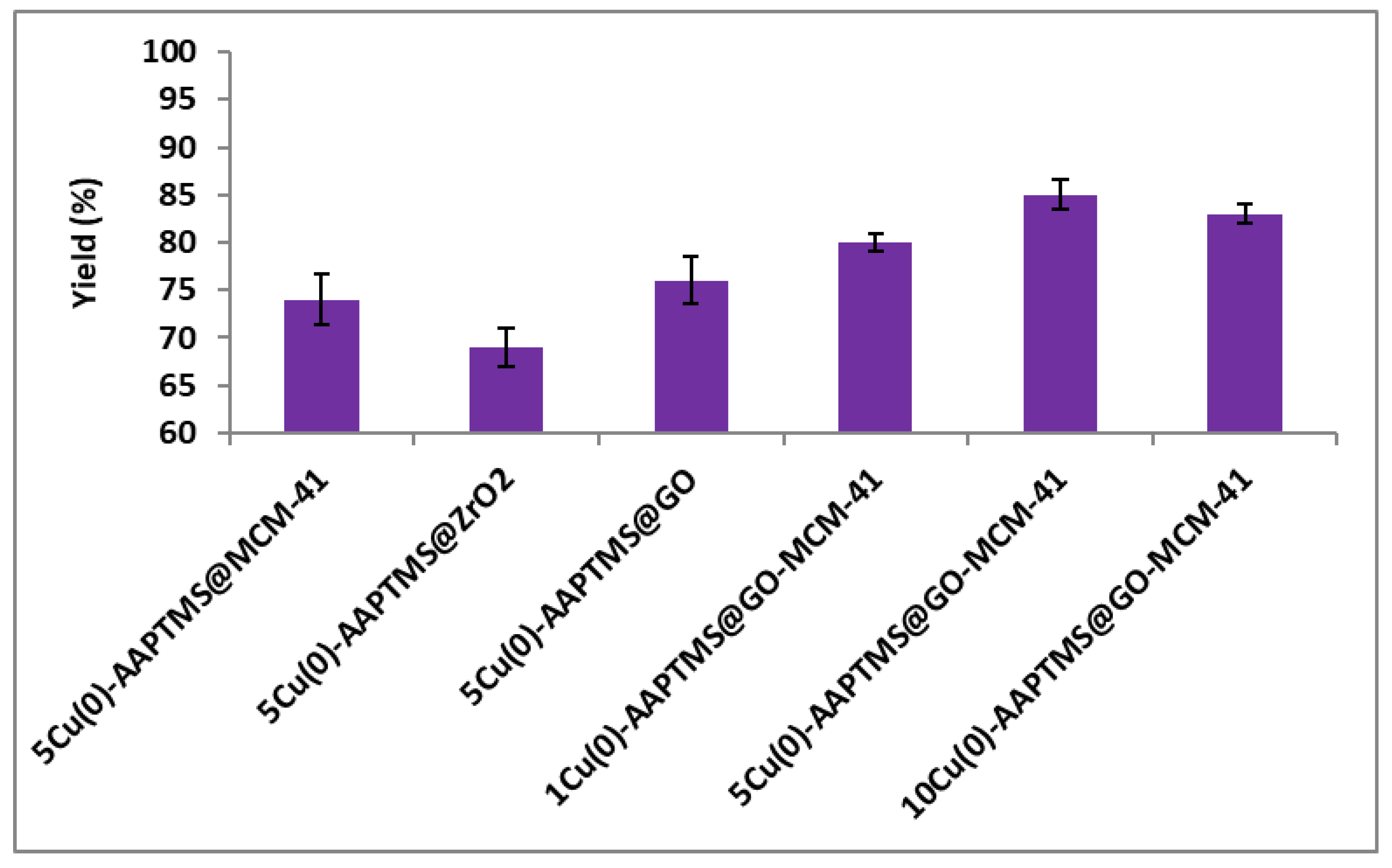
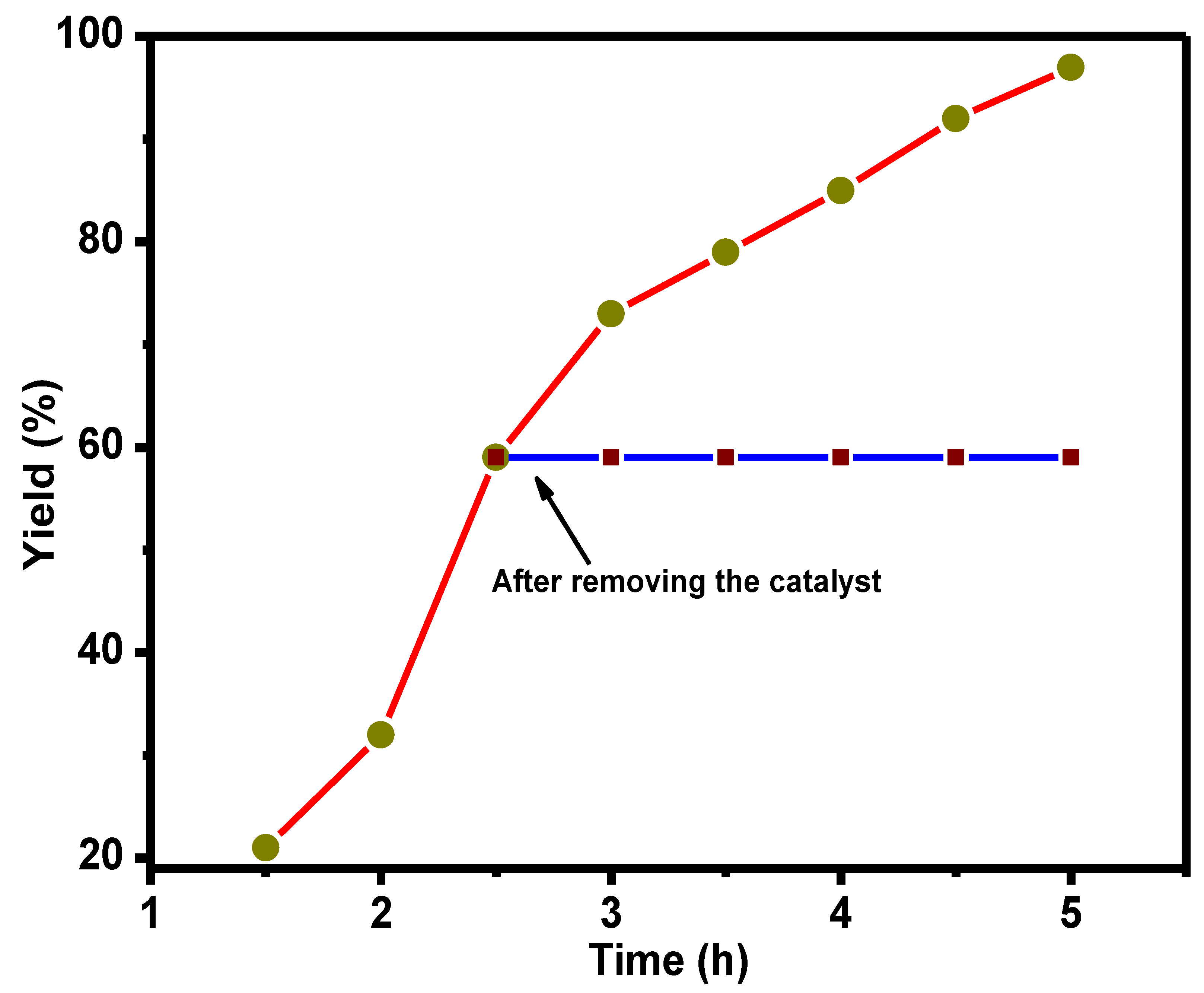
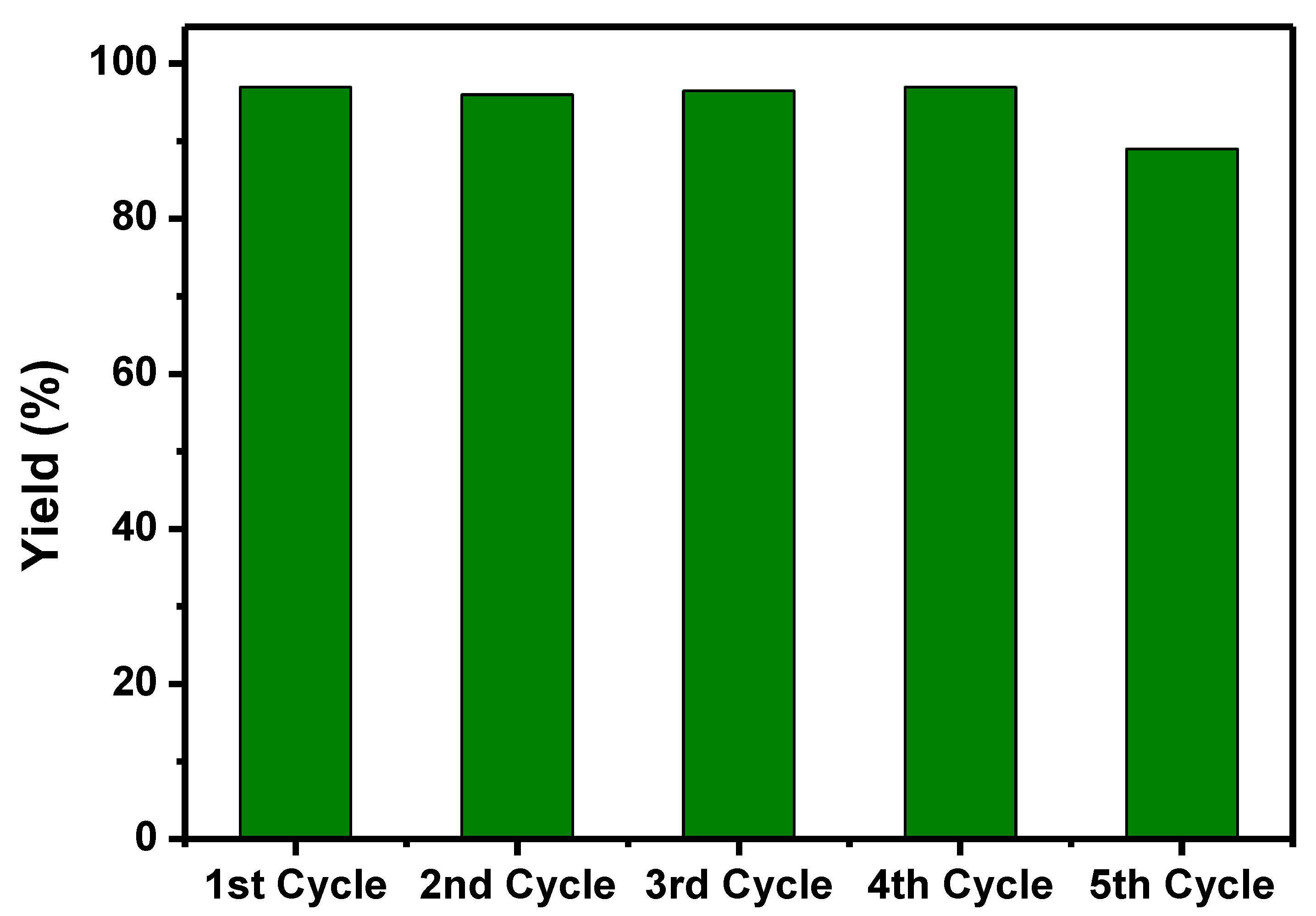
3.2. Catalyst Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senanayake, S.; Stacchiola, D.; Rodriguez, J.A. Unique Properties of Ceria Nanoparticles Supported on Metals: Novel Inverse Ceria/Copper Catalysts for CO Oxidation and the Water-Gas Shift Reaction. Acc. Chem. Res. 2013, 46, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, S.; Groppo, E.; Agostini, G.; Van Bokhoven, J.A.; Lamberti, C. Reactivity of Surface Species in Heterogeneous Catalysts Probed by In Situ X-ray Absorption Techniques. Chem. Rev. 2013, 113, 1736–1850. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilisation, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Branco, P.; Parghi, K.D.; Shrikhande, J.J.; Pandey, R.K.; Ghumman, C.A.A.; Bundaleski, N.; Teodoro, O.; Jayaram, R.V. Synthesis and characterization of versatile MgO–ZrO2 mixed metal oxide nanoparticles and their applications. Catal. Sci. Technol. 2011, 1, 1653–1664. [Google Scholar] [CrossRef]
- Zaera, F. Nanostructured materials for applications in heterogeneous catalysis. Chem. Soc. Rev. 2013, 42, 2746–2762. [Google Scholar] [CrossRef] [PubMed]
- Evano, G.; Blanchard, N.; Toumi, M. Copper-Mediated Coupling Reactions and Their Applications in Natural Products and Designed Biomolecules Synthesis. Chem. Rev. 2008, 108, 3054–3131. [Google Scholar] [CrossRef]
