Marble Waste Sludges as Effective Nanomaterials for Cu (II) Adsorption in Aqueous Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterizations
2.2. Batch Adsorption Tests
2.3. Dinamic Adsorption Tests
3. Results
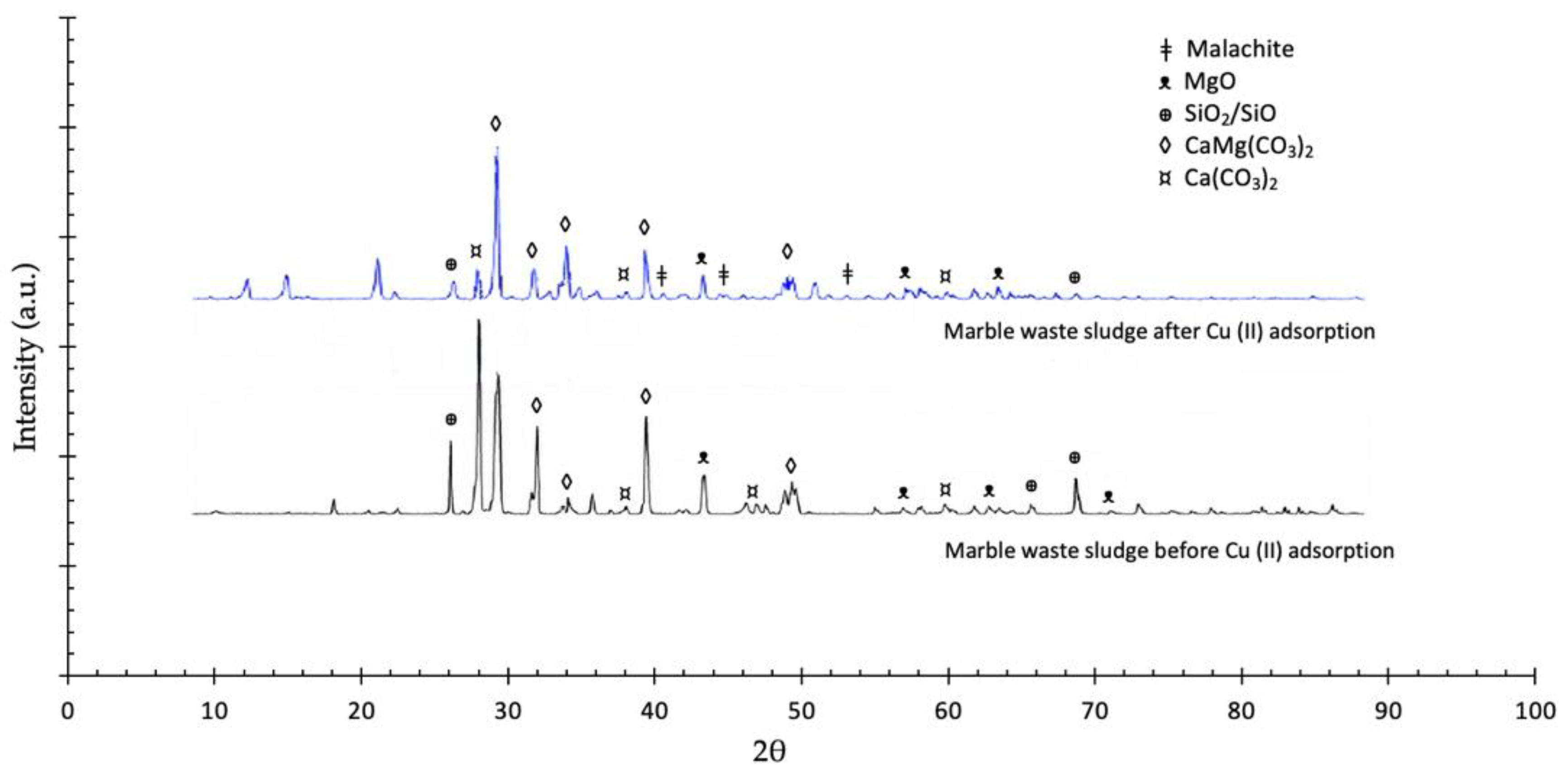
3.1. Characterizations
3.2. Batch Adsorption Tests
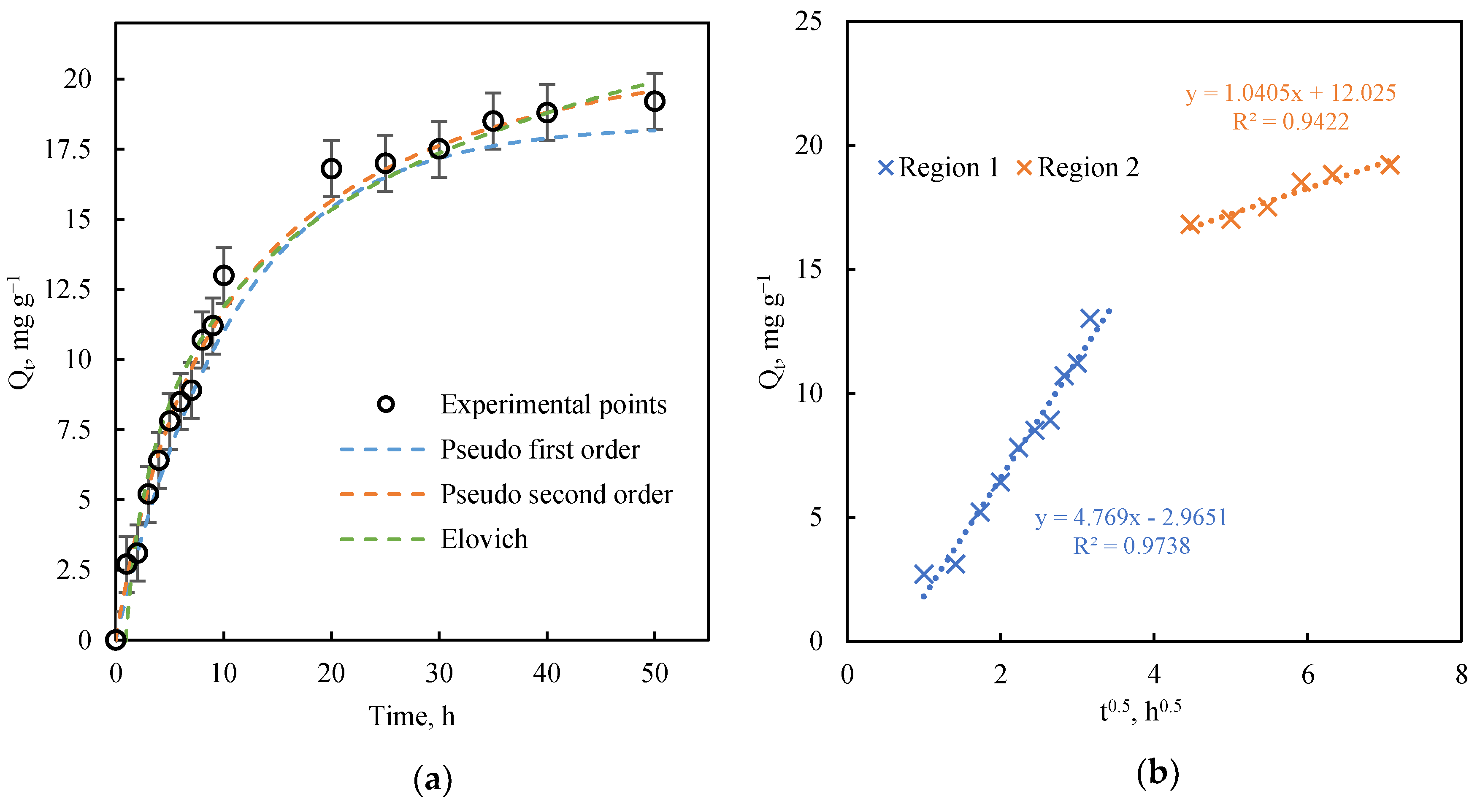
3.2.1. Kinetics Study
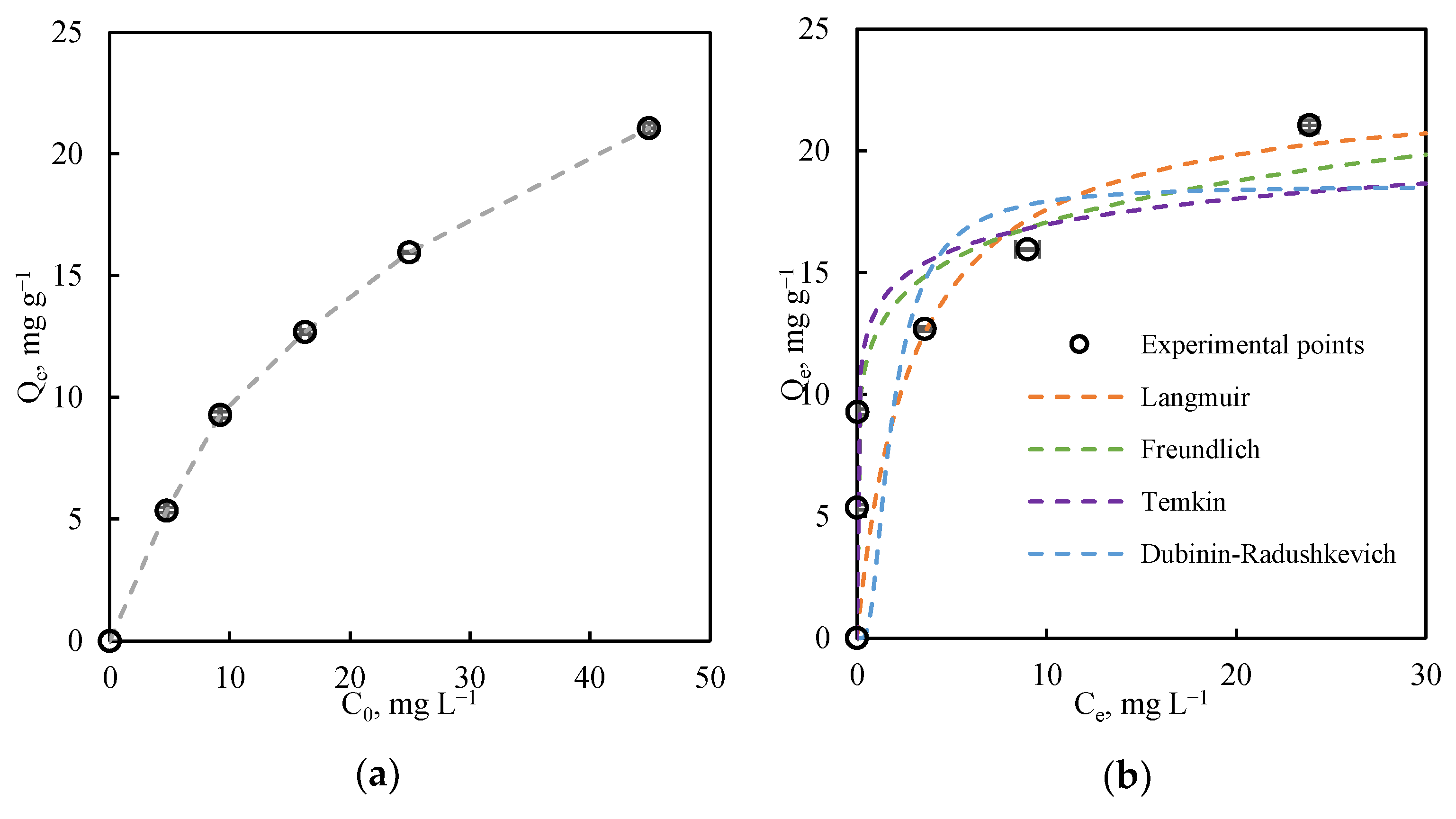
3.2.2. Equilibrium Study
3.3. Adsorption Mechanism
3.4. Influence of Operational Variables
3.4.1. Influence of pH
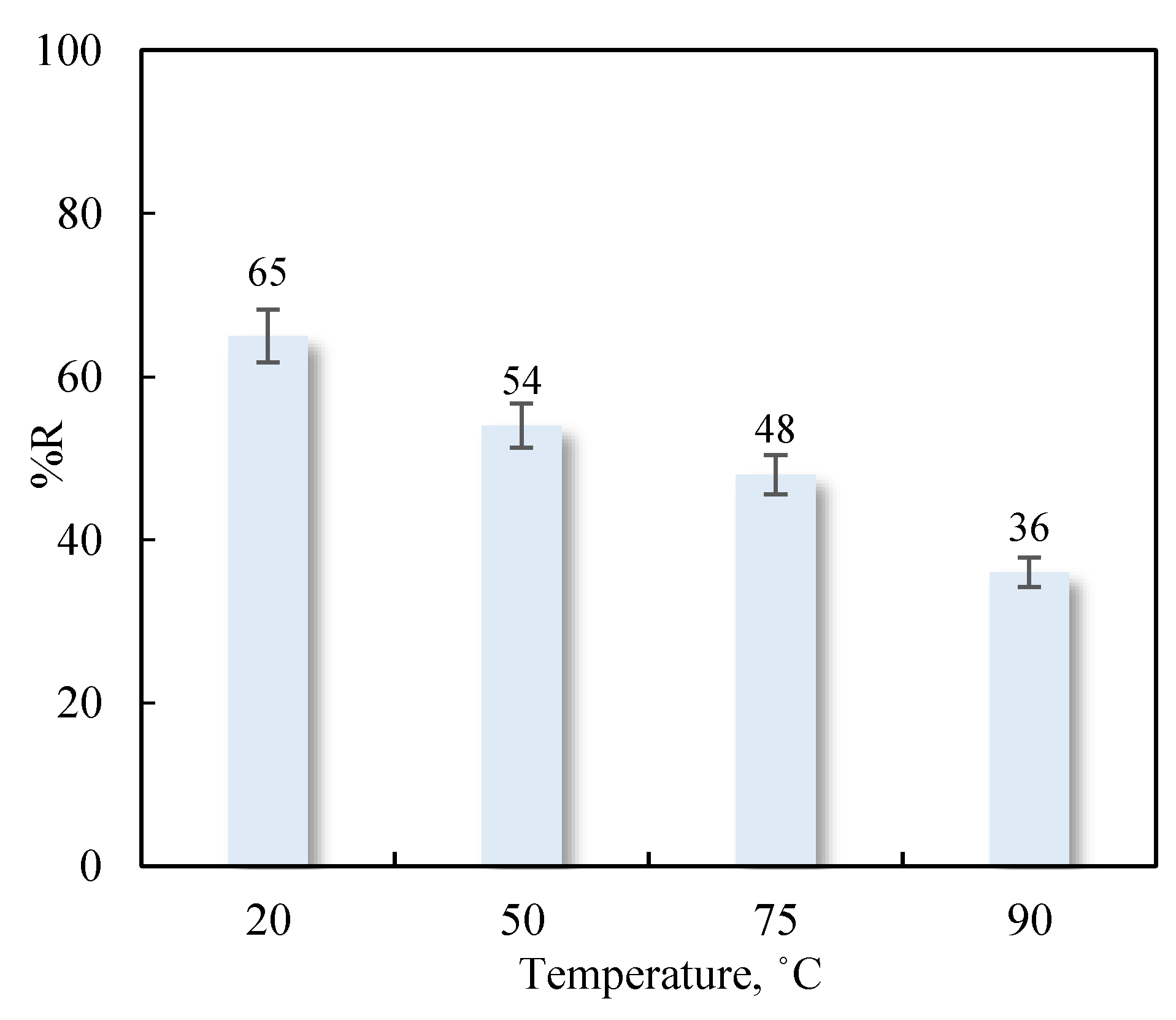
3.4.2. Influence of Temperature
3.4.3. Influence of Ionic Strength
3.4.4. Influence of Chemical Composition of Water
3.5. Dynamic Adsorption Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- European Environment Agency. Available online: https://industry.eea.europa.eu/pollutants/pollutant-index (accessed on 1 July 2021).
- European Environment Agency-Environmental Pressures of Heavy Metal Releases from Europe’s Industry. Available online: https://www.eea.europa.eu/publications/environmental-pressures-of-heavy-metal/heavy-metal-pollution (accessed on 1 July 2021).
- Guidelines for Drinking Water Quality, 4th Edition, WHO. Available online: https://www.who.int/water_sanitation_health/publications/2011/9789241548151_ch12.pdf (accessed on 1 July 2021).
- Office of Water U.S. Environmental Protection Agency. 2018 Edition of the Drinking Water Standards and Health Advisories Tables. 2018. Available online: https://www.epa.gov/sites/production/files/2018-03/documents/dwtable2018.pdf (accessed on 1 July 2021).
