Recent Progress in Graphene-Based Electrocatalysts for Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Synthesis and Preparation of Graphene
2.1. Chemical Vapor Deposition (CVD)
2.2. Graphene Oxide Reduction
2.3. Hydrothermal Carbonization
2.4. Ball Milling
3. Strategies and Methods to Improve Catalytic Properties
3.1. Heteroatom Doping
3.2. Surface Functionalization
3.3. Defect and Edge Tailoring
3.4. Porous Structure and Morphology Engineering
4. Graphene-Based HER Electrocatalysts
4.1. Non-Doped Graphene-Based
4.1.1. Powder Catalysts
4.1.2. 3D-Type Catalysts
4.2. Doped Graphene-Based Electrocatalysts
4.2.1. Single-Element Doped Graphene Powder Catalysts
4.2.2. 3D-Type Catalysts
4.2.3. Dual-Doped Graphene Powder Catalysts
5. Graphene Quantum Dot Catalysts
5.1. 3D-Type Catalysts
5.2. Dual-Action Catalysts
| Electrocatalyst | Electrolyte | Onset Potential/Overpotential | Tafel Slope (mV·dec−1) | Stability | Ref. |
|---|---|---|---|---|---|
| ReSe2 nanoflakes/rGO | 0.5 M H2SO4 | 145.3 (vs. REH) at 10 mA cm−2 | 40.7 | 24 h | Yan et al. [86] |
| Ni0.85Se nanospheres/rGO | 1 M KOH | 128 (vs. REH) at 10 mA cm−2 | 91 | 18 h, 1000 CV | Zhu et al. [91] |
| Conducting scaffold-supported 3D rGO-CNT/MoS2 nanostructure | 0.5 M H2SO4 | 123.75 (vs. REH) at 100 mA cm−2 | 31 | Bolar et al. [96] | |
| 1 M KOH | 217.61 (vs. REH) at 50 mA cm−2 | 52 | |||
| 3D Pd nanosponge-shaped networks wrapped by graphene dots | 0.5 M H2SO4 | 32 (vs. REH) at 10 mA cm−2 | 33 | 15 h, 3000 CV | Nguyen et al. [99] |
| 3D graphene hollow nanospheres supported ruthenium phosphides | 1.0 M KOH | 25.5 (vs. REH) at 10 mA cm−2 | 34.4 | 10 h, 1000 CV | Li et al. [101] |
| Ru Nanoclusters/N-graphene | 1.0 M KOH | 25.9 (vs. REH) at 10 mA cm−2 | 32.6 | 2000 CV | Li et al. [113] |
| FeCoNiB@Boron-doped vertically aligned graphene arrays | 1.0 M KOH | 31 (vs. REH) at 10 mA cm−2 | 30 | 10 h | Jiang et al. [119] |
| Plasma-etched, S-doped graphene | 0.5 M H2SO4 | 178 (vs. REH) at 10 mA cm−2 | 86 | 20 h, 3000 CV | Tian et al. [120] |
| 3D porous NG derivative-integrated MoS2 nanosheet | 0.5 M H2SO4 | 157 (vs. REH) at 10 mA cm−2 | 45.8 | 10 h, 1000 CV | Zang et al. [122] |
| 3D FeP NT/PG | 0.5 M H2SO4 | 69 (vs. REH) at 10 mA cm−2 | 52.4 | 40 h, 1000 CV | Yu et al. [125] |
| A self-supporting P–Fe3O4@3DG bulk composite | 1.0 M KOH | 123 (vs. REH) at 10 mA cm−2 | 65 | 50 h | Li et al. [126] |
| Ni2P nanoparticles/N,B-graphene | 1.0 M KOH | 92 mV (vs. RHE) at 10 mA cm−2 | 48.3 | 20 h, 3000 CV | Sun et al. [130] |
| Dispersed tungsten (W)-optimized MoP nanoparticles on N,P-doped graphene oxide | 1.0 M KOH | 70 mV (vs. RHE) at 10 mA cm−2 | 60.3 | 16 h | Chen et al. [129] |
| Ni-Ni3P@NPC/rGO | 0.5 M H2SO4 | 113 mV (vs. RHE) at 20 mA cm−2 | 57.93 mV dec−1 | 25 h | Li et al. [140] |
| Cobalt phosphide decorated/N,B-3D-graphene | 0.5 M H2SO4 | 118 mV (vs. RHE) at 10 mA cm−2 | 50 | 50 h, 1000 CV | Karaman et al. [143] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, M.Z. Review of solutions to global warming, air pollution, and energy security. Energy Environ. Sci. 2009, 2, 148–173. [Google Scholar] [CrossRef]
- Cong, Y.; Huang, S.; Mei, Y.; Li, T.T. Metal—Organic Frameworks-Derived Self-Supported Carbon-Based Composites for Electrocatalytic Water Splitting. Chem. A Eur. J. 2021, 27, 15866–15888. [Google Scholar] [CrossRef] [PubMed]
- Dusastre, V. Hydrogen to the rescue. Nat. Mater. 2018, 17, 565. [Google Scholar]
- Haryanto, A.; Fernando, S.; Murali, N.; Adhikari, S. Current Status of Hydrogen Production Techniques by Steam Reforming of Ethanol: A Review. Energy Fuels 2005, 19, 2098–2106. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [Green Version]
- Kosmala, T.; Baby, A.; Lunardon, M.; Perilli, D.; Liu, H.; Durante, C.; Di Valentin, C.; Agnoli, S.; Granozzi, G. Operando visualization of the hydrogen evolution reaction with atomic-scale precision at different metal–graphene interfaces. Nat. Catal. 2021, 4, 850–859. [Google Scholar] [CrossRef]
- Wang, J.; Xu, F.; Jin, H.; Chen, Y.; Wang, Y. Non-noble metal-based carbon composites in hydrogen evolution reaction: Fundamentals to applications. Adv. Mater. 2017, 29, 1605838. [Google Scholar]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar]
- Dinh, C.-T.; Jain, A.; de Arquer, F.P.G.; De Luna, P.; Li, J.; Wang, N.; Zheng, X.; Cai, J.; Gregory, B.Z.; Voznyy, O.; et al. Multi-site electrocatalysts for hydrogen evolution in neutral media by destabilization of water molecules. Nat. Energy 2019, 4, 107–114. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, F.; Fan, X.; Zhao, W.; Cui, M.; Li, X.; Liang, R.; Ou, Q.; Zhang, S. Fabrication of amorphous molybdenum sulfide/nitrogen-doped reduced graphene oxide nanocomposites with a tailored composition and hydrogen evolution activity via plasma treatment. Carbon 2022, 187, 386–395. [Google Scholar] [CrossRef]