- Losada-Garcia, N.; Rodriguez-Otero, A.; Palomo, J.M. Tailorable synthesis of heterogeneous enzyme–copper nanobiohybrids and their application in the selective oxidation of benzene to phenol. Catal. Sci. Technol. 2020, 10, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Huang, W.; Xu, Y.; Ye, X.; Wu, M.; Shao, Q.; Ou, G.; Peng, Z.; Shi, J.; Chen, J.; et al. Catalytic oxidation of gaseous benzene with ozone over zeolite-supported metal oxide nanoparticles at room temperature. Catal. Today 2015, 258, 627–633. [Google Scholar] [CrossRef]
- Ahmed, A.; Elvati, P.; Violi, A. Size-and phase-dependent structure of copper (II) oxide nanoparticles. RSC Adv. 2015, 5, 35033–35041. [Google Scholar] [CrossRef]
- Mondal, J.; Biswas, A.; Chiba, S.; Zhao, Y. Cu0 Nanoparticles Deposited on Nanoporous Polymers: A Recyclable Heterogeneous Nanocatalyst for Ullmann Coupling of Aryl Halides with Amines in Water. Sci. Rep. 2015, 5, 8294. [Google Scholar] [CrossRef] [Green Version]
- Baig, N.; Varma, R. Copper Modified Magnetic Bimetallic Nano-catalysts Ligand Regulated Catalytic Activity. Curr. Org. Chem. 2013, 17, 2227–2237. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [Green Version]
- Nerl, H.; Cheng, C.; E Goode, A.; Bergin, S.D.; Lich, B.; Gass, M.; E Porter, A. Imaging methods for determining uptake and toxicity of carbon nanotubesin vitroandin vivo. Nano Med. 2011, 6, 849–865. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Tung, V.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Yu, D.; Dai, L. Self-Assembled Graphene/Carbon Nanotube Hybrid Films for Supercapacitors. J. Phys. Chem. Lett. 2010, 1, 467–470. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, D.; Zeng, C.; Miao, Z.; Dai, L. Biocompatible Graphene Oxide-Based Glucose Biosensors. Langmuir 2010, 26, 6158–6160. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.; Booth, T.