Evaluation of the Safety and Toxicity of the Original Copper Nanocomposite Based on Poly-N-vinylimidazole
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly-N-vinylimidazole
2.3. Synthesis of CuNPs Nanocomposite
2.4. Instruments for Characterization of Polymer and NC(Cu-PVI)
2.5. Preparation of Primary Culture of Rat Hepatocytes
2.6. Incubation of Rat Hepatocytes with NC(Cu-PVI)
2.7. Biochemical Research
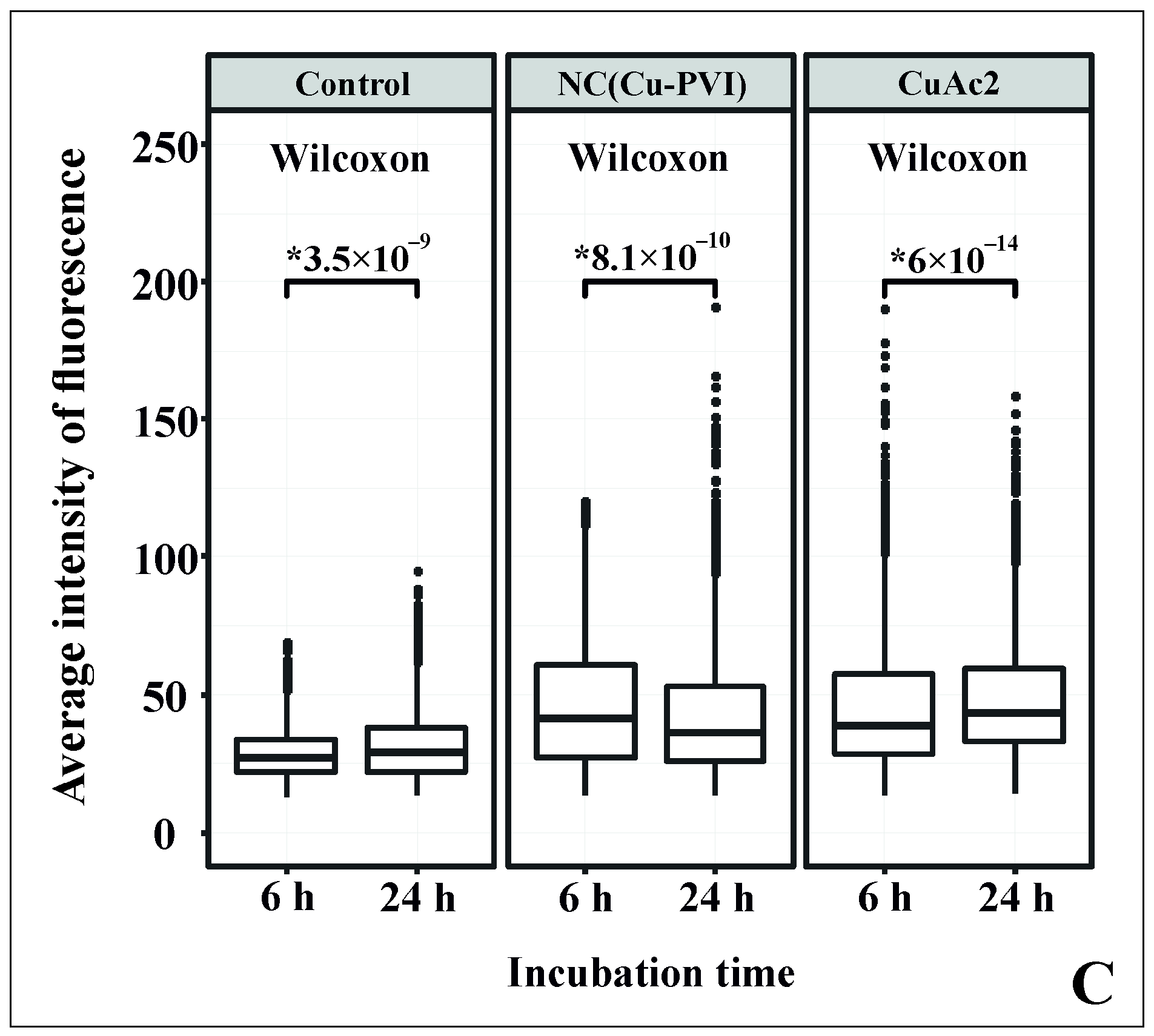
2.8. Immunofluorescence Studies
3. Results and Discussion
3.1. Characterization of Poly-N-vinylimidazole
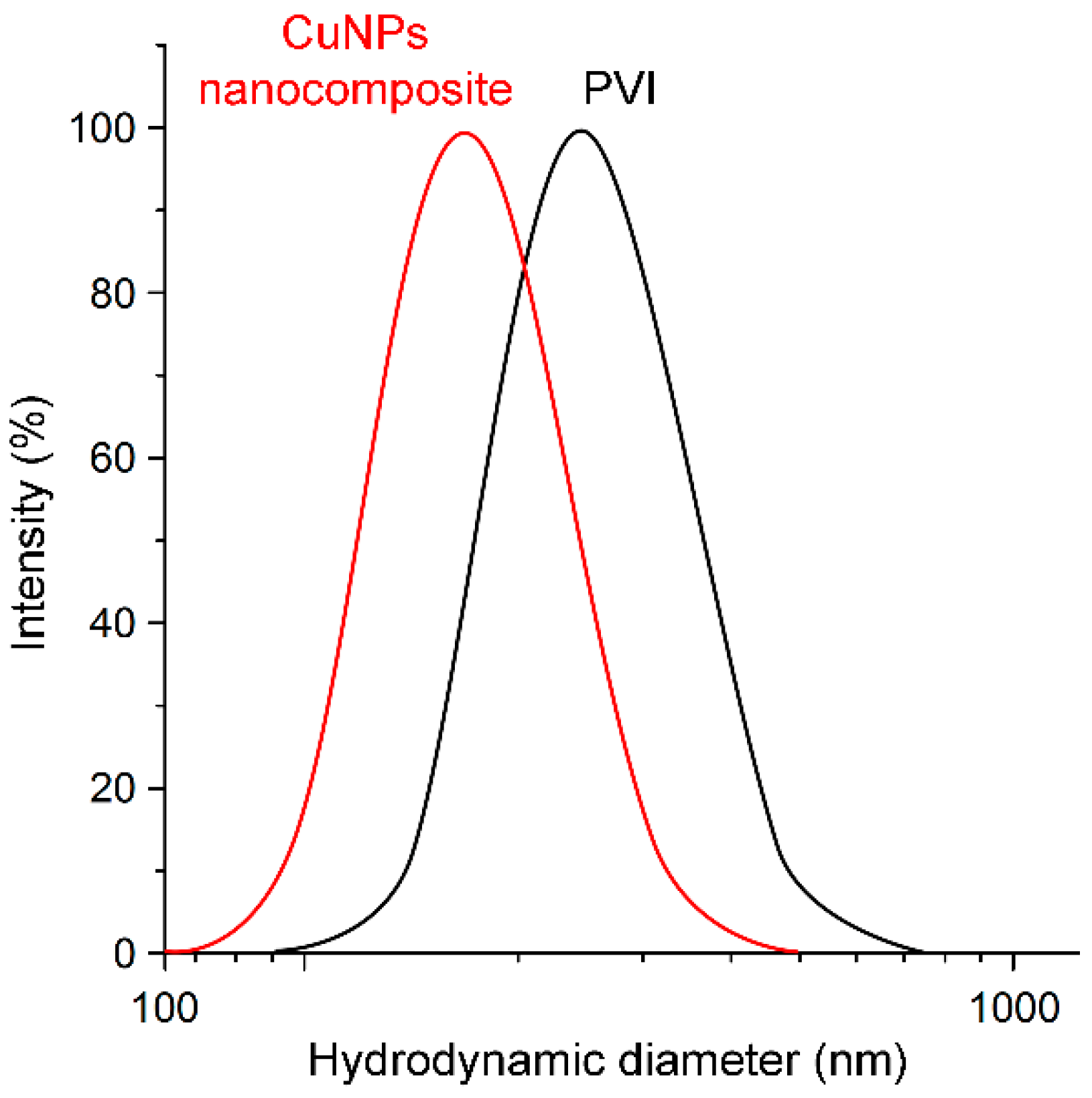
3.2. Synthesis and Characterization of Polymeric CuNPs Nanocomposite
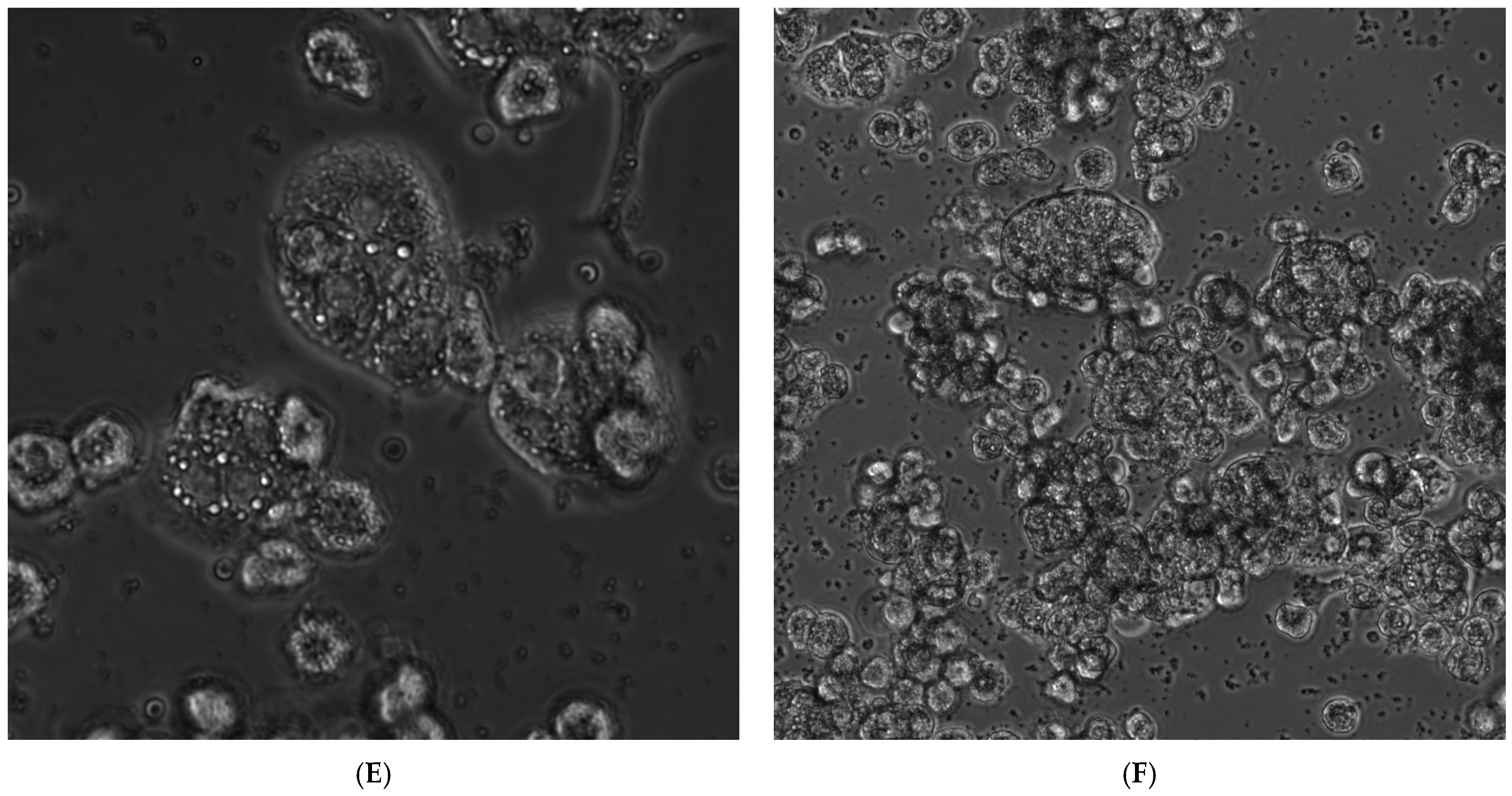
3.3. Influence of NC(Cu-PVI) on the Morphology of Hepatocytes
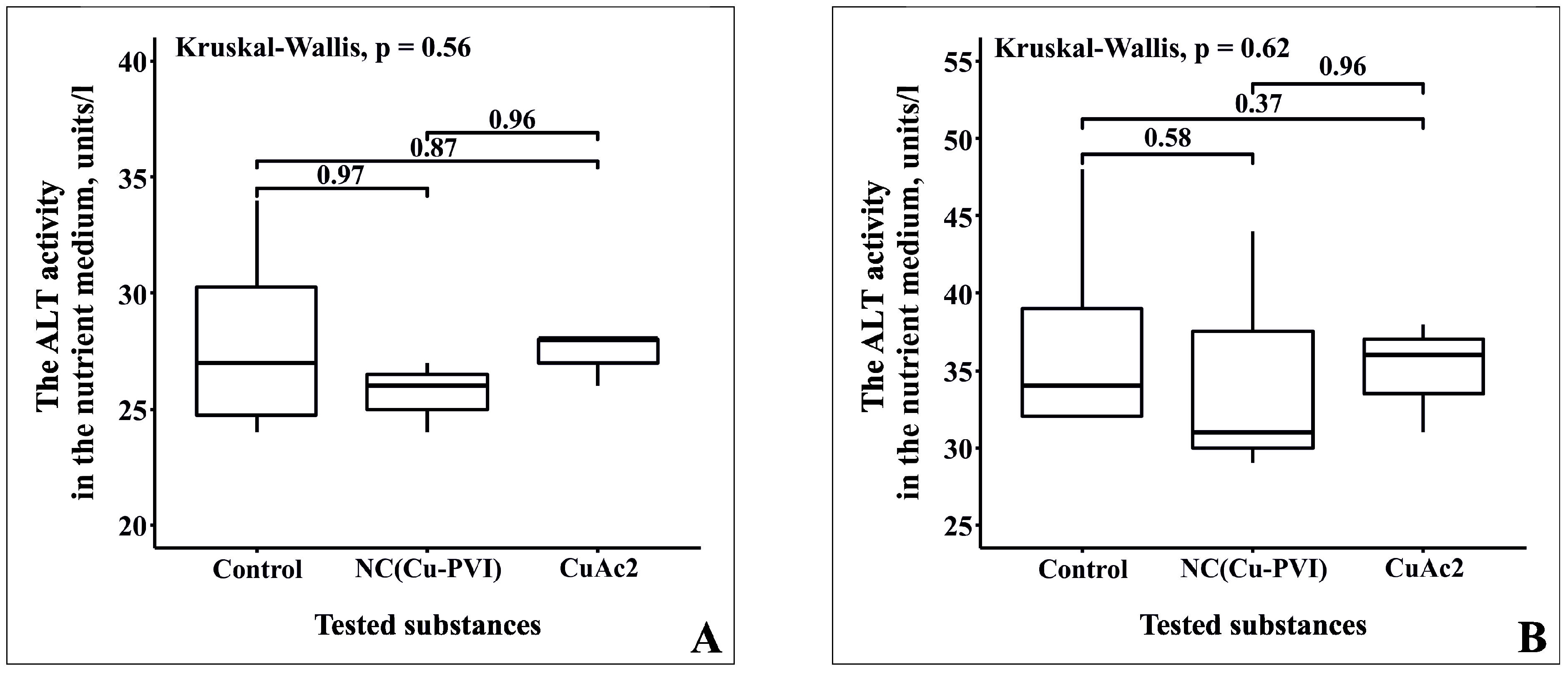
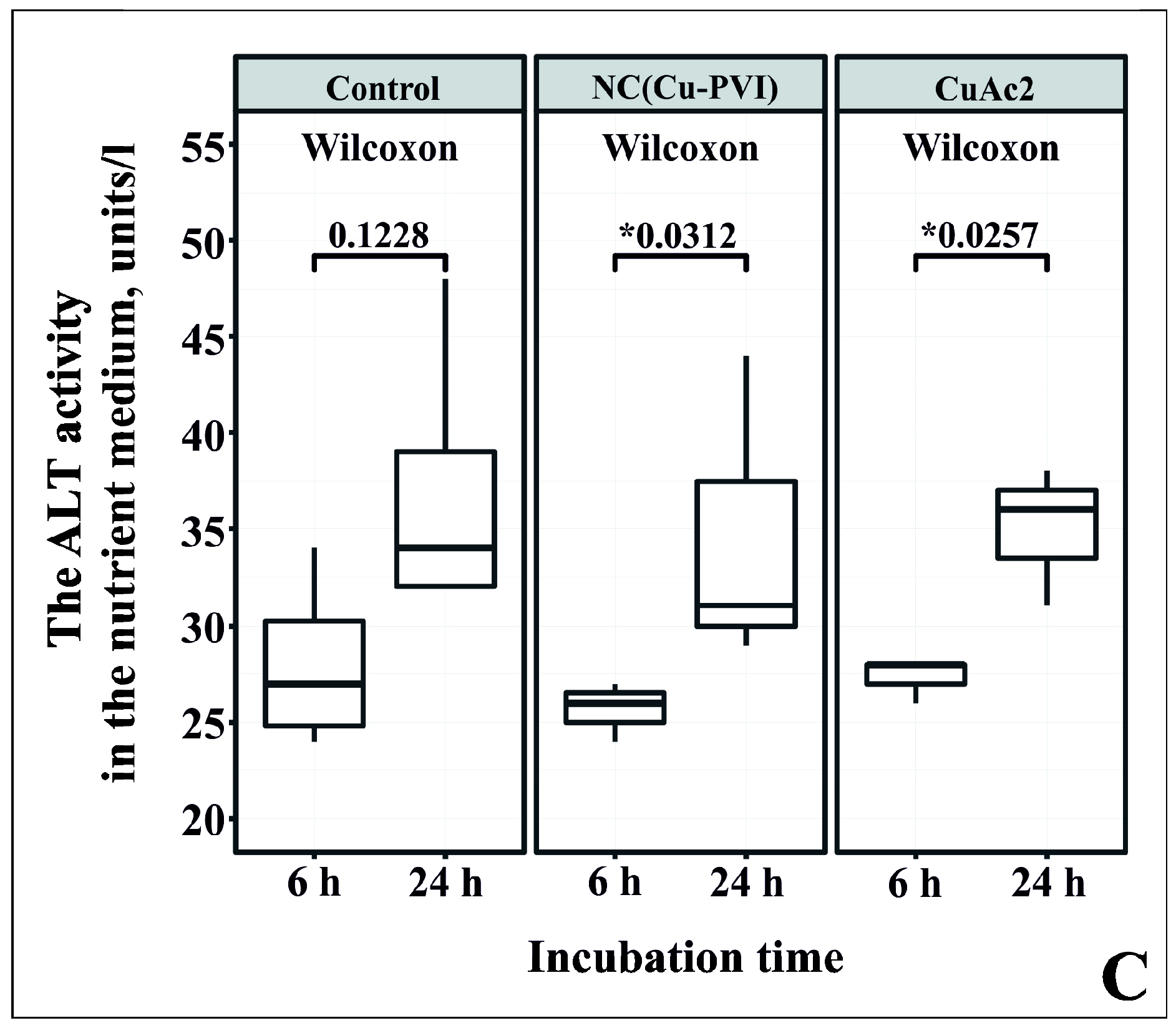
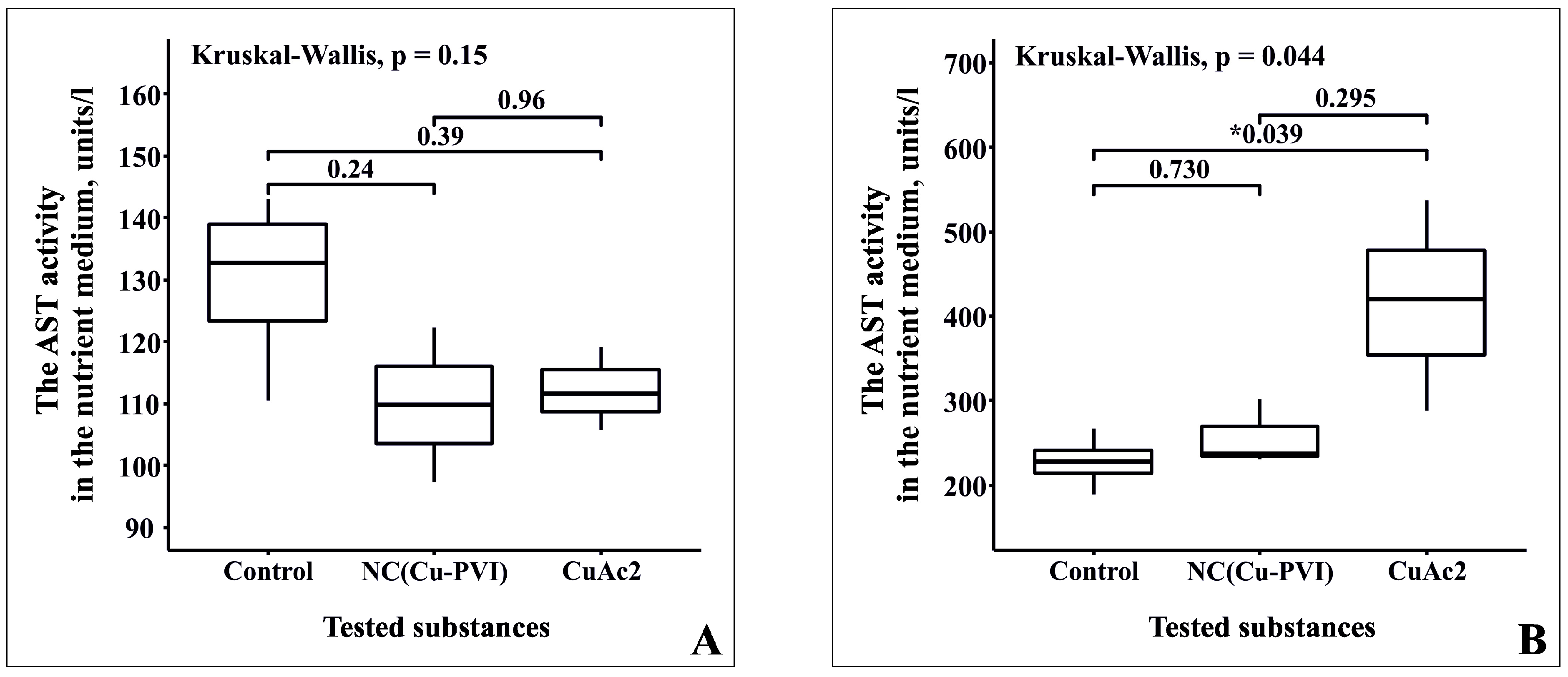
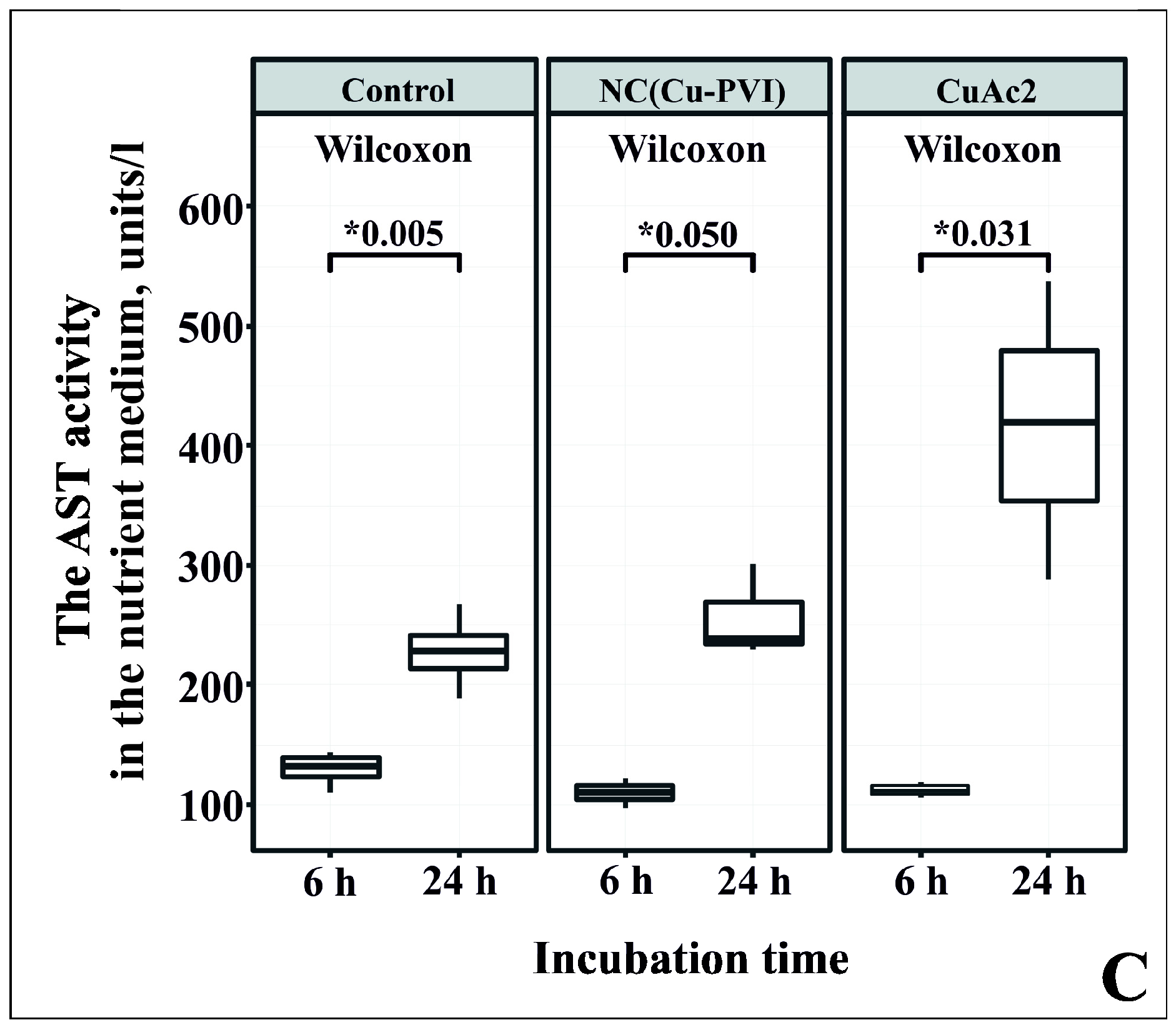
3.4. Analysis of Cytotoxicity NC(Cu-PVI) Based on the Activity of AST and ALT
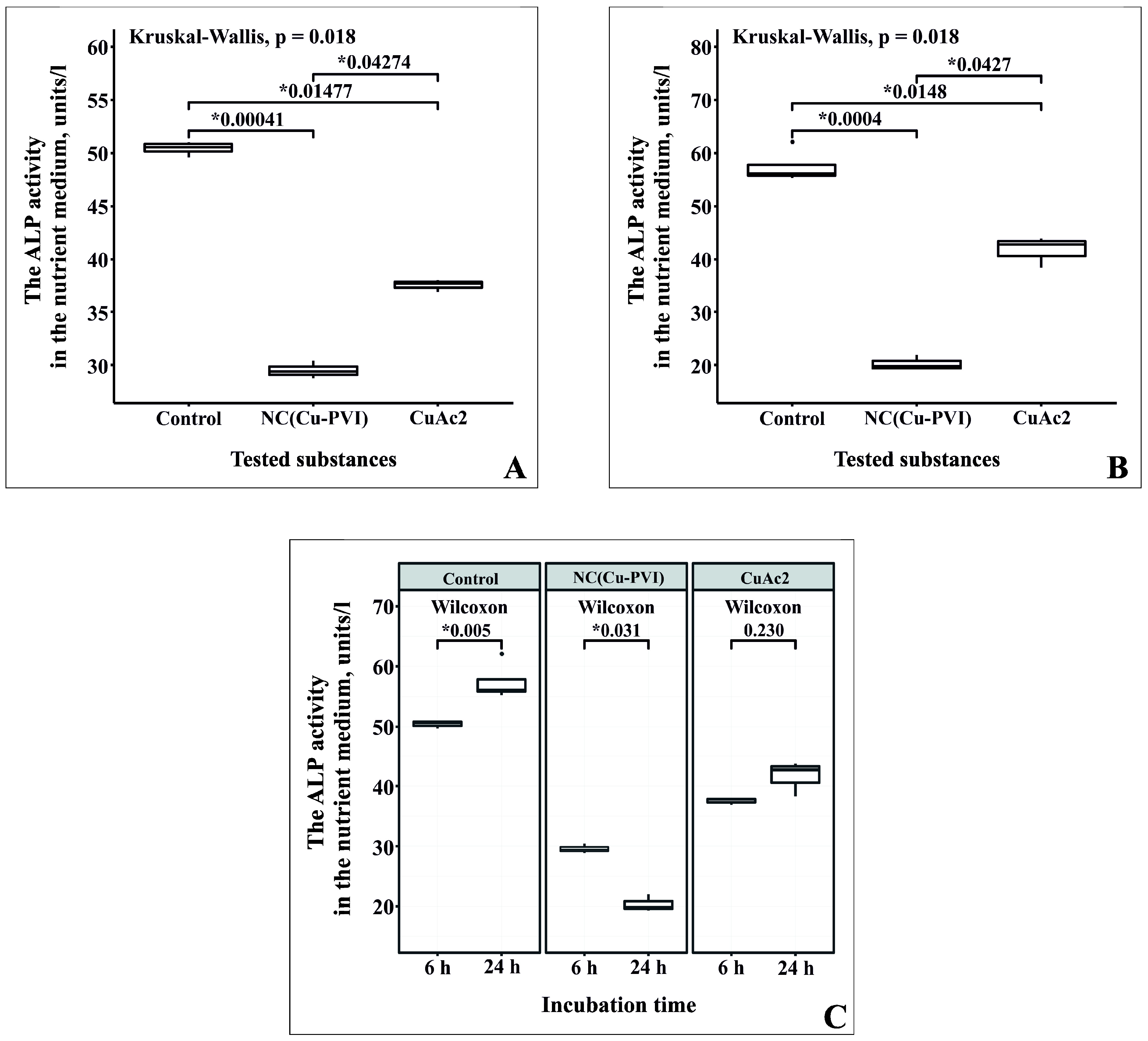
3.5. Effect of NC(Cu-PVI) on ALP Activity
3.6. Influence of NC(Cu-PVI) on the Activity of Oxidative Phosphorylation in Hepatocytes
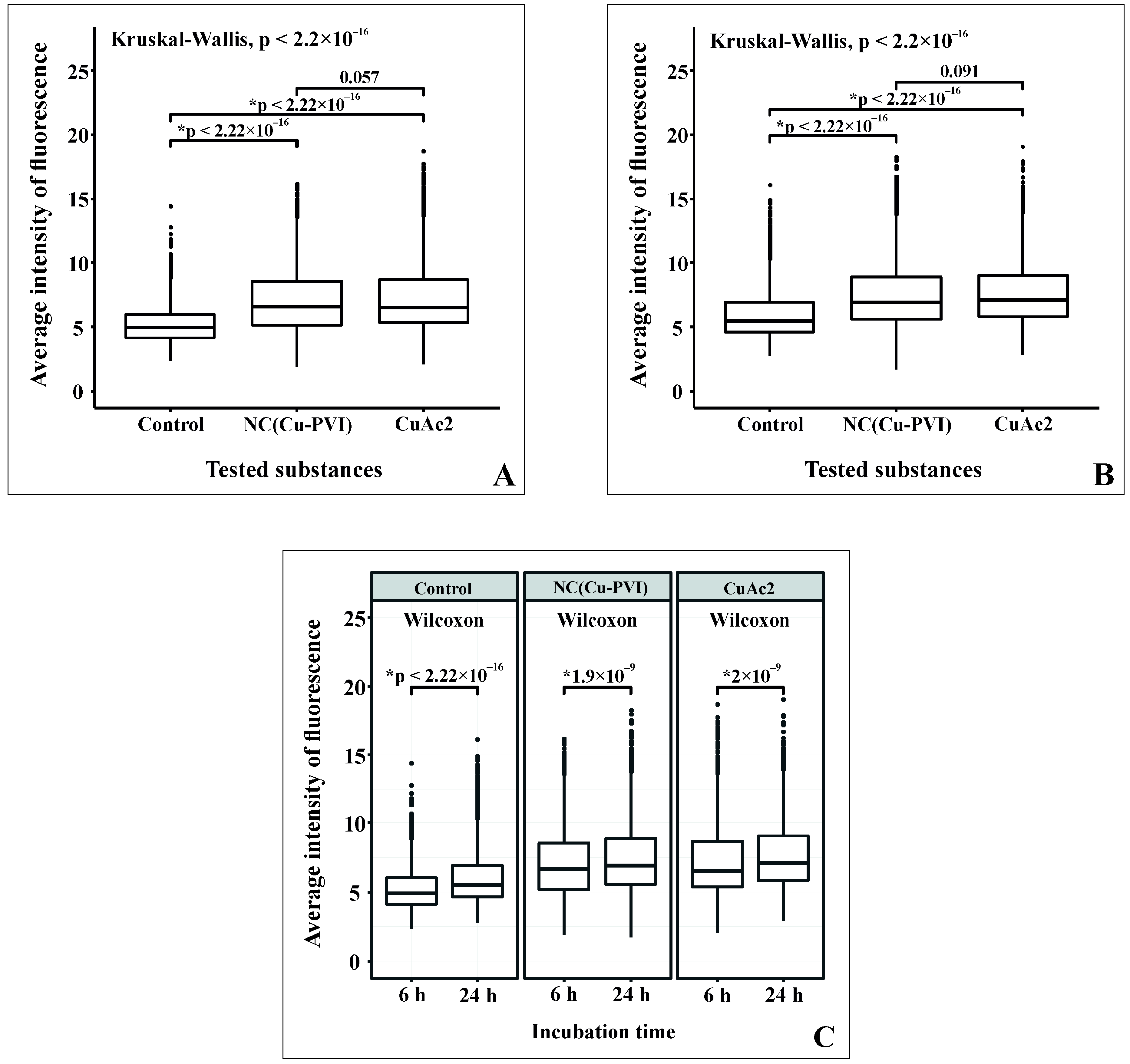
3.7. Influence of NC(Cu-PVI) on the Activity of Glutathione Synthetase in Hepatocytes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, L.; Panderi, I.; Yan, D.D.; Szulak, K.; Li, Y.; Chen, Y.-T.; Ma, H.; Niesen, D.B.; Seeram, N.; Ahmed, A.; et al. A comparative study of hollow copper sulfide nanoparticles and hollow gold nanospheres on degradability and toxicity. ACS Nano 2013, 7, 8780–8793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Altarelli, M.; Ben-Hamouda, N.; Schneider, A.; Berger, M.M. Copper deficiency: Causes, manifestations, and treatment. Nutr. Clin. Pract. 2019, 34, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Saporito-Magriñá, C.M.; Musacco-Sebio, R.N.; Andrieux, G.; Kook, L.; Orrego, M.T.; Tuttolomondo, M.V.; Desimone, M.F.; Boerries, M.; Borner, C.; Repetto, M.G. Copper-induced cell death and the protective role of glutathione: The implication of impaired protein folding rather than oxidative stress. Metallomics 2018, 10, 1743–1754. [Google Scholar] [CrossRef]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of copper on mitochondrial function and metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef] [PubMed]
- Peña, M.M.; Lee, J.; Thiele, D.J. A delicate balance: Homeostatic control of copper uptake and distribution. J. Nutr. 1999, 129, 1251–1260. [Google Scholar] [CrossRef] [Green Version]
- Letchumanan, D.; Sok, S.P.M.; Ibrahim, S.; Nagoor, N.H.; Arshad, N.M. Plant-based biosynthesis of copper/copper oxide nanoparticles: An update on their applications in biomedicine, mechanisms, and toxicity. Biomolecules 2021, 11, 564. [Google Scholar] [CrossRef]