- Ola, O.; Thummavichai, K.; Chen, Y.; Wang, N.; Niu, Q.; Wang, J.; Sun, S.; Zhu, Y. Layered tungsten-based composites and their pseudocapacitive and electrocatalytic performance. Mater. Chem. Front. 2022, 6, 737–747. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Gu, C.; Zhang, L.; Wang, X.; Tu, J. Active Co@ CoO core/shell nanowire arrays as efficient electrocatalysts for hydrogen evolution reaction. Chem. Eng. J. 2022, 429, 132226. [Google Scholar] [CrossRef]
- Ni, Y.; Ma, X.; Wang, S.; Wang, Y.; Song, F.; Cao, M.; Hu, C. Heterostructured nickel/vanadium nitrides composites for efficient electrocatalytic hydrogen evolution in neutral medium. J. Power Sources 2022, 521, 230934. [Google Scholar] [CrossRef]
- Wang, J.; Kong, H.; Zhang, J.; Hao, Y.; Shao, Z.; Ciucci, F. Carbon-based electrocatalysts for sustainable energy applications. Prog. Mater. Sci. 2021, 116, 100717. [Google Scholar] [CrossRef]
- Li, J.; Zheng, G. One-dimensional earth-abundant nanomaterials for water-splitting electrocatalysts. Adv. Sci. 2017, 4, 1600380. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Mo, J.; Stefanov, B.I.; Lau, T.H.; Chen, T.; Wu, S.; Wang, Z.; Gong, X.-Q.; Wilkinson, I.; Schmid, G.n.; Tsang, S.C.E. Superior performance of Ag over Pt for hydrogen evolution reaction in water electrolysis under high overpotentials. ACS Appl. Energy Mater. 2019, 2, 1221–1228. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Stern, L.-A.; Hu, X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 2014, 43, 6555–6569. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Zhao, Z.; Zhang, Y.; Sun, Y.; Xing, Y.; Lv, F.; Yang, Y.; Zhang, X.; Hwang, S.; Qin, Y.; et al. PdMo bimetallene for oxygen reduction catalysis. Nature 2019, 574, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-J.; Li, M.-X.; Yu, J.-H.; Ge, X.-B.; Liu, Y.-H.; Wang, W.-H. Low-Iridium-Content IrNiTa Metallic Glass Films as Intrinsically Active Catalysts for Hydrogen Evolution Reaction. Adv. Mater. 2020, 32, 1906384. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, M.; Yang, G.; Song, W.; Zhong, W.; Wang, X.; Wang, M.; Sun, T.; Tang, Y. Heterogeneous Bimetallic Mo-NiPx/NiSy as a Highly Efficient Electrocatalyst for Robust Overall Water Splitting. Adv. Funct. Mater. 2021, 31, 2101532. [Google Scholar] [CrossRef]
- Qian, G.; Yu, G.; Lu, J.; Luo, L.; Wang, T.; Zhang, C.; Ku, R.; Yin, S.; Chen, W.; Mu, S. Ultra-thin N-doped-graphene encapsulated Ni nanoparticles coupled with MoO2 nanosheets for highly efficient water splitting at large current density. J. Mater. Chem. A 2020, 8, 14545–14554. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, J.; Li, C.; Liu, R. Heterogeneity in a Metal–Organic Framework in-situ Guides Engineering Co@CoO Heterojunction for Electrocatalytic H2 Production in tandem with Glucose Oxidation. J. Mater. Chem. A 2022, 10, 4791–4799. [Google Scholar] [CrossRef]
- Ng, S.; Sturala, J.; Vyskocil, J.; Lazar, P.; Martincova, J.; Plutnar, J.; Pumera, M. Two-Dimensional Functionalized Germananes as Photoelectrocatalysts. ACS Nano 2021, 15, 11681–11693. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Hähnel, A.; Naumann, V.; Lin, C.; Azimi, S.; Schweizer, S.L.; Maijenburg, A.W.; Wehrspohn, R.B. Bifunctional heterostructure assembly of NiFe LDH nanosheets on NiCoP nanowires for highly efficient and stable overall water splitting. Adv. Funct. Mater. 2018, 28, 1706847. [Google Scholar] [CrossRef]
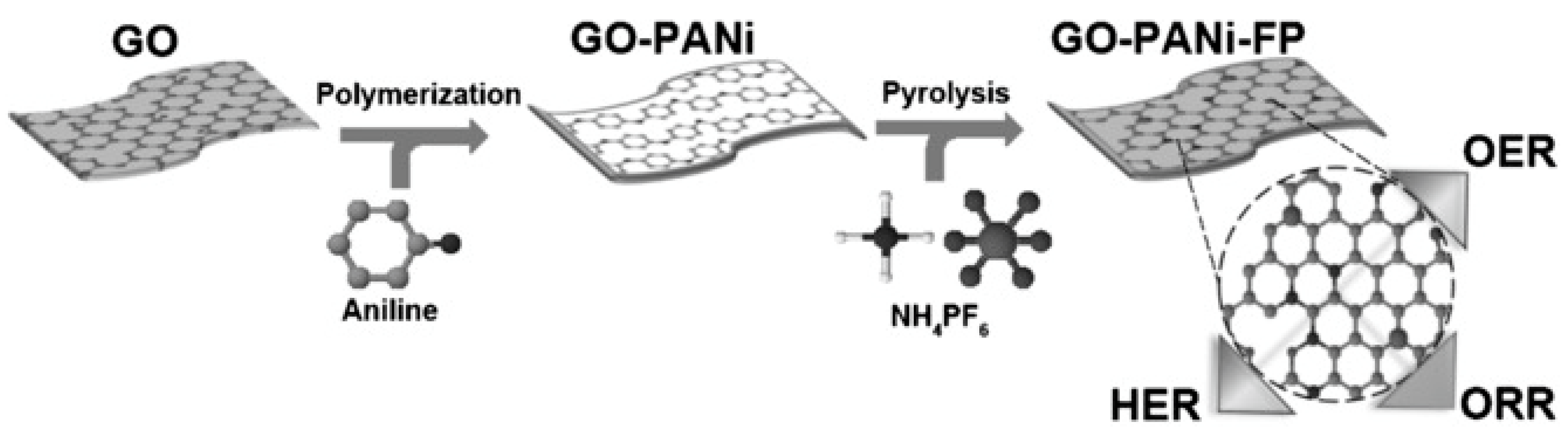
- Jung, J.Y.; Hong, Y.L.; Kim, J.-G.; Kim, M.J.; Kim, Y.-K.; Kim, N.D. New insight of tailor-made graphene oxide for the formation of atomic Co-N sites toward hydrogen evolution reaction. Appl. Surf. Sci. 2021, 563, 150254. [Google Scholar] [CrossRef]
- Shen, B.; Huang, H.; Liu, H.; Jiang, Q.; He, H. Bottom-up construction of three-dimensional porous MXene/nitrogen-doped graphene architectures as efficient hydrogen evolution electrocatalysts. Int. J. Hydrog. Energy 2021, 46, 29984–29993. [Google Scholar] [CrossRef]
- He, H.; Chen, Y.; Yang, C.; Yang, L.; Jiang, Q.; Huang, H. Constructing 3D interweaved MXene/graphitic carbon nitride nanosheets/graphene nanoarchitectures for promoted electrocatalytic hydrogen evolution. J. Energy Chem. 2022, 67, 483–491. [Google Scholar] [CrossRef]
- Huang, H.; Yan, M.; Yang, C.; He, H.; Jiang, Q.; Yang, L.; Lu, Z.; Sun, Z.; Xu, X.; Bando, Y. Graphene nanoarchitectonics: Recent advances in graphene-based electrocatalysts for hydrogen evolution reaction. Adv. Mater. 2019, 31, 1903415. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, D.; Ouyang, Y.; Wu, H.; Jiang, M.; Wang, F.; Zhang, L.Y. Synthesis of hollow cobalt phosphide nanocrystals with ultrathin shells anchored on reduced graphene oxide as an electrocatalyst toward hydrogen evolution. Appl. Surf. Sci. 2020, 506, 144975. [Google Scholar] [CrossRef]
- Chen, M.; Jian, X.; Wu, H.; Huang, J.; Liu, W.; Liu, Y. Facile synthesis of Mn-doped MoS2 nanosheets on carbon nanotubes as efficient electrocatalyst for hydrogen evolution reaction. Nanotechnology 2020, 31, 205403. [Google Scholar] [CrossRef] [PubMed]
- Ola, O.; Niu, Q.; Chen, Y.; Xia, Y.; Zhu, Y. Carbon nanotube reinforced nanocomposites for energy conversion and storage. J. Power Sources 2019, 443, 227277. [Google Scholar] [CrossRef]
- Li, J.-S.; Zhou, Y.-W.; Huang, M.-J. Engineering Mo x C nanoparticles confined in N,P-codoped porous carbon hollow spheres for enhanced hydrogen evolution reaction. Dalton Trans. 2021, 50, 499–503. [Google Scholar] [CrossRef]
- Puente Santiago, A.R.; He, T.; Eraso, O.; Ahsan, M.A.; Nair, A.N.; Chava, V.S.; Zheng, T.; Pilla, S.; Fernandez-Delgado, O.; Du, A. Tailoring the interfacial interactions of van der Waals 1T-MoS2/C60 heterostructures for high-performance hydrogen evolution reaction electrocatalysis. J. Am. Chem. Soc. 2020, 142, 17923–17927. [Google Scholar] [CrossRef]
- Wang, X.-D.; Xu, Y.-F.; Rao, H.-S.; Xu, W.-J.; Chen, H.-Y.; Zhang, W.-X.; Kuang, D.-B.; Su, C.-Y. Novel porous molybdenum tungsten phosphide hybrid nanosheets on carbon cloth for efficient hydrogen evolution. Energy Environ. Sci. 2016, 9, 1468–1475. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Z.; Zhang, M.; Tan, Y.; Jia, S.; Liu, A. Green electroless plating of cuprous oxide nanoparticles onto carbon nanotubes as efficient electrocatalysts for hydrogen evolution reaction. Appl. Surf. Sci. 2021, 548, 149218. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Wu, M.; Feng, X.; Redfern, S.A.; Shang, Y.; Yong, X.; Feng, T.; Wu, K.; Liu, Z. Carbon-quantum-dots-loaded ruthenium nanoparticles as an efficient electrocatalyst for hydrogen production in alkaline media. Adv. Mater. 2018, 30, 1800676. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, J.; Xiao, H.; Hu, T.; Jia, J.; Wu, H. Facile in situ synthesis of a carbon quantum dot/graphene heterostructure as an efficient metal-free electrocatalyst for overall water splitting. Chem. Commun. 2019, 55, 1635–1638. [Google Scholar] [CrossRef]
- Xiao, H.; Xue, S.; Zhang, J.; Zhao, M.; Ma, J.; Chen, S.; Zheng, Z.; Jia, J.; Wu, H. Facile electrolytic synthesis of Pt and carbon quantum dots coloaded multiwall carbon nanotube as highly efficient electrocatalyst for hydrogen evolution and ethanol oxidation. Chem. Eng. J. 2021, 408, 127271. [Google Scholar] [CrossRef]
- Naqvi, S.T.R.; Rasheed, T.; Majeed, S.; Hussain, D.; Fatima, B.; ul Haq, M.N.; Nawaz, R.; Ahmad, N.; Noon, T. Nitrogen doped carbon quantum dots conjugated with AgNi alloy nanoparticles as potential electrocatalyst for efficient water splitting. J. Alloys Compd. 2020, 847, 156492. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-E.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Mishra, R.K.; Ha, S.K.; Huczko, A. Evolution of graphene oxide and graphene: From imagination to industrialization. ChemNanoMat 2018, 4, 598–620. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Kumar, V.; Huczko, A.; Oraon, R.; Adhikari, A.D.; Nayak, G. Magical allotropes of carbon: Prospects and applications. Crit. Rev. Solid State Mater. Sci. 2016, 41, 257–317. [Google Scholar] [CrossRef]
- Liao, L.; Peng, H.; Liu, Z. Chemistry makes graphene beyond graphene. J. Am. Chem. Soc. 2014, 136, 12194–12200. [Google Scholar] [CrossRef]
- Ola, O.; Chen, Y.; Thummavichai, K.; Zhu, Y. In situ fabrication of dendritic tin-based carbon nanostructures for hydrogen evolution reaction. Sustain. Energy Fuels 2020, 4, 5223–5228. [Google Scholar] [CrossRef]
- Chen, K.; Shi, L.; Zhang, Y.; Liu, Z. Scalable chemical-vapour-deposition growth of three-dimensional graphene materials towards energy-related applications. Chem. Soc. Rev. 2018, 47, 3018–3036. [Google Scholar] [CrossRef]
- Wei, D.; Peng, L.; Li, M.; Mao, H.; Niu, T.; Han, C.; Chen, W.; Wee, A.T.S. Low temperature critical growth of high quality nitrogen doped graphene on dielectrics by plasma-enhanced chemical vapor deposition. ACS Nano 2015, 9, 164–171. [Google Scholar] [CrossRef]
- Li, X.; Chi, M.; Mahurin, S.M.; Liu, R.; Chuang, Y.-J.; Dai, S.; Pan, Z. Graphitized hollow carbon spheres and yolk-structured carbon spheres fabricated by metal-catalyst-free chemical vapor deposition. Carbon 2016, 101, 57–61. [Google Scholar] [CrossRef] [Green Version]
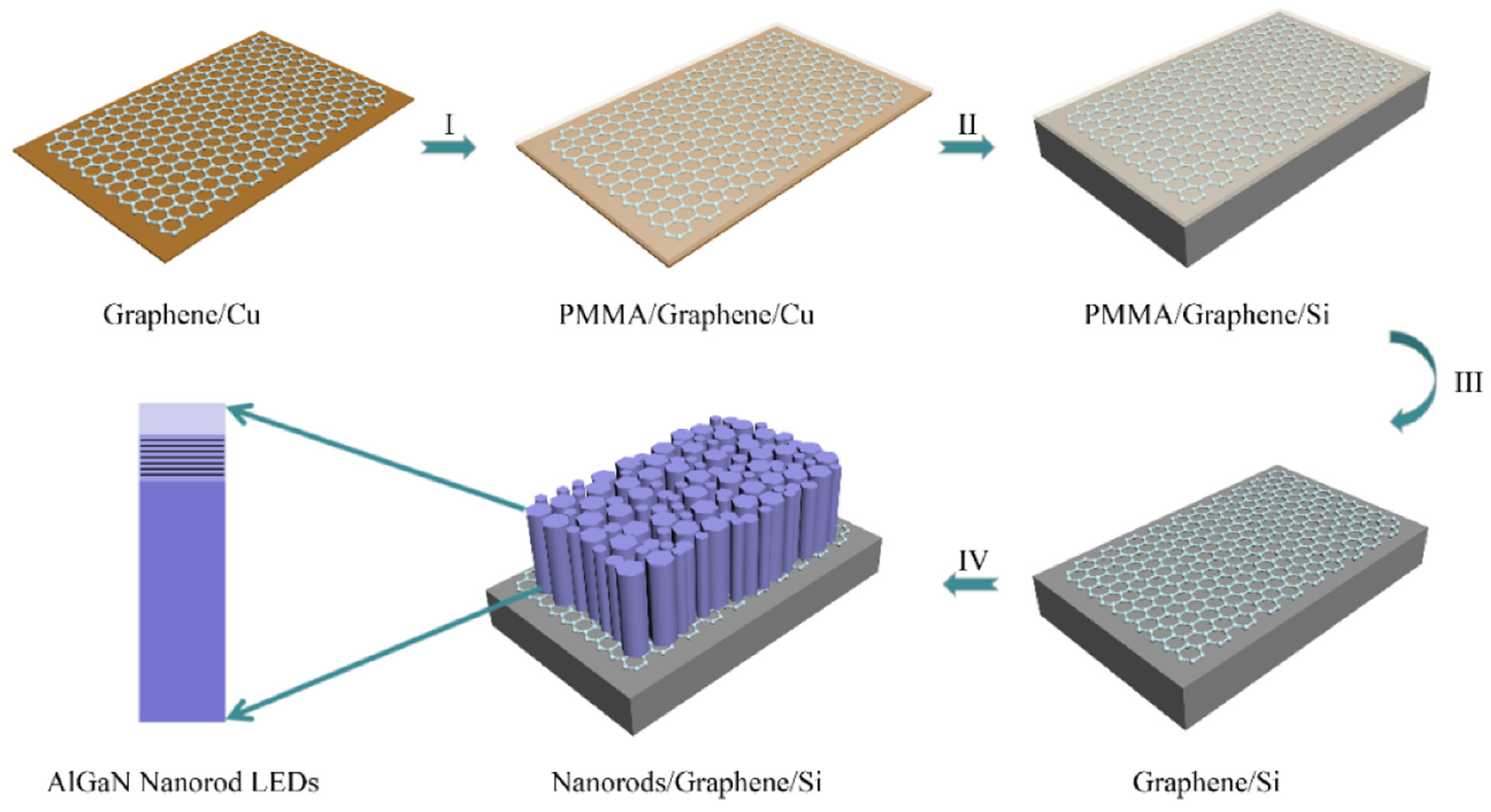
- Ren, F.; Yin, Y.; Wang, Y.; Liu, Z.; Liang, M.; Ou, H.; Ao, J.; Wei, T.; Yan, J.; Yuan, G. Direct growth of AlGaN nanorod LEDs on graphene-covered Si. Materials 2018, 11, 2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hummers Jr, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
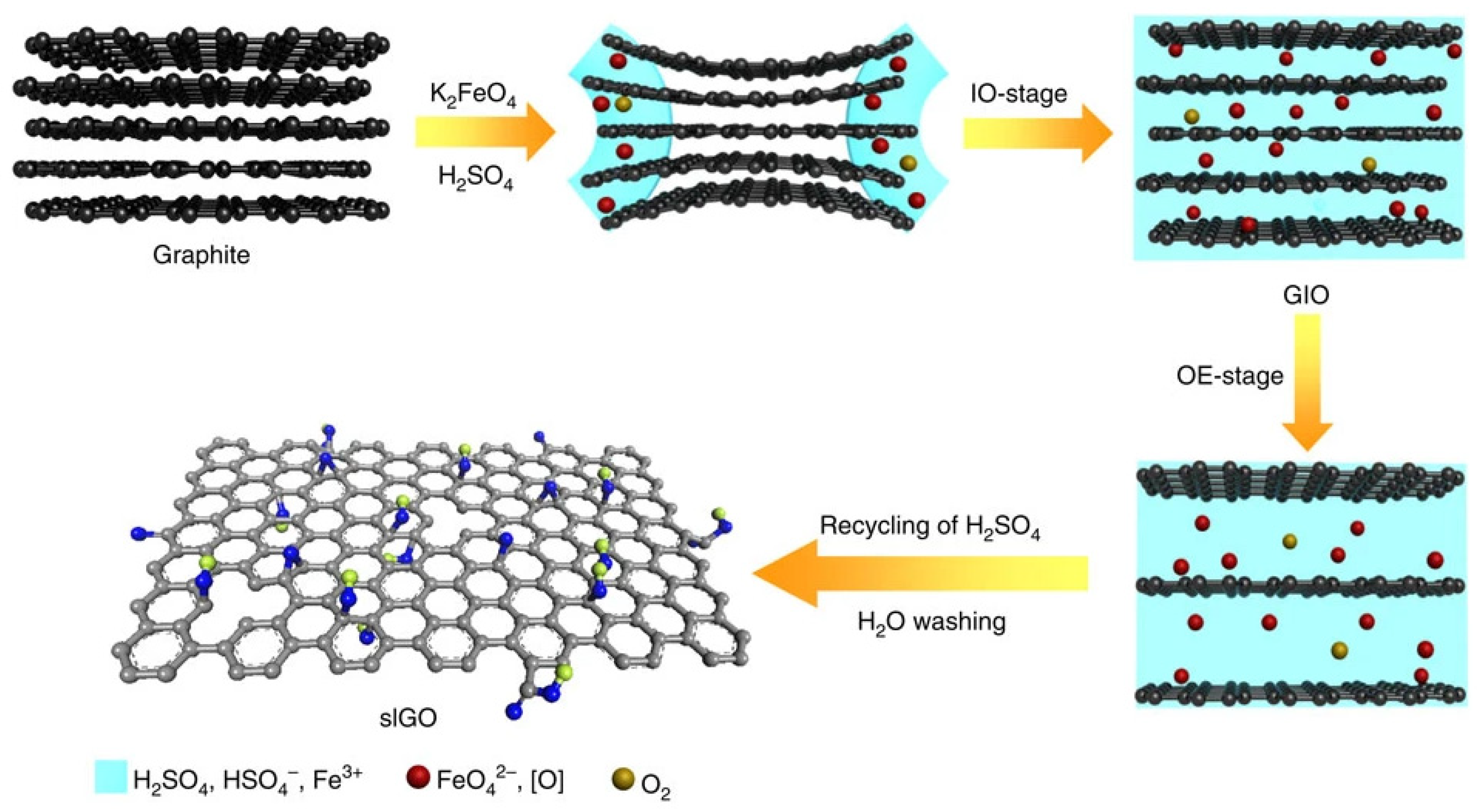