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-Based Materials: Synthesis, Characterization, Properties, and Applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Pary, F.F.; Tirumala, R.T.A.; Andiappan, M.; Nelson, T.L. Copper(i) oxide nanoparticle-mediated C–C couplings for synthesis of polyphenylenediethynylenes: Evidence for a homogeneous catalytic pathway. Catal. Sci. Technol. 2021, 11, 2414–2421. [Google Scholar] [CrossRef]
- Sonei, M.S.S.; Taghavi, F.; Khojastehnezhad, A.; Gholizadeh, M. Copper-Functionalized Silica-Coated Magnetic Nanoparticles for an Efficient Suzuki Cross-Coupling Reaction. ChemistrySelect 2021, 6, 359–368. [Google Scholar] [CrossRef]
- Lee, J.K.; Smith, K.B.; Hayner, C.M.; Kung, H.H. Silicon nanoparticles–graphene paper composites for Li ion battery anodes. Chem. Commun. 2010, 46, 2025–2027. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.; Thomas, A.W. Modern Synthetic Methods for Copper-Mediated C(aryl)-O, C(aryl)-N, and C(aryl)-S Bond Formation. Angew. Chem. Int. Ed. 2003, 42, 5400–5449. [Google Scholar] [CrossRef]
- Rana, S.; Varadwaj, G.B.B.; Jonnalagadda, S.B.; Varadwaj, B.B. Ni nanoparticle supported reduced graphene oxide as a highly active and durable heterogeneous material for coupling reactions. Nanoscale Adv. 2019, 1, 1527–1530. [Google Scholar] [CrossRef] [Green Version]
- Rana, S.; Mallick, S.; Parida, K.M. Facile Method for Synthesis of Polyamine-Functionalized Mesoporous Zirconia and Its Catalytic Evaluation toward Henry Reaction. Ind. Eng. Chem. Res. 2011, 50, 2055–2064. [Google Scholar] [CrossRef]