- Guimarães, T.; Paquini, L.D.; Ferraz, B.R.L.; Profeti, L.P.R.; Profeti, D. Efficient removal of Cu(II) and Cr(III) contaminants from aqueous solutions using marble waste powder. J. Environ. Chem. Eng. 2020, 8, 103972. [Google Scholar] [CrossRef]
- Pohl, A. Removal of Heavy Metal Ions from Water and Wastewaters by Sulfur-Containing Precipitation Agents. Water Air Soil Pollut. 2020, 231, 503. [Google Scholar] [CrossRef]
- Al-Enezi, G.; Hamoda, M.F.; Fawzi, N. Ion Exchange Extraction of Heavy Metals from Wastewater Sludges. J. Environ. Sci. Health 2004, 39, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Wu, T.; Hsu, P.C.; Xie, J.; Zhao, J.; Liu, K.; Sun, J.; Xu, J.; Tang, J.; Ye, Z.; et al. Direct/alternating current electrochemical method for removing and recovering heavy metal from water using graphene oxide electrode. ACS Nano 2019, 13, 6431–6437. [Google Scholar] [CrossRef] [PubMed]
- Wołowiec, M.; Komorowska-Kaufman, M.; Pruss, A.; Rzepa, G.; Bajda, T. Removal of heavy metals and metalloids from water using drinking water treatment residuals as adsorbents: A review. Minerals 2019, 9, 487. [Google Scholar] [CrossRef] [Green Version]
- Boujelben, N.; Bouzid, J.; Elouear, Z. Adsorption of nickel and copper onto natural iron oxide-coated sand from aqueous solutions: Study in single and binary systems. J. Hazard. Mater. 2009, 163, 376–382. [Google Scholar] [CrossRef]
- Ramos, V.C.; Han, W.; Yeung, K.L. A comparative study between ionic liquid coating and counterparts in bulk for toluene absorption. Green Chem. Eng. 2020, 1, 147–154. [Google Scholar] [CrossRef]
- Ramos, V.C.; Han, W.; Zhang, X.; Zhang, S.; Yeung, K.L. Supported ionic liquids for air purification. Curr. Opin. Green Sustain. Chem. 2020, 25, 100391. [Google Scholar] [CrossRef]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef]
- Martin-Martínez, J.M. Adsorción Física de Gases y Vapores por Carbones; Universidad de Alicante: Alicante, Spain, 1990. [Google Scholar]
- Stoeckli, H.F. Microporous carbons and their characterization: The present state of the art. Carbon 1990, 28, 1–6. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef]
- Saxena, S.; D’Souza, S.F. Heavy metal pollution abatement using rock phosphate mineral. Environ. Int. 2006, 32, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Ronda, A.; Martín-Lara, M.A.; Osegueda, O.; Castillo, V.; Blázquez, G. Scale-up of a packed bed column for wastewater treatment. Water Sci. Technol. 2018, 77, 1386–1396. [Google Scholar] [CrossRef]
- Abas, S.N.A.; Ismail, M.H.S.; Kamal, M.L.; Izhar, S. Adsorption process of heavy metals by low-cost adsorbent: A review. World Appl. Sci. J. 2013, 28, 1518–1530. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Alwared, A.I.; Sadiq, N.A. Competitive removal of lead copper and cadmium ions by sorptive flotation using marble wastes. Int. J. Environ. Waste Manag. 2019, 23, 156–178. [Google Scholar] [CrossRef]
- Wazwaz, A.; Al-Salaymeh, A.; Khan, M.S. Removing heavy metals through different types of soils and marble powder found in Oman. J. Ecol. Eng. 2019, 20, 136–142. [Google Scholar] [CrossRef]
- Javed, I.; Mateen, F.; Rafique, U.; Tabassum, N.; Balkhair, K.S.; Ashraf, M.A. Synthesis of zeolite from marble powder waste: A greener approach and its application for the removal of inorganic metals from wastewater. Desalination Water Treat. 2016, 57, 10422–10431. [Google Scholar] [CrossRef]
- Tozsin, G. Inhibition of acid mine drainage and immobilization of heavy metals from copper flotation tailings using a marble cutting waste. Int. J. Miner. Metall. Mater. 2016, 23, 1–6. [Google Scholar] [CrossRef]
- Rana, A.; Kalla, P.; Verma, H.K.; Mohnot, J.K. Recycling of dimensional stone waste in concrete: A review. J. Clean. Prod. 2016, 135, 312–331. [Google Scholar] [CrossRef]
- Demirel, B.; Alyamaç, K.E. Waste marble powder/dust. In Waste and Supplementary Cementitious Materials in Concrete: Characterisation, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 181–197. [Google Scholar] [CrossRef]
- Raklami, A.; Tahiri, A.I.; Bechtaoui, N.; Pajuelo, E.; Baslam, M.; Meddich, A.; Oufdou, K. Restoring the plant productivity of heavy metal-contaminated soil using phosphate sludge, marble waste, and beneficial microorganisms. J. Environ. Sci. 2021, 99, 210–221. [Google Scholar] [CrossRef]
- Farmaki, S.; Vorrisi, E.; Karakasi, O.; Moutsatsou, A. The role of limestone and dolomite Tailings’ particle size in retention of heavy metals from liquid waste. Min. Miner. Depos. 2018, 12, 95–103. [Google Scholar] [CrossRef]
- Mehta, D.; Mondal, P.; George, S. Utilization of marble waste powder as a novel adsorbent for removal of fluoride ions from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 932–942. [Google Scholar] [CrossRef]
- Ghazy, S.E.; Gabr, I.M.; Gad, A.H.M. Cadmium(II) sorption from water samples by powdered marble wastes. Chem. Speciat. Bioavailab. 2008, 20, 249–260. [Google Scholar] [CrossRef]
- Ramavandi, B.; Ahmadi, M.; Faradmal, J.; Maleki, S.; Asgari, G. Optimization of Fluoride Adsorption from Aqueous Solution by Marble Powder Using Taguchi Model. J. Maz. Univ. Med Sci. 2014, 24, 113–121. [Google Scholar]
- Zogorski, J.S.; Faust, S.D. Operational parameters for optimum removal of phenolic compounds from polluted waters by columns of activated carbon. Water AICHE Symp. Ser. Am. Inst. Chem. Eng. 1977, 73, 54–65. [Google Scholar]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie Der Sogenannten adsorptiongeloster stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Elovich, S.Y.; Larinov, O.G. Theory of Adsorption from Solutions of Non Electrolytes on Solid (I) Equation Adsorption from Solutions and the Analysis of Its Simplest Form, (II) Verification of the Equation of Adsorption Isotherm from Solutions. Izv. Akad. Nauk. SSSR Otd. Khimicheskikh Nauk. 1962, 2, 209–216. [Google Scholar]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of adsorption on carbonfrom solution. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Walker, G.M.; Connor, G.; Allen, S.J. Copper (II) removal onto dolomitic sorbents. Chem. Eng. Res. Des. 2004, 82, 961–966. [Google Scholar] [CrossRef]
- Aziz, H.A.; Adlan, M.N.; Ariffin, K.S. Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: Post treatment by high quality limestone. Bioresour. Technol. 2008, 99, 1578–1583. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M. Langmuir, Freundlich, Temkin and Dubinin-Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk. Available online: www.iosrjournals.org (accessed on 15 July 2021).
- Lin, T.Y.; Chai, W.S.; Chen, S.J.; Shih, J.Y.; Koyande, A.K.; Liu, B.L.; Chang, Y.K. Removal of soluble microbial products and dyes using heavy metal wastes decorated on eggshell. Chemosphere 2021, 270, 128615. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Jegan, J.; Palanivelu, K.; Velan, M. Removal and recovery of copper from aqueous solution by eggshell in a packed column. Miner. Eng. 2005, 18, 545–547. [Google Scholar] [CrossRef]
- Ahmad, R.; Kumar, R.; Haseeb, S. Adsorption of Cu2+ from aqueous solution onto iron oxide coated eggshell powder: Evaluation of equilibrium, isotherms, kinetics, and regeneration capacity. Arab. J. Chem. 2012, 5, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Pehlivan, E.; Özkan, A.M.; Dinç, S.; Parlayici, Ş. Adsorption of Cu2+ and Pb2+ ion on dolomite powder. J. Hazard. Mater. 2009, 167, 1044–1049. [Google Scholar] [CrossRef]
- Nashtifan, S.G.; Azadmehr, A.; Maghsoudi, A. Comparative and competitive adsorptive removal of Ni2+ and Cu2+ from aqueous solution using iron oxide-vermiculite composite. Appl. Clay Sci. 2017, 140, 38–49. [Google Scholar] [CrossRef]
- Huang, Y.H.; Hsueh, C.L.; Cheng, H.P.; Su, L.C.; Chen, C.Y. Thermodynamics and kinetics of adsorption of Cu(II) onto waste iron oxide. J. Hazard. Mater. 2007, 144, 406–411. [Google Scholar] [CrossRef]
- Chen, C.; Liu, H.; Chen, T.; Chen, D.; Frost, R.L. An insight into the removal of Pb(II), Cu(II), Co(II), Cd(II), Zn(II), Ag(I), Hg(I), Cr(VI) by Na(I)-montmorillonite and Ca(II)-montmorillonite. Appl. Clay Sci. 2015, 118, 239–247. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Guo, L.; Zhang, Y.; Lou, Z.; Wang, Y. Cu(II) removal from aqueous solution by Spartina alterniflora derived biochar. Bioresour. Technol. 2013, 141, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Bouhamed, F.; Elouear, Z.; Bouzid, J. Adsorptive removal of copper(II) from aqueous solutions on activated carbon prepared from Tunisian date stones: Equilibrium, kinetics and thermodynamics. J. Taiwan Inst. Chem. Eng. 2012, 43, 741–749. [Google Scholar] [CrossRef]
- Figgis, B.N.; Sobolev, A.N.; Simmons, C.J.; Hitchman, M.A.; Stratemeier, H.; Riley, M.J. (NH4)2[Cu(H2O)6](SO4)2 Structural Science Bonding effects and the crystal structures of (NH4)2[Cu(H2O)6](SO4)2 and its H218O substituted form at 9.5 K. Acta Crystallogr. Sect. B Struct. Sci. 2000, 56, 438–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Table of Solubility Product Constants at 25 °C. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1002/9781119421252.app3 (accessed on 2 July 2021).
- Han, C.; Li, H.; Li, Y.; Zhu, J.; Zhi, C. Proton-assisted calcium-ion storage in aromatic organic molecular crystal with coplanar stacked structure. Nat. Commun. 2021, 12, 2400. [Google Scholar] [CrossRef]
- RUFF-R050508 Database. Available online: https://rruff.info/malachite/R050508 (accessed on 2 July 2021).
- Mokhtar, H.H.; Boukoussa, B.; Hamacha, R.; Bengueddach, A.; EL Abed, D. CuCO3–CuO nanocomposite as a novel and environmentally friendly catalyst for triazole synthesis. RSC Adv. 2015, 5, 93438–93446. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ngo, H.H.; Guo, W.S.; Nguyen, T.V. Removal of copper from water by adsorption onto banana peel as bioadsorbent. Int. J. Geomate 2012, 2, 227–234. [Google Scholar] [CrossRef]
- Frantz, S.T.; Silveira, N.; Quadro, M.S.; Andreazza, R.; Barcelos, A.A.; Cadaval, T.R.; Pinto, L.A. Cu (II) adsorption from copper mine water by chitosan films and the matrix effects. Environ. Sci. Pollut. Res. 2017, 24, 5908–5917. [Google Scholar] [CrossRef]
- Glatstein, D.A.; Francisca, F.M. Influence of pH and ionic strength on Cd, Cu and Pb removal from water by adsorption in Na-bentonite. Appl. Clay Sci. 2015, 118, 61–67. [Google Scholar] [CrossRef]
- Fan, Q.; Li, Z.; Zhao, H.; Jia, Z.; Xu, J.; Wu, W. Adsorption of Pb(II) on palygorskite from aqueous solution: Effects of pH, ionic strength and temperature. Appl. Clay Sci. 2009, 45, 111–116. [Google Scholar] [CrossRef]
- Quinlivan, P.A.; Li, L.; Knappe, D.R. Effects of activated carbon characteristics on the simultaneous adsorption of aqueous organic micropollutants and natural organic matter. Water Res. 2005, 39, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, H.E. Dissolved Organic Matter (DOM). In Encyclopedia of Geochemistry. Encyclopedia of Earth Sciences Series; White, W., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]




| Adsorbent | Adsorbates | Performance | Ref. |
|---|---|---|---|
| Residual marble powder | Pb (II), Cu (II), Cd (II) | 24.695, 19.4675, and 7.91 mg g−1 90 min, pH 5–6 | [22] |
| Marble powder mixtures | Cu, Zn, Mn, Zr | Removed 51–96% of heavy metals | [23] |
| Marble sludge | Cd, Cu, Pb, Zn | 0.030, 0.53, 0.022 and 0.36 mg g−1 | [29] |
| Zeolite synthesized from marble powder | Zn, Ni, Pb, Cr, Cd, Cu | Removed 75–99% of heavy metals | [24] |
| Marble powder | F− | 1.20 mg g−1, pH = 7 | [30] |
| Marble powder | Acid mine drainage Cd, Cr, Cu, Ni, Pb, Zn | Removed 80% of heavy metals | [25] |
| Marble powder waste | Cd (II) | Removed 99.45% of Cd (II) 20 mg L−1, pH = 7 | [31] |
| Crushed marble | F− | 0.7 mg L−1, 5 min, pH = 2, 25 °C | [32] |
| Sludge Sample | SiO2 % | Al2O3 % | Fe2O3 % | MnO % | MgO % | CaO % | Na2O % | K2O % | TiO2 % | P2O5 % | LOI * % | Addition % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | 0.04 | <LLD | 0.01 | <LLD | 0.32 | 55.52 | 0.07 | 0.01 | <LLD | 0.01 | 43.33 | 99.30 |
| M2 | 0.21 | 0.02 | 0.06 | 0.01 | 1.03 | 55.33 | 0.06 | 0.02 | <LLD | 0.01 | 43.40 | 100.13 |
| M3 | 0.28 | 0.06 | 0.20 | 0.02 | 1.12 | 54.95 | 0.07 | 0.03 | <LLD | 0.01 | 43.51 | 100.25 |
| M4 | 0.28 | 0.06 | 0.36 | 0.03 | 0.84 | 55.15 | 0.07 | 0.03 | <LLD | 0.01 | 43.24 | 100.07 |
| M5 | 0.27 | 0.06 | 0.21 | 0.03 | 1.07 | 54.92 | 0.07 | 0.02 | <LLD | 0.01 | 43.40 | 100.06 |
| M6 | 0.25 | 0.05 | 0.05 | 0.01 | 1.01 | 55.27 | 0.07 | 0.03 | <LLD | 0.01 | 43.40 | 100.01 |
| Model Parameters | R2 | |
|---|---|---|
| Pseudo-first order | ||
| k1, h−1 | 0.091 | 0.987 |
| Qe, mg g−1 | 18.361 | |
| Pseudo-second order | ||
| k2, g mg−1 h−1 | 0.004 | 0.992 |
| Qe, mg g−1 | 23.529 | |
| Elovich | ||
| α, mg g−1 h−1 | 5.410 | 0.972 |
| β, g mg−1 | 0.201 | |
| Intraparticle diffusion | First region | |
| k, mg g−1 h−0.5 | 4.769 | 0.974 |
| C, mg g−1 | −2.965 | |
| Second region | ||
| k, mg g−1 h−0.5 | 1.041 | 0.942 |
| C, mg g−1 | 12.025 |
| Model Parameters | R2 | |
|---|---|---|
| Langmuir | 0.834 | |
| KL, L mg−1 | 0.342 | |
| Qm, mg g−1 | 22.745 | |
| Freundlich | 0.909 | |
| Kf, mg g−1 | 12.458 | |
| n | 7.308 | |
| Temkin | 0.779 | |
| B, J mol−1 | 1.539 | |
| AT, L mg−1 | 6175.302 | |
| Dubinin | 0.689 | |
| Kad, mol2 kJ−2 | 6.35 × 10−7 | |
| Qm, mg g−1 | 18.572 |
| Adsorbent | Q (mg g−1) | pH | Initial Concentration of Cu (II) (mg L−1) | Ref |
|---|---|---|---|---|
| Marble waste sludge | 20–23 | 6 | 45 | This work |
| Marble powder | 222.84 | 6 | 2000 | [5] |
| Egg shell | 150 | 6 | 30,000 | [42] |
| 5.03 | 6.5 | 100 | [43] | |
| Egg shell powder coated with iron oxide | 44.84 | 6 | 100 | [44] |
| Limestone | 0.59 | 9 | 100 | [29] |
| 0.0145 | 8.5 | 2 | [40] | |
| Dolomite | 0.60 | 10 | 100 | [29] |
| 8.26 | - | 2400 | [45] | |
| Iron oxide-vermiculite compound | 59.70 | 5 | 500 | [46] |
| Waste iron oxide | 17.08 | 6 | 35 | [47] |
| Ca-montmorillonite | 9.86 | - | 164 | [48] |
| Spartina alterniflora-derived biochar | 49.14 | 6 | 290 | [49] |
| Activated carbon derived from Tunisian date stones | 31.25 | 5 | 100 | [50] |
| Type of Water | pH | [HCO3−], meq L−1 | TOC, mg L−1 | Qm, mg g−1 |
|---|---|---|---|---|
| Ultrapure water | 5.8 | 0.0 | 0.0 | 22.75 |
| Surface water | 8.3 | 6.4 | 11.9 | 12.4 |
| Groundwater | 7.5 | 8.8 | 10.3 | 8.65 |
| Wastewater | 7.8 | 7.2 | 17.0 | 6.75 |
| Type of Water | X0.02, mg g−1 | V0.02, L | Φ | HMTZ, cm | Du, % |
|---|---|---|---|---|---|
| Ultrapure water | 8.88 | 0.57 | 7.37 | 5.62 | 81.52 |
| Surface water | 7.06 | 0.45 | 2.33 | 6.43 | 81.53 |
| Groundwater | 5.06 | 0.32 | 5.14 | 7.56 | 80.80 |
| Wastewater | 3.34 | 0.21 | 9.35 | 8.64 | 17.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, V.C.; Utrilla, J.R.; Sánchez, A.R.; Ramón, M.V.L.; Polo, M.S. Marble Waste Sludges as Effective Nanomaterials for Cu (II) Adsorption in Aqueous Media. Nanomaterials 2021, 11, 2305. https://doi.org/10.3390/nano11092305
Ramos VC, Utrilla JR, Sánchez AR, Ramón MVL, Polo MS. Marble Waste Sludges as Effective Nanomaterials for Cu (II) Adsorption in Aqueous Media. Nanomaterials. 2021; 11(9):2305. https://doi.org/10.3390/nano11092305
Chicago/Turabian StyleRamos, Ventura Castillo, José Rivera Utrilla, Antonio Ruiz Sánchez, María Victoria López Ramón, and Manuel Sánchez Polo. 2021. "Marble Waste Sludges as Effective Nanomaterials for Cu (II) Adsorption in Aqueous Media" Nanomaterials 11, no. 9: 2305. https://doi.org/10.3390/nano11092305