- Peng, L.; Xu, Z.; Liu, Z.; Wei, Y.; Sun, H.; Li, Z.; Zhao, X.; Gao, C. An iron-based green approach to 1-h production of single-layer graphene oxide. Nat. Commun. 2015, 6, 5716. [Google Scholar] [CrossRef] [Green Version]
- Scardaci, V.; Compagnini, G. Raman Spectroscopy Data Related to the Laser Induced Reduction of Graphene Oxide. Data Brief 2021, 107306. [Google Scholar] [CrossRef]
- Liang, M.; Zhang, K.; Lei, P.; Wang, B.; Shu, C.-M.; Li, B. Fuel properties and combustion kinetics of hydrochar derived from co-hydrothermal carbonization of tobacco residues and graphene oxide. Biomass Convers. Bioefin. 2020, 10, 189–201. [Google Scholar] [CrossRef]
- Yeon, J.S.; Park, S.H.; Suk, J.; Lee, H.; Park, H.S. Confinement of sulfur in the micropores of honeycomb-like carbon derived from lignin for lithium-sulfur battery cathode. Chem. Eng. J. 2020, 382, 122946. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.H.; Antonietti, M.; Titirici, M.M. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, Y.; Lei, H.; Wang, C.; Zhao, Y.; Huo, E.; Lin, X.; Zhang, Q.; Qian, M.; Mateo, W. Synthesis of graphene-like carbon from biomass pyrolysis and its applications. Chem. Eng. J. 2020, 399, 125808. [Google Scholar] [CrossRef]
- Yang, L.; Hu, M.; Lv, Q.; Zhang, H.; Yang, W.; Lv, R. Salt and sugar derived high power carbon microspheres anode with excellent low-potential capacity. Carbon 2020, 163, 288–296. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, S.; Liang, Y.; Wu, S.; Li, Z.; Luo, S.; Cui, Z. Self-supported Ni3Se2@ NiFe layered double hydroxide bifunctional electrocatalyst for overall water splitting. J. Colloid Interface Sci. 2021, 587, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Tan, M.; Li, Z.; He, L.; Gao, B.; Chen, Y.; Zheng, Y.; Lin, B. Ethylenediamine-assisted phase engineering of 1T/2H–MoS2/graphene for efficient and stable electrocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2021, 46, 11688–11700. [Google Scholar] [CrossRef]
- Yang, Z.; Qian, K.; Lv, J.; Yan, W.; Liu, J.; Ai, J.; Zhang, Y.; Guo, T.; Zhou, X.; Xu, S. Encapsulation of Fe3O4 nanoparticles into N, S co-doped graphene sheets with greatly enhanced electrochemical performance. Sci. Rep. 2016, 6, 27957. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Liang, Y.; Lu, Z.; Lou, H.; Zhang, X.; Liu, S.; Zheng, B.; Liu, R.; Fu, R.; Wu, D. Mechanochemistry: A green, activation-free and top-down strategy to high-surface-area carbon materials. ACS Sustain. Chem. Eng. 2017, 5, 8535–8540. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials. Bioresour. Technol. 2020, 312, 123613. [Google Scholar] [CrossRef]
- Caicedo, F.M.C.; López, E.V.; Agarwal, A.; Drozd, V.; Durygin, A.; Hernandez, A.F.; Wang, C. Synthesis of graphene oxide from graphite by ball milling. Diam. Relat. Mater. 2020, 109, 108064. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, Y.; Gao, B.; Cao, X. N-doped biochar synthesized by a facile ball-milling method for enhanced sorption of CO2 and reactive red. Chem. Eng. J. 2019, 368, 564–572. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Shin, Y.-R.; Sohn, G.-J.; Choi, H.-J.; Bae, S.-Y.; Mahmood, J.; Jung, S.-M.; Seo, J.-M.; Kim, M.-J.; Chang, D.W. Edge-carboxylated graphene nanosheets via ball milling. Proc. Natl. Acad. Sci. USA 2012, 109, 5588–5593. [Google Scholar] [CrossRef] [Green Version]
- Si, Z.; Lv, Z.; Lu, L.; Liu, M.; Chen, Y.; Jin, H.; Tian, X.; Dai, K.; Liu, J.; Song, W. Nitrogen-doped Graphene Chainmail Wrapped IrCo Alloy Particles on Nitrogen-doped Graphene Nanosheet for Highly Active and Stable Full Water Splitting. ChemCatChem 2019, 11, 5457–5465. [Google Scholar] [CrossRef]
- Sun, J.; Li, S.; Zhao, Q.; Huang, C.; Wu, Q.; Chen, W.; Xu, Q.; Yao, W. Atomically confined calcium in nitrogen-doped graphene as an efficient heterogeneous catalyst for hydrogen evolution. Iscience 2021, 24, 102728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dai, L. Nitrogen, phosphorus, and fluorine tri-doped graphene as a multifunctional catalyst for self-powered electrochemical water splitting. Angew. Chem. Int. Ed. 2016, 55, 13296–13300. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liao, Z.; Yang, W.; Zhou, H.; Fu, C.; Gong, Y.; Chen, L.; Kuang, Y. Different types of nitrogen species in nitrogen-doped carbon material: The formation mechanism and catalytic role on oxygen reduction reaction. Electrochim. Acta 2017, 245, 957–966. [Google Scholar] [CrossRef]
- Yan, Y.B.; Miao, J.W.; Yang, Z.H.; Xiao, F.X.; Yang, H.B.; Liu, B.; Yang, Y.H. Carbon nanotube catalysts: Recent advances in synthesis, characterization and applications. Chem. Soc. Rev. 2015, 44, 3295–3346. [Google Scholar] [CrossRef]
- Zhang, L.H.; Shi, Y.; Wang, Y.; Shiju, N.R. Nanocarbon catalysts: Recent understanding regarding the active sites. Adv. Sci. 2020, 7, 1902126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, H.; Zhou, D.; Li, F.; Cao, D.; Chen, X. Defect engineering in MoSe2 for the hydrogen evolution reaction: From point defects to edges. ACS Appl. Mater. Interfaces 2017, 9, 42688–42698. [Google Scholar] [CrossRef]