- Kommidi, D.R.; Rana, S.; Singh, P.; Shintre, S.A.; Koorbanally, N.A.; Jonnalagadda, S.B.; Pagadala, R.; Moodley, B. Novel carbapenem chalcone derivatives: Synthesis, cytotoxicity and molecular docking studies. Org. Biomol. Chem. 2015, 13, 4344–4350. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Varadwaj, G.B.B.; Jonnalagadda, S.B. Pd nanoparticle supported reduced graphene oxide and its excellent catalytic activity for the Ullmann C–C coupling reaction in a green solvent. RSC Adv. 2019, 9, 13332–13335. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, Y.; Rao, K.V.; Sai, V.S.; Pavani, T. A facile biosynthesis of copper nanoparticles: A micro-structural and antibacterial activity investigation. J. Saudi Chem. Soc. 2017, 21, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Gao, Z.; Li, Y. Immobilization of Pd(II) on MOFs as a highly active heterogeneous catalyst for Suzuki–Miyaura and Ullmann-type coupling reactions. Catal. Today 2015, 245, 122–128. [Google Scholar] [CrossRef]
- Li, H.; Chai, W.; Zhang, F.; Chen, J. Water-medium Ullmann reaction over a highly active and selective Pd/Ph-SBA-15 catalyst. Green Chem. 2007, 9, 1223–1228. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, H.; Zhao, Q.; Klingstedt, M.; Terasaki, O.; Zhao, D. Ordered Mesoporous Pd/Silica−Carbon as a Highly Active Heterogeneous Catalyst for Coupling Reaction of Chlorobenzene in Aqueous Media. J. Am. Chem. Soc. 2009, 131, 4541–4550. [Google Scholar] [CrossRef]
- Karimi, B.; Esfahani, F.K. Unexpected golden Ullmann reaction catalyzed by Au nanoparticles supported on periodic mesoporous organosilica (PMO). Chem. Commun. 2011, 47, 10452–10454. [Google Scholar] [CrossRef]