- Horn, D.; Barrientos, A. Mitochondrial copper metabolism and delivery to cytochrome c oxidase. IUBMB Life 2008, 60, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Garza, N.M.; Griffin, A.T.; Zulkifli, M.; Qiu, C.; Kaplan, C.D.; Gohil, V.M. A genome-wide copper-sensitized screen identifies novel regulators of mitochondrial cytochrome c oxidase activity. J. Biol. Chem. 2021, 296, 100485. [Google Scholar] [CrossRef]
- Freedman, J.H.; Ciriolo, M.R.; Peisach, J. The role of glutathione in copper metabolism and toxicity. J. Biol. Chem. 1989, 264, 5598–5605. [Google Scholar] [CrossRef]
- Rae, T.D.; Schmidt, P.J.; Pufahl, R.A.; Culotta, V.C.; O’Halloran, T.V. Undetectable intracellular free copper: The requirement of a copper chaperone for superoxide dismutase. Science 1999, 284, 805–808. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Kang, Z.; Qiao, N.; Wang, C.; Tang, Z. Cu-induced mitochondrial dysfunction is mediated by abnormal mitochondrial fission through oxidative stress in primary chicken embryo hepatocytes. J. Trace Elem. Med. Biol. 2021, 65, 126721. [Google Scholar] [CrossRef]
- Tseng, H.-L.; Li, C.-J.; Huang, L.-H.; Chen, C.-Y.; Tsai, C.-H.; Lin, C.-N.; Hsu, H.-Y. Quercetin 3-O-methyl ether protects FL83B cells from copper induced oxidative stress through the PI3K/Akt and MAPK/Erk pathway. Toxicol. Appl. Pharmacol. 2012, 264, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, H.; Jian, Z.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Copper induces oxidative stress and apoptosis in the mouse liver. Oxid. Med. Cell. Longev. 2020, 2020, 1359164. [Google Scholar] [CrossRef] [Green Version]
- Johncilla, M.; Mitchell, K.A. Pathology of the liver in copper overload. Semin. Liver Dis. 2011, 31, 239–244. [Google Scholar] [CrossRef]
- Hosseini, M.J.; Shaki, F.; Ghazi-Khansari, M.; Pourahmad, J. Toxicity of copper on isolated liver mitochondria: Impairment at complexes I, II, and IV leads to increased ROS production. Cell Biochem. Biophys. 2014, 70, 367–381. [Google Scholar] [CrossRef]
- Lorincz, M.T. Wilson disease and related copper disorders. Handb. Clin. Neurol. 2018, 147, 279–292. [Google Scholar]
- Yuan, X.Z.; Yang, R.M.; Wang, X.P. Management perspective of Wilson’s disease: Early diagnosis and individualized therapy. Curr. Neuropharmacol. 2021, 19, 465–485. [Google Scholar] [CrossRef]
- Badea, M.; Uivarosi, V.; Olar, R. Improvement in the pharmacological profile of copper biological active complexes by their incorporation into organic or inorganic matrix. Molecules 2020, 25, 5830. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yahia, L.; Sacher, E. Antimicrobial properties of the Ag, Cu nanoparticle system. Biology 2021, 10, 137. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in copper complexes as anticancer agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef] [PubMed]