- Hao, J.; Wei, F.; Zhang, X.; Li, L.; Zhang, C.; Liang, D.; Ma, X.; Lu, P. Defect and doping engineered penta-graphene for catalysis of hydrogen evolution reaction. Nanoscale Res. Lett. 2021, 16, 130. [Google Scholar] [CrossRef]
- Tian, Y.; Mei, R.; Xue, D.-z.; Zhang, X.; Peng, W. Enhanced electrocatalytic hydrogen evolution in graphene via defect engineering and heteroatoms co-doping. Electrochim. Acta 2016, 219, 781–789. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Pan, Z.; Lai, Y.; Lu, Y.; Wang, Y.; Song, S. Boosting hydrogen evolution electrocatalysis through defect engineering: A strategy of heat and cool shock. Chem. Eng. J. 2021, 426, 131524. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, R. Defect engineering of two-dimensional materials for efficient electrocatalysis. J. Mater. 2018, 4, 95–107. [Google Scholar] [CrossRef]
- Tang, T.; Wang, Z.; Guan, J. A review of defect engineering in two-dimensional materials for electrocatalytic hydrogen evolution reaction. Chin. J. Catal. 2022, 43, 636–678. [Google Scholar] [CrossRef]
- Sharma, R.; Baik, J.H.; Perera, C.J.; Strano, M.S. Anomalously large reactivity of single graphene layers and edges toward electron transfer chemistries. Nano Lett. 2010, 10, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Kumatani, A.; Miura, C.; Kuramochi, H.; Ohto, T.; Wakisaka, M.; Nagata, Y.; Ida, H.; Takahashi, Y.; Hu, K.; Jeong, S. Chemical dopants on edge of holey graphene accelerate electrochemical hydrogen evolution reaction. Adv. Sci. 2019, 6, 1900119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, G.; Zhang, Y.; Dong, J.; Xu, L. Fabrication of Ni2P/Ni5P4 nanoparticles embedded in three-dimensional N-doped graphene for acidic hydrogen evolution reaction. Mater. Lett. 2021, 299, 130071. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Y.; Chen, P.; Zhang, S.; Jiang, H.; Yang, L.; Wang, Y.; Zhang, L.; Shen, J.; Zhao, X. A Freestanding 3D Heterostructure Film Stitched by MOF-Derived Carbon Nanotube Microsphere Superstructure and Reduced Graphene Oxide Sheets: A Superior Multifunctional Electrode for Overall Water Splitting and Zn–Air Batteries. Adv. Mater. 2020, 32, 2003313. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xu, S.; Li, H.; Selvam, N.C.S.; Lee, J.Y.; Lee, H.; Yoo, P.J. Perpendicularly anchored ReSe2 nanoflakes on reduced graphene oxide support for highly efficient hydrogen evolution reactions. Chem. Eng. J. 2021, 405, 126728. [Google Scholar] [CrossRef]
- Hu, K.; Ohto, T.; Nagata, Y.; Wakisaka, M.; Aoki, Y.; Fujita, J.-i.; Ito, Y. Catalytic activity of graphene-covered non-noble metals governed by proton penetration in electrochemical hydrogen evolution reaction. Nat. Commun. 2021, 12, 203. [Google Scholar] [CrossRef]
- Flis-Kabulska, I.; Flis, J. Electrodeposits of nickel with reduced graphene oxide (Ni/rGO) and their enhanced electroactivity towards hydrogen evolution in water electrolysis. Mater. Chem. Phys. 2020, 241, 122316. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Z.; Shen, C.; Liu, H.; Pang, X.; Gao, M.; Mu, J.; Cao, F.; Li, G. Ni/NiO heterostructures encapsulated in oxygen-doped graphene as multifunctional electrocatalysts for the HER, UOR and HMF oxidation reaction. Catal. Sci. Technol. 2021, 11, 2480–2490. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Ali, M.; Karuppasamy, K.; Santhoshkumar, P.; Hwang, J.-H.; Jung, J.; Kim, H.-S. Theoretical evaluation and experimental investigation of layered 2H/1T-phase MoS2 and its reduced graphene-oxide hybrids for hydrogen evolution reactions. J. Alloys Compd. 2021, 868, 159272. [Google Scholar] [CrossRef]
- Zhu, M.; Yan, Y.; Yan, Q.; Yin, J.; Cheng, K.; Ye, K.; Zhu, K.; Yan, J.; Cao, D.; Wang, G. In situ growth of Ni0· 85Se on graphene as a robust electrocatalyst for hydrogen evolution reaction. Int. J. Hydrog. Energy 2020, 45, 10486–10493. [Google Scholar] [CrossRef]
- Kuang, P.; Sayed, M.; Fan, J.; Cheng, B.; Yu, J. 3D graphene-based H2-production photocatalyst and electrocatalyst. Adv. Energy Mater. 2020, 10, 1903802. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, Y.; Zhang, T.; Yang, Y.; Wei, L.; Chen, Y. Monolithic 3D cross-linked polymeric graphene materials and the likes: Preparation and their redox catalytic applications. J. Am. Chem. Soc. 2018, 140, 11538–11550. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, S. Recent progress in electrode fabrication for electrocatalytic hydrogen evolution reaction: A mini review. Chem. Eng. J. 2020, 393, 124726. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Chambers, B.A.; Andersson, G.G.; Qiao, S.Z. 3D WS2 nanolayers@heteroatom-doped graphene films as hydrogen evolution catalyst electrodes. Adv. Mater. 2015, 27, 4234–4241. [Google Scholar] [CrossRef]