- Varadwaj, G.B.B.; Rana, S.; Parida, K. Pd(0) Nanoparticles Supported Organofunctionalized Clay Driving C–C Coupling Reactions under Benign Conditions through a Pd(0)/Pd(II) Redox Interplay. J. Phys. Chem. C 2014, 118, 1640–1651. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Maham, M.; Rostami-Vartooni, A.; Bagherzadeh, M.; Sajadi, S.M. Barberry fruit extract assisted in situ green synthesis of Cu nanoparticles supported on a reduced graphene oxide–Fe3O4 nanocomposite as a magnetically separable and reusable catalyst for the O-arylation of phenols with aryl halides under ligand-free conditions. RSC Adv. 2015, 5, 64769–64780. [Google Scholar] [CrossRef]
- Miao, T.; Wang, L. Immobilization of copper in organic–inorganic hybrid materials: A highly efficient and reusable catalyst for the Ullmann diaryl etherification. Tetrahedron Lett. 2007, 48, 95–99. [Google Scholar] [CrossRef]
- Arundhathi, R.; Sreedhar, B.; Parthasarathy, G. Highly efficient heterogenous catalyst for O-arylation of phenols with aryl halides using natural ferrous chamosite. Appl. Clay Sci. 2011, 51, 131–137. [Google Scholar] [CrossRef]
- Zhai, Z.; Guo, X.; Jiao, Z.; Jin, G.; Guo, X.-Y. Graphene-supported Cu2O nanoparticles: An efficient heterogeneous catalyst for C–O cross-coupling of aryl iodides with phenols. Catal. Sci. Technol. 2014, 4, 4196–4199. [Google Scholar] [CrossRef]
- Pan, K.; Ming, H.; Yu, H.; Huang, H.; Liu, Y.; Kang, Z. Copper nanoparticles modified silicon nanowires with enhanced cross-coupling catalytic ability. Dalton Trans. 2012, 41, 2564–2566. [Google Scholar] [CrossRef] [PubMed]


| Entry | Solvents | Yield (%) C-C Coupling Product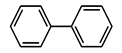 |
|---|---|---|
| 1 | Toluene | 79 |
| 2 | Benzene | 68 |
| 3 | DMF | 99 |
| 4 | THF | 98 |