- Scott-Fordsmand, J.J.; Weeks, J.M.; Hopkin, S.P. Importance of contamination history for understanding toxicity of copper to earthworm Eisenia fetica (Oligochaeta: Annelida), using neutral-red retention assay. Environ. Toxicol. Chem. 2000, 19, 1774–1780. [Google Scholar] [CrossRef]
- Veerapandian, M.; Sadhasivam, S.; Choi, J.; Yun, K. Glucosamine functionalized copper nanoparticles: Preparation, characterization and enhancement of anti-bacterial activity by ultraviolet irradiation. Chem. Eng. J. 2012, 209, 558–567. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef] [Green Version]
- Andleeb, A.; Andleeb, A.; Asghar, S.; Zaman, G.; Tariq, M.; Mehmood, A.; Nadeem, M.; Hano, C.; Lorenzo, J.M.; Abbasi, B.H. A systematic review of biosynthesized metallic nanoparticles as a promising anti-cancer-strategy. Cancers 2021, 13, 2818. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-M.; Rahuman, A.A.; Marimuthu, S.; Kirthi, A.V.; Anbarasan, K.; Padmini, P.; Rajakumar, G. Green synthesis of copper nanoparticles using Eclipta prostrata leaves extract and their antioxidant and cytotoxic activities. Exp. Ther. Med. 2017, 14, 18–24. [Google Scholar] [PubMed] [Green Version]
- Nagajyothi, P.C.; Muthuraman, P.; Sreekanth, T.V.M.; Kim, D.H.; Shim, J. Green synthesis: In-vitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells. Arab. J. Chem. 2017, 10, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Evaluation of antioxidant and anticancer activity of copper oxide nanoparticles synthesized using medicinally important plant extracts. Biomed. Pharmacother. 2017, 89, 1067–1077. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Tawfeeq, A.T.; Jaaffer, M.D. Biogenic synthesis of copper oxide nanoparticles using olea europaea leaf extract and evaluation of their toxicity activities: An in vivo and in vitro study. Biotechnol. Prog. 2018, 34, 218–230. [Google Scholar] [CrossRef]
- Harne, S.; Sharma, A.; Dhaygude, M.; Joglekar, S.; Kodam, K.; Hudlikar, M. Novel route for rapid biosynthesis of copper nanoparticles using aqueous extract of Calotropis procera L. latex and their cytotoxicity on tumor cells. Colloids Surf. B Biointerfaces 2012, 95, 284–288. [Google Scholar] [CrossRef]
- Sankar, R.; Maheswari, R.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 234–239. [Google Scholar] [CrossRef]
- Prasad, P.R.; Kanchi, S.; Naidoo, E.B. In-vitro evaluation of copper nanoparticles cytotoxicity on prostate cancer cell lines and their antioxidant, sensing and catalytic activity: One-pot green approach. J. Photochem. Photobiol. B Biol. 2016, 161, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, S.; Ahmeda, A.; Salehabadi, Y.; Zangeneh, A.; Zangeneh, M.M. Synthesis, characterization, and evaluation of cytotoxicity, antioxidant, antifungal, antibacterial, and cutaneous wound healing effects of copper nanoparticles using the aqueous extract of Strawberry fruit and L-Ascorbic acid. Polyhedron 2020, 180, 114425. [Google Scholar] [CrossRef]