- Bolar, S.; Shit, S.; Samanta, P.; Murmu, N.C.; Kolya, H.; Kang, C.-W.; Kuila, T. Conducting scaffold supported defect rich 3D rGO-CNT/MoS2 nanostructure for efficient HER electrocatalyst at variable pH. Compos. Part B Eng. 2022, 230, 109489. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Pillai, V.V.; Alhassan, S.M. Three dimensional (3D) nanostructured assembly of MoS2-WS2/Graphene as high performance electrocatalysts. Int. J. Hydrog. Energy 2020, 45, 10475–10485. [Google Scholar] [CrossRef]
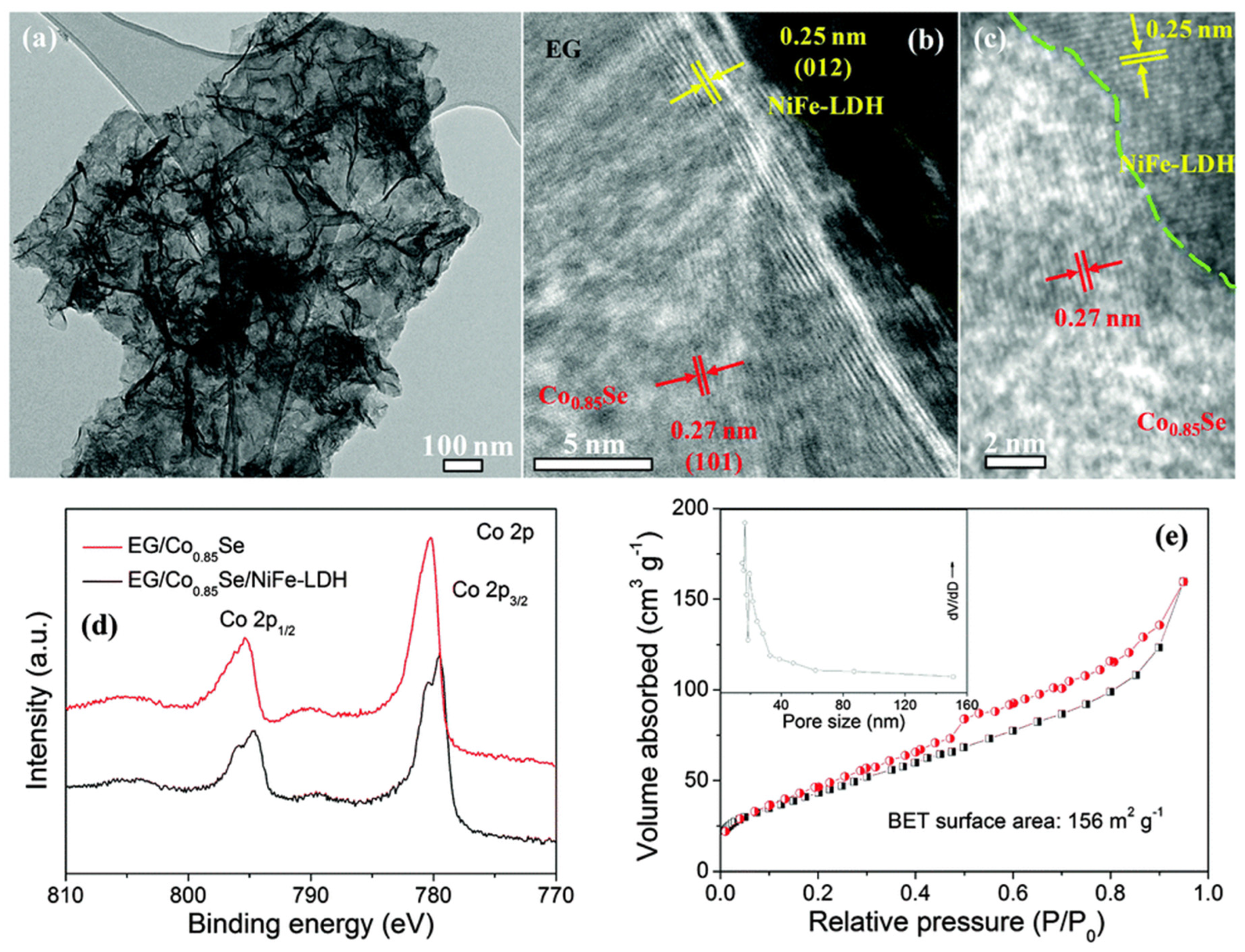
- Hou, Y.; Lohe, M.R.; Zhang, J.; Liu, S.; Zhuang, X.; Feng, X. Vertically oriented cobalt selenide/NiFe layered-double-hydroxide nanosheets supported on exfoliated graphene foil: An efficient 3D electrode for overall water splitting. Energy Environ. Sci. 2016, 9, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.-T.; Ha, H.; Nguyen, N.-A.; An, H.; Kim, H.Y.; Choi, H.-S. In situ engineering of Pd nanosponge armored with graphene dots using Br–toward high-performance and stable electrocatalyst for the hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2020, 12, 15500–15506. [Google Scholar] [CrossRef]
- Gu, Y.; Xi, B.; Wei, R.; Fu, Q.; Qain, Y.; Xiong, S. Sponge assembled by graphene nanocages with double active sites to accelerate alkaline her kinetics. Nano Lett. 2020, 20, 8375–8383. [Google Scholar] [CrossRef]
- Li, J.-S.; Li, J.-Y.; Huang, M.-J.; Kong, L.-X.; Wu, Z. Anchoring RuxP on 3D hollow graphene nanospheres as efficient and pH-universal electrocatalysts for the hydrogen evolution reaction. Carbon 2020, 161, 44–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, X.; Liu, B.; Deng, S.; Xie, D.; Liu, Q.; Wang, Y.; Wu, J.; Wang, X.; Tu, J. Multiscale graphene-based materials for applications in sodium ion batteries. Adv. Energy Mater. 2019, 9, 1803342. [Google Scholar] [CrossRef]
- Xu, X.; Liang, H.; Ming, F.; Qi, Z.; Xie, Y.; Wang, Z. Prussian blue analogues derived penroseite (Ni, Co) Se2 nanocages anchored on 3D graphene aerogel for efficient water splitting. ACS Catal. 2017, 7, 6394–6399. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Chen, S.; Ren, J.; Jia, Y.A.; Chen, C.; Komarneni, S.; Yang, D.; Yao, X. Electronic structure tuning in Ni3FeN/r-GO aerogel toward bifunctional electrocatalyst for overall water splitting. ACS Nano 2018, 12, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, S.; Kariper, İ.A. Graphene and graphene oxide based aerogels: Synthesis, characteristics and supercapacitor applications. J. Energy Storage 2020, 27, 101038. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Q.; Yan, M.; Wei, Y.; Zong, J.; Zhang, J.; Wu, Y.; Huang, H. Three-dimensional boron-and nitrogen-codoped graphene aerogel-supported Pt nanoparticles as highly active electrocatalysts for methanol oxidation reaction. ACS Sustain. Chem. Eng. 2018, 6, 6644–6653. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, K.; Yang, J.; Zheng, Y.; Hübner, R.; Ou, Z.; Dong, X.; He, L.; Wang, H.; Li, J. Tungsten Oxide/Reduced Graphene Oxide Aerogel with Low-Content Platinum as High-Performance Electrocatalyst for Hydrogen Evolution Reaction. Small 2021, 17, 2102159. [Google Scholar] [CrossRef]
- Cao, K.W.; Sun, H.Y.; Xue, Q.; Ding, Y.; Wang, T.J.; Li, F.M.; Xu, G.R.; Chen, P.; Yang, Y.; Chen, Y. Functionalized Ultrafine Rhodium Nanoparticles on Graphene Aerogels for the Hydrogen Evolution Reaction. ChemElectroChem 2021, 8, 1759–1765. [Google Scholar] [CrossRef]
- Wei, W.; Li, H.; Sun, W.; Wang, J.; Fan, X.; Jiang, G.; Jiang, Z.; Xie, J. NiCoP nanoparticles encapsulated in cross-linked graphene aerogel to efficient hydrogen evolution reaction. J. Mater. Sci. Mater. Electron. 2020, 31, 13521–13530. [Google Scholar] [CrossRef]
- Choi, H.; Lee, S.; Kim, M.-C.; Park, Y.; Jang, A.; Ahn, W.; Sohn, J.-I.; Park, J.-B.; Hong, J.; Lee, Y.-W. Hierarchically Ordinated Two-Dimensional MoS2 Nanosheets on Three-Dimensional Reduced Graphene Oxide Aerogels as Highly Active and Stable Catalysts for Hydrogen Evolution Reaction. Catalysts 2021, 11, 182. [Google Scholar] [CrossRef]
- Pawar, R.C.; Kang, S.; Khan, H.; Han, H.; Lee, C.S. Study of multi-faceted CoS2 introduced graphene aerogel hybrids via chemical approach for an effective electrocatalytic water splitting. Curr. Appl. Phys. 2021, 32, 78–85. [Google Scholar] [CrossRef]
- Ali, A.; Shen, P.K. Recent progress in graphene-based nanostructured electrocatalysts for overall water splitting. Electrochem. Energy Rev. 2020, 3, 370–394. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Y.; Zhang, Z.; Yu, Q.; Li, C.; Zhang, Q.; Zheng, Z.; Liu, H.; Liu, B.; Dou, S. Implanting Ru nanoclusters into N-doped graphene for efficient alkaline hydrogen evolution. Carbon 2021, 183, 362–367. [Google Scholar] [CrossRef]