| 5 | Water | 97 |
| Entry | Solvents | Yield (%) C-O Coupling Product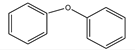 |
|---|---|---|
| 1 | Toluene | 63 |
| 2 | Benzene | 49 |
| 3 | DMF | 94 |
| 4 | THF | 90 |
| 5 | Water | 89 |
| Entry | Solvents | Yield (%) C-N Coupling Product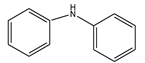 |
|---|---|---|
| 1 | Toluene | 57 |
| 2 | Benzene | 25 |
| 3 | DMF | 85 |
| 4 | THF | 65 |
| 5 | Water | 84 |
| Sl. No. | Substrate | a Yield (%) C-C Coupling Product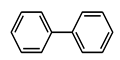 | b Yield (%) C-O Coupling Product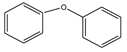 | c Yield (%) C-N Coupling Product |
|---|---|---|---|---|
| 1 | 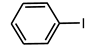 | 94 | 85 | |
| 2 | 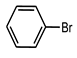 | 90 | 71 | 62 |
| 3 |  | 81 | 29 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, S.; Varadwaj, G.B.B.; Jonnalagadda, S.B. Green Synthesis of Cu Nanoparticles in Modulating the Reactivity of Amine-Functionalized Composite Materials towards Cross-Coupling Reactions. Nanomaterials 2021, 11, 2260. https://doi.org/10.3390/nano11092260
Rana S, Varadwaj GBB, Jonnalagadda SB. Green Synthesis of Cu Nanoparticles in Modulating the Reactivity of Amine-Functionalized Composite Materials towards Cross-Coupling Reactions. Nanomaterials. 2021; 11(9):2260. https://doi.org/10.3390/nano11092260
Chicago/Turabian StyleRana, Surjyakanta, G. Bishwa Bidita Varadwaj, and Sreekanth B. Jonnalagadda. 2021. "Green Synthesis of Cu Nanoparticles in Modulating the Reactivity of Amine-Functionalized Composite Materials towards Cross-Coupling Reactions" Nanomaterials 11, no. 9: 2260. https://doi.org/10.3390/nano11092260