- Abou-Yousef, H.; Abdel-Aziz, M.S. Efficient alternative of antimicrobial nanocomposites based on cellulose acetate/Cu-NPs. Soft Mater. 2018, 16, 141–150. [Google Scholar] [CrossRef]
- Araújo, I.M.S.; Silva, R.R.; Pachecoc, G.; Lustri, W.R.; Tercjak, A. Hydrothermal synthesis of bacterial cellulose–copper oxide nanocomposites and evaluation of their antimicrobial activity. Carbohydr. Polym. 2018, 179, 341–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.Y.; Hu, X.H.; Zhang, Y.W.; Wahid, F.; Chu, L.Q.; Jia, S.R.; Zhong, C. Development and antibacterial activities of bacterial cellulose/graphene oxide-CuO nanocomposite films. Carbohydr. Polym. 2020, 229, 115456. [Google Scholar] [CrossRef]
- Zhong, T.; Oporto, G.S.; Jaczynski, J.; Jiang, C. Nanofibrillated cellulose and copper nanoparticles embedded in polyvinyl alcohol films for antimicrobial applications. BioMed Res. Int. 2015, 2015, 456834. [Google Scholar] [CrossRef] [Green Version]
- Usman, M.; El Zowalaty, M.; Shameli, K.; Zainuddin, N.; Salama, M.; Ibrahim, N.A. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 2013, 8, 4467–4479. [Google Scholar]
- Basumallick, S.; Rajasekaran, P.; Tetard, L.; Santra, S. Hydrothermally derived water-dispersible mixed valence copper-chitosan nanocomposite as exceptionally potent antimicrobial agent. J. Nanoparticle Res. 2014, 16, 2675. [Google Scholar] [CrossRef]
- Bogdanović, U.; Vodnik, V.; Mitrić, M.; Dimitrijević, S.; Škapin, S.D.; Žunič, V.; Budimir, M.; Stoiljković, M. Nanomaterial with high antimicrobial efficacy—copper/polyaniline nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 1955–1966. [Google Scholar] [CrossRef]
- Jaramillo, A.F.; Riquelme, S.A.; Sánchez-Sanhueza, G.; Medina, C.; Solís-Pomar, F.; Rojas, D.; Montalba, C.; Melendrez, M.F.; Pérez-Tijerina, E. Comparative study of the antimicrobial effect of nanocomposites and composite based on poly(butylene adipate-co-terephthalate) using Cu and Cu/Cu2O nanoparticles and CuSO. Nanoscale Res. Lett. 2019, 14, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Escobar, A.; Ruíz-Baltazar, Á.J.; Reyes-López, S.Y. Novel route of synthesis of PCL-CuONPs composites with antimicrobial properties. Dose Response 2019, 17, 1559325819869502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaramillo, A.F.; Riquelme, S.; Montoya, L.F.; Sánchez-Sanhueza, G.; Medinam, C.; Rojas, D.; Salazar, F.; Sanhueza, J.; Meléndrez, M.F. Influence of the concentration of copper nanoparticles on the thermo-mechanical and antibacterial properties of nanocomposites based on poly(butylene adipate-co-terephthalate). Polym. Compos. 2019, 40, 1870–1882. [Google Scholar] [CrossRef]
- Yang, Z.; Hao, X.; Chen, S.; Ma, Z.; Wang, W.; Wang, C.; Yue, L.; Sun, H.; Shao, Q.; Murugadoss, V.; et al. Long-term antibacterial stable reduced graphene oxide nanocomposites loaded with cuprous oxide nanoparticles. J. Colloid Interface Sci. 2019, 533, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Subashini, K.; Prakash, S.; Sujatha, V. Polymer nanocomposite prepared using copper oxide nanoparticles derived from Sterculia foetida leaf extract with biological applications. Mater. Res. Express 2020, 7, 115308. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y.; Huang, J.; Zhang, Z.; Zhao, D.; Zhang, X.; Zhang, B. A novel colloidal deposition method to prepare copper nanoparticles/polystyrene nanocomposite with antibacterial activity and its comparison to the liquid-phase in situ reduction method. Chem. Pap. 2020, 74, 471–483. [Google Scholar] [CrossRef]
- Sabbah, I.A.; Zaky, M.F.; Hendawy, M.E.; Negm, N.A. Synthesis, characterization and antimicrobial activity of colloidal coppernanoparticles stabilized by cationic thiol polyurethane surfactants. J. Polym. Res. 2018, 25, 252–264. [Google Scholar] [CrossRef]
- Selivanova, A.V.; Tyurin, V.S.; Beletskaya, I.P. Palladium nanoparticles supported on poly(N-vinyl- imidazole-co-N-vinylcaprolactam) as an effective recyclable catalyst for the Suzuki reaction. Chem. Plus Chem. 2014, 79, 1278–1283. [Google Scholar] [CrossRef]
- Dağaş, D.E.; Danelyan, G.V.; Ghaffarlou, M.; Zezina, E.; Abramchuk, S.S.; Feldman, V.I.; Güven, O.; Zezin, A.A. Generation of spatially ordered metal–polymer nanostructures in the irradiated dispersions of poly(acrylic acid)–poly(vinylimidazole)–Cu2+ complexes. Colloid Polym. Sci. 2020, 298, 193–202. [Google Scholar] [CrossRef]
- Isikli, S.; Tuncagil, S.; Bozkurt, A.; Toppare, L. Immobilization of invertase in a novel proton conducting poly(vinylphosphonic acid-poly(1-vinylimidazole) network. J. Macromol. Sci. Part A 2010, 47, 639–646. [Google Scholar] [CrossRef]
- Asayama, S.; Nishinohara, S.; Kawakami, H. Zinc-chelated poly(1-vinylimidazole) and a carbohydrate ligand polycation form DNA ternary complexes for gene delivery. Bioconjugate Chem. 2011, 22, 1864–1868. [Google Scholar] [CrossRef]
- Shulman, M.; Nahmias, Y. Long-term culture and coculture of primary rat and human hepatocytes. Methods Mol. Biol. 2013, 945, 287–302. [Google Scholar] [PubMed] [Green Version]
- Hewitt, N.J.; Lechon, M.J.; Houston, J.B.; Hallifax, D.; Brown, H.S.; Maurel, P.; Kenna, J.G.; Gustavsson, L.; Lohmann, C.; Skonberg, C.; et al. Primary hepatocytes: Current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab. Rev. 2007, 39, 159–234. [Google Scholar] [CrossRef]
- Shurygin, M.G.; Shurygina, I.A.; Kanya, O.V.; Dremina, N.N.; Lushnikova, E.L.; Nepomnyashchikh, R.D. Morphological evaluation of oxidative phosphorylation system in myocardial infarction under conditions of modified vascular endothelial growth factor concentration. Bull. Exp. Biol. Med. 2015, 159, 402–405. [Google Scholar] [CrossRef]
- Shurygina, I.A.; Trukhan, I.S.; Dremina, N.N.; Shurygin, M.G. Changes in oxidative phosphorylation activity in fibroblasts at p38 MAPK pathway inhibition. Int. J. Biomed. 2019, 9, 350–355. [Google Scholar] [CrossRef]
- Ismail, M.; Gul, S.; Khan, M.I.; Khan, M.A.; Asiri, A.M.; Khan, S.B. Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange. Green Process. Synth. 2018, 8, 135–143. [Google Scholar] [CrossRef]
- Saravanakumara, K.; Shanmugam, S.; Varukattuc, N.B.; Ali, D.M.; Kathiresane, K.; Wang, M.-H. Biosynthesis and characterization of copper oxide nanoparticles from indigenous fungi and its effect of photothermolysis on human lung carcinoma. J. Photochem. Photobiol. B Biol. 2019, 190, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumara, K.; Sathiyaseelan, A.; Vijaya, A.; Mariadoss, A.; Xiaowen, H.; Wang, M.-H. Physical and bioactivities of biopolymeric films incorporated with cellulose, sodium alginate and copper oxide nanoparticles for food packaging application. Int. J. Biol. Macromol. 2020, 153, 207–214. [Google Scholar] [CrossRef]
- Vijaya, A.; Mariadoss, A.; Saravanakumara, K.; Sathiyaseelan, A.; Venkatachalam, K.; Wang, M.-H. Folic acid functionalized starch encapsulated green synthesized copper oxide nanoparticles for targeted drug delivery in breast cancer therapy. Int. J. Biol. Macromol. 2020, 164, 2073–2084. [Google Scholar]
- Akintelu, S.A.; Folorunso, A.S.; Folorunso, F.A.; Oyebamiji, A.K. Green synthesis of copper oxide nanoparticles for biomedical application and environmental remediation. Heliyon 2020, 6, e04508. [Google Scholar] [CrossRef]
- Garrido, L.V.Q.; Gonçalves, J.; Rocha, J.C.; Bastos, E.L.; Toma, H.E.; Zamarion, V.M. Intriguing Plasmonic and Fluorescence Duality in Copper Nanoparticles. Plasmonics 2020, 15, 1213–1219. [Google Scholar] [CrossRef]
- Aguilar, M.; Esparza, R.; Rosas, G. Synthesis of Cu nanoparticles by chemical reduction method. Trans. Nonferrous Met. Soc. China 2019, 29, 1510–1515. [Google Scholar] [CrossRef]
- Jeong, H.; Stika, C.S. Methods to study mechanisms underlying altered hepatic drug elimination during pregnancy. Semin. Perinatol. 2020, 44, 151228. [Google Scholar] [CrossRef]
- Guelfi, M.; Maioli, M.A.; Tavares, M.A.; Mingatto, F.E. Citotoxicity of fipronil on hepatocytes isolated from rat and effects of its biotransformation. Braz. Arch. Biol. Technol. 2015, 58, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.X.; Lin, Y.X.; Liu, F.P.; Chang, Y.C.; Li, R.; Li, C.W.; Li, Y.; He, J.S.; Ma, X.; Li, Z. Hepatoprotective effects of ethanol extracts from Folium Syringae against acetaminophen-induced hepatotoxicity in vitro and in vivo. J. Chin. Med. Assoc. 2017, 80, 623–629. [Google Scholar] [CrossRef]
- Mizoi, K.; Arakawa, H.; Yano, K.; Koyama, S.; Kojima, H.; Ogihara, T. Utility of three-dimensional cultures of primary human hepatocytes (spheroids) as pharmacokinetic models. Biomedicines 2020, 8, 374. [Google Scholar] [CrossRef] [PubMed]
- Kiraççakali, A.N.; Oğuz, A.R. Determination of cytotoxic, genotoxic, and oxidative damage from deltamethrin on primary hepatocyte culture of Lake Van fish. Alburnus tarichi. Chem. Ecol. 2020, 36, 651–662. [Google Scholar] [CrossRef]
- Gowda, S.; Desai, P.B.; Hull, V.V.; Math, A.A.; Vernekar, S.N.; Kulkarni, S.S. A review on laboratory liver function tests. Pan Afr. Med. J. 2009, 3, 17. [Google Scholar] [PubMed]
- Thapa, B.R.; Walia, A. Liver function tests and their interpretation. Indian J. Pediatr. 2007, 74, 663–671. [Google Scholar] [CrossRef]
- Leary, S.C.; Winge, D.R.; Cobine, P.A. “Pulling the plug” on cellular copper: The role of mitochondria in copper export. Biochim. Biophys. Acta 2009, 1793, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndrepepa, G. Aspartate aminotransferase and cardiovascular disease–a narrative review. J. Lab. Precis. Med. 2021, 6, 6. [Google Scholar] [CrossRef]
- Shribman, S.; Warner, T.T.; Dooley, J.S. Clinical presentations of Wilson disease. Ann. Transl. Med. 2019, 7, S60. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Monteagudo, A.; Ripollés, E.; Berenguer, M.; Espinós, C. Wilson’s disease: Facing the challenge of diagnosing a rare disease. Biomedicines 2021, 9, 1100. [Google Scholar] [CrossRef]
- Contreras-Zentella, M.L.; Hernández-Muñoz, R. Is liver enzyme release really associated with cell necrosis induced by oxidant stress? Oxid. Med. Cell. Longev. 2016, 2016, 3529149. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, C.R.; Gahl, W.A. Disorders of metal metabolism. Transl. Sci. Rare Dis. 2017, 2, 101–139. [Google Scholar] [CrossRef] [Green Version]
- Shaver, W.A.; Bhatt, H.; Combes, B. Low serum alkaline phosphatase activity in Wilson’s disease. Hepatology 1986, 6, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline phosphatase: An overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Lucena-Valera, A.; Perez-Palacios, D.; Muñoz-Hernandez, R.; Romero-Gómez, M.; Ampuero, J. Wilson’s disease: Revisiting an old friend. World J. Hepatol. 2021, 13, 634–649. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.J.; Evans, W.J. Inactivation of intestinal alkaline phosphatase by inositol hexaphosphate-Cu (II) coordinate complexes. J. Inorg. Biochem. 1991, 42, 161–175. [Google Scholar] [CrossRef]
- Martin, C.J. Reaction of the coordinate complexes of inositol hexaphosphate with first row transition series cations and Cd(II) with calf intestinal alkaline phosphatase. J. Inorg. Biochem. 1995, 58, 89–107. [Google Scholar] [CrossRef]
- Chen, X.; Medeiros, D.M.; Jennings, D. Mitochondrial membrane potential is reduced in copper-deficient C2C12 cells in the absence of apoptosis. Biol. Trace Elem. Res. 2005, 106, 51–64. [Google Scholar] [CrossRef]
- Zeng, H.; Saari, J.T.; Johnson, W.T. Copper deficiency decreases complex IV but not complex I, II, III, or V in the mitochondrial respiratory chain in rat heart. J. Nutr. 2007, 137, 14–148. [Google Scholar] [CrossRef] [Green Version]
- Johnson, W.T.; Brown-Borg, H.M. Cardiac cytochrome-c oxidase deficiency occurs during late postnatal development in progeny of copper-deficient rats. Exp. Biol. Med. 2006, 231, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.M.; Jensen, E.L.; Rossel, Y.; Puas, G.I.; Gonzalez-Ibanez, A.M.; Bustos, R.I.; Ferrick, D.A.; Elorza, A.A. Non-cytotoxic copper overload boosts mitochondrial energy metabolism to modulate cell proliferation and differentiation in the human erythroleukemic cell line K562. Mitochondrion 2016, 29, 18–30. [Google Scholar] [CrossRef]
- Babak, M.V.; Ahn, D. Modulation of intracellular copper levels as the mechanism of action of anticancer copper complexes: Clinical relevance. Biomedicines 2021, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Barile, A.; Arciello, M.; Rossi, L. Copper homeostasis as target of both consolidated and innovative strategies of anti-tumor therapy. J. Trace Elem. Med. Biol. 2019, 55, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Marí, M.; de Gregorio, E.; de Dios, C.; Roca-Agujetas, V.; Cucarull, B.; Tutusaus, A.; Morales, A.; Colell, A. Mitochondrial glutathione: Recent insights and role in disease. Antioxidants 2020, 9, 909. [Google Scholar] [CrossRef] [PubMed]
- Maryon, E.B.; Molloy, S.A.; Kaplan, J.H. Rate and regulation of copper transport by human copper transporter 1 (hCTR1). J. Biol. Chem. 2013, 288, 18035–18046. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, J.H.; Maryon, E.B. How mammalian cells acquire copper: An essential but potentially toxic metal. Biophys. J. 2016, 110, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.L.; Wu, S.; Zhang, R.Y.; Zhang, C.G.; He, H.; Jiang, H.C.; Nie, Z.Y.; Qiu, G.Z. Effects of copper exposure on expression of glutathione-related genes in Acidithiobacillus ferrooxidans. Curr. Microbiol. 2011, 62, 1460–1466. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shurygina, I.A.; Prozorova, G.F.; Trukhan, I.S.; Korzhova, S.A.; Dremina, N.N.; Emel’yanov, A.I.; Say, O.V.; Kuznetsova, N.P.; Pozdnyakov, A.S.; Shurygin, M.G. Evaluation of the Safety and Toxicity of the Original Copper Nanocomposite Based on Poly-N-vinylimidazole. Nanomaterials 2022, 12, 16. https://doi.org/10.3390/nano12010016
Shurygina IA, Prozorova GF, Trukhan IS, Korzhova SA, Dremina NN, Emel’yanov AI, Say OV, Kuznetsova NP, Pozdnyakov AS, Shurygin MG. Evaluation of the Safety and Toxicity of the Original Copper Nanocomposite Based on Poly-N-vinylimidazole. Nanomaterials. 2022; 12(1):16. https://doi.org/10.3390/nano12010016
Chicago/Turabian StyleShurygina, Irina A., Galina F. Prozorova, Irina S. Trukhan, Svetlana A. Korzhova, Nataliya N. Dremina, Artem I. Emel’yanov, Olesya V. Say, Nadezhda P. Kuznetsova, Alexander S. Pozdnyakov, and Michael G. Shurygin. 2022. "Evaluation of the Safety and Toxicity of the Original Copper Nanocomposite Based on Poly-N-vinylimidazole" Nanomaterials 12, no. 1: 16. https://doi.org/10.3390/nano12010016