- Yang, C.; Shen, K.; Zhao, R.; Xiang, H.; Wu, J.; Zhong, W.; Zhang, Q.; Li, X.; Yang, N. Balance Effect: A Universal Strategy for Transition Metal Carbides to Enhance Hydrogen Evolution. Adv. Funct. Mater. 2021, 2108167. [Google Scholar] [CrossRef]
- Wang, A.; Zhao, C.; Yu, M.; Wang, W. Trifunctional Co nanoparticle confined in defect-rich nitrogen-doped graphene for rechargeable Zn-air battery with a long lifetime. Appl. Catal. B Environ. 2021, 281, 119514. [Google Scholar] [CrossRef]
- Deng, R.; Lin, L.; Li, L.; Wu, J. Novel Ni2P-microporous nickel phosphite supported on nitrogen-doped graphene composite electrocatalyst for efficient hydrogen evolution reaction. Nanotechnology 2021, 32, 505703. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ai, J.; Sun, M.; Han, F.; Li, Z.; Peng, Q.; Wang, Q.D.; Liu, J.; Liu, L. Phosphorous-Doped Graphite Layers with Outstanding Electrocatalytic Activities for the Oxygen and Hydrogen Evolution Reactions in Water Electrolysis. Adv. Funct. Mater. 2020, 30, 1910741. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, G.; Gao, Y.; Huang, X.; Chen, W. Applying surface strain and coupling with pure or N/B-doped graphene to successfully achieve high HER catalytic activity in 2D layered SnP3-based nanomaterials: A first-principles investigation. Inorg. Chem. Front. 2020, 7, 647–658. [Google Scholar] [CrossRef]
- Jiang, B.; Liang, K.; Yang, Z.; Guo, K.; Shaik, F.; Zheng, J. FeCoNiB@ Boron-doped vertically aligned graphene arrays: A self-supported electrocatalyst for overall water splitting in a wide pH range. Electrochim. Acta 2021, 386, 138459. [Google Scholar] [CrossRef]
- Tian, Y.; Wei, Z.; Wang, X.; Peng, S.; Zhang, X.; Liu, W.-m. Plasma-etched, S-doped graphene for effective hydrogen evolution reaction. Int. J. Hydrog. Energy 2017, 42, 4184–4192. [Google Scholar] [CrossRef]
- Sun, X.; Gao, X.; Chen, J.; Wang, X.; Chang, H.; Li, B.; Song, D.; Li, J.; Li, H.; Wang, N. Ultrasmall Ru nanoparticles highly dispersed on sulfur-doped graphene for HER with high electrocatalytic performance. ACS Appl. Mater. Interfaces 2020, 12, 48591–48597. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Qin, Y.; Wang, T.; Li, F.; Shao, Q.; Cao, N. 1T/2H mixed phase MoS2 nanosheets integrated by a 3D nitrogen-doped graphene derivative for enhanced electrocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 2020, 12, 55884–55893. [Google Scholar] [CrossRef] [PubMed]
- Ion-Ebrașu, D.; Andrei, R.D.; Enache, S.; Căprărescu, S.; Negrilă, C.C.; Jianu, C.; Enache, A.; Boerașu, I.; Carcadea, E.; Varlam, M. Nitrogen functionalization of cvd grown three-dimensional graphene foam for hydrogen evolution reactions in alkaline media. Materials 2021, 14, 4952. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Jiang, Z.; Tian, X.; Zhu, H.; Jiang, Z.-J. Effects of nitrogen-doping structural changes of spherical hollow graphene on the growth of MoS2+x nanosheets and the enhanced hydrogen evolution reaction. J. Alloys Compd. 2021, 884, 161073. [Google Scholar] [CrossRef]
- Yu, C.; Shi, Y.; Yan, F.; Zhao, Y.; Zhu, C.; Zhang, X.; Zhang, X.; Chen, Y. Three-dimensional FeP nanotube arrays fabricated through electrostatic-repulsion-limited-nucleation strategy for high-efficiency hydrogen evolution. Chem. Eng. J. 2021, 423, 130240. [Google Scholar] [CrossRef]
- Li, S.; Jian, X.; Liu, J.; Guo, S.; Zhou, C.; Zhang, P.; Yang, Y.; Chen, L. Phosphorus-doped Fe3O4 nanoflowers grown on 3D porous graphene for robust pH-Universal hydrogen evolution reaction. Int. J. Hydrog. Energy 2020, 45, 4435–4443. [Google Scholar] [CrossRef]
- Yang, J.; Guo, D.; Zhao, S.; Lin, Y.; Yang, R.; Xu, D.; Shi, N.; Zhang, X.; Lu, L.; Lan, Y.Q. Cobalt Phosphides Nanocrystals Encapsulated by P-Doped Carbon and Married with P-Doped Graphene for Overall Water Splitting. Small 2019, 15, 1804546. [Google Scholar] [CrossRef]
- Kurys, Y.I.; Mazur, D.O.; Koshechko, V.G.; Pokhodenko, V.D. Nanocomposite Based on N, P-Doped Reduced Graphene Oxide, Mo2C, and Mo2N as Efficient Electrocatalyst for Hydrogen Evolution in a Wide pH Range. Electrocatalysis 2021, 12, 469–477. [Google Scholar] [CrossRef]
- Chen, C.; Luo, W.; Li, H.; Hu, T.; Zhao, Y.; Zhao, Z.; Sun, X.; Zai, H.; Qi, Y.; Wu, M. Optimized MoP with Pseudo-Single-Atom Tungsten for Efficient Hydrogen Electrocatalysis. Chem. Mater. 2021, 33, 3639–3649. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, K.; Zhao, Z.; Li, X.; Chen, G.; Li, C. Strongly coupled dual zerovalent nonmetal doped nickel phosphide nanoparticles/N, B-graphene hybrid for pH-Universal hydrogen evolution catalysis. Appl. Catal. B Environ. 2020, 278, 119284. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Li, L.H.; Xing, T.; Chen, Y.; Jaroniec, M.; Qiao, S.Z. Toward design of synergistically active carbon-based catalysts for electrocatalytic hydrogen evolution. ACS Nano 2014, 8, 5290–5296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shit, S.; Samanta, P.; Bolar, S.; Murmu, N.C.; Kuila, T. Alteration in electrocatalytic water splitting activity of reduced graphene oxide through simultaneous and individual doping of Lewis acid/base center. Electrochim. Acta 2020, 362, 137146. [Google Scholar] [CrossRef]
- Kuo, W.-S.; Shao, Y.-T.; Huang, K.-S.; Chou, T.-M.; Yang, C.-H. Antimicrobial amino-functionalized nitrogen-doped graphene quantum dots for eliminating multidrug-resistant species in dual-modality photodynamic therapy and bioimaging under two-photon excitation. ACS Appl. Mater. Interfaces 2018, 10, 14438–14446. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Song, Y.; Wang, J.; Wan, H.; Zhang, Y.; Ning, Y.; Yang, B. Photoluminescence mechanism in graphene quantum dots: Quantum confinement effect and surface/edge state. Nano Today 2017, 13, 10–14. [Google Scholar] [CrossRef]
- Guo, T.; Wang, L.; Sun, S.; Wang, Y.; Chen, X.; Zhang, K.; Zhang, D.; Xue, Z.; Zhou, X. Layered MoS2@graphene functionalized with nitrogen-doped graphene quantum dots as an enhanced electrochemical hydrogen evolution catalyst. Chin. Chem. Lett. 2019, 30, 1253–1260. [Google Scholar] [CrossRef]
- Sim, Y.; Kim, S.J.; Janani, G.; Chae, Y.; Surendran, S.; Kim, H.; Yoo, S.; Seok, D.C.; Jung, Y.H.; Jeon, C. The synergistic effect of nitrogen and fluorine co-doping in graphene quantum dot catalysts for full water splitting and supercapacitor. Appl. Surf. Sci. 2020, 507, 145157. [Google Scholar] [CrossRef]
- Favaro, M.; Cattelan, M.; Price, S.W.; Russell, A.E.; Calvillo, L.; Agnoli, S.; Granozzi, G. In situ study of graphene oxide quantum dot-MoSx nanohybrids as hydrogen evolution catalysts. Surfaces 2020, 3, 225–236. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, J.; Zhao, M.; Ma, J.; Li, Y.; Hu, T.; Zheng, Z.; Jia, J.; Wu, H. Electric field-assisted synthesis of Pt, carbon quantum dots-coloaded graphene hybrid for hydrogen evolution reaction. J. Power Sources 2020, 451, 227770. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, Z.; Zeng, Z.; Wang, W.; Kong, L.; Liu, J.; Chen, P. Graphene quantum dots assisted exfoliation of atomically-thin 2D materials and as-formed 0D/2D van der Waals heterojunction for HER. Carbon 2021, 184, 554–561. [Google Scholar] [CrossRef]
- Li, G.; Wang, J.; Yu, J.; Liu, H.; Cao, Q.; Du, J.; Zhao, L.; Jia, J.; Liu, H.; Zhou, W. Ni-Ni3P nanoparticles embedded into N, P-doped carbon on 3D graphene frameworks via in situ phosphatization of saccharomycetes with multifunctional electrodes for electrocatalytic hydrogen production and anodic degradation. Appl. Catal. B Environ. 2020, 261, 118147. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, B.; Xiong, B.; Ye, J.; Yang, Q.; Fan, P.; Nie, M.; Jin, Y.; Fang, L.; Tian, W.Q. Temperature differentiated synthesis of hierarchically structured N, S-Doped carbon nanotubes/graphene hybrids as efficient electrocatalyst for hydrogen evolution reaction. J. Alloys Compd. 2020, 848, 156528. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Qin, Y.; Ding, D.; Bu, Y.; Chu, F.; Kong, Y.; Liu, M. Co, N-codoped graphene as efficient electrocatalyst for hydrogen evolution reaction: Insight into the active centre. J. Power Sources 2017, 363, 260–268. [Google Scholar] [CrossRef]
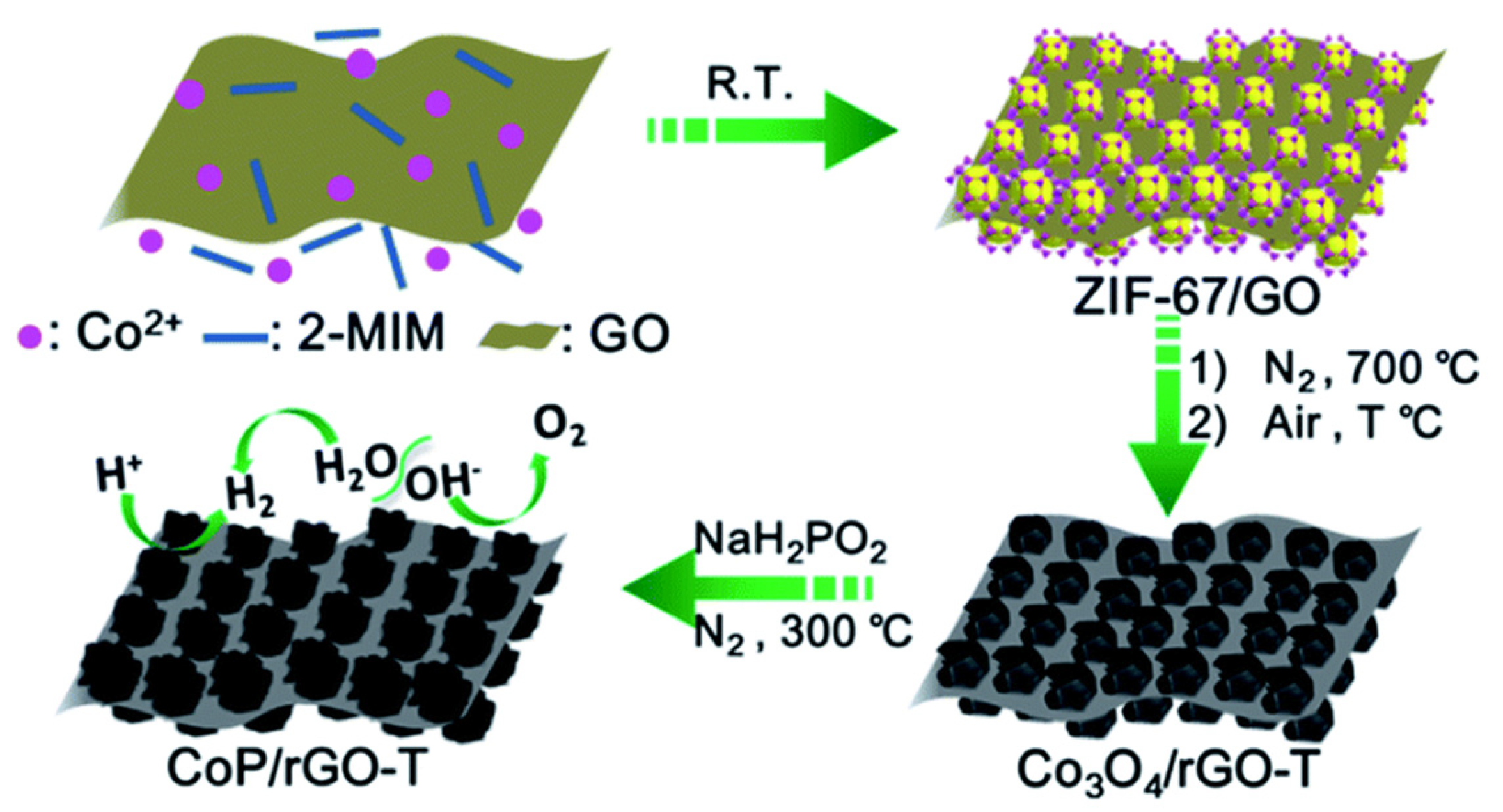
- Karaman, C.; Karaman, O.; Atar, N.; Yola, M.L. Tailoring of cobalt phosphide anchored nitrogen and sulfur co-doped three dimensional graphene hybrid: Boosted electrocatalytic performance towards hydrogen evolution reaction. Electrochim. Acta 2021, 380, 138262. [Google Scholar] [CrossRef]
- Navadeepthy, D.; Rebekah, A.; Viswanthan, C.; Ponpandian, N. Boosting the kinetics of oxygen and hydrogen evolution in alkaline water splitting using nickel ferrite/N-graphene nanocomposite as a bifunctional electrocatalyst. Int. J. Hydrog. Energy 2021, 46, 21512–21524. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, X.; Zhu, B.; Fan, L.; Chai, X.; Zhang, Q.; Liu, J.; He, C.; Lin, Z. Crafting MoC2-doped bimetallic alloy nanoparticles encapsulated within N-doped graphene as roust bifunctional electrocatalysts for overall water splitting. Nano Energy 2018, 50, 212–219. [Google Scholar] [CrossRef]
- Bu, F.; Chen, W.; Aboud, M.F.A.; Shakir, I.; Gu, J.; Xu, Y. Microwave-assisted ultrafast synthesis of adjustable bimetal phosphide/graphene heterostructures from MOFs for efficient electrochemical water splitting. J. Mater. Chem. A 2019, 7, 14526–14535. [Google Scholar] [CrossRef]
- Jiao, L.; Zhou, Y.-X.; Jiang, H.-L. Metal–organic framework-based CoP/reduced graphene oxide: High-performance bifunctional electrocatalyst for overall water splitting. Chem. Sci. 2016, 7, 1690–1695. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Lin, S.; Yu, Y.; Meng, F.; Du, G.; Xu, B. In-situ phosphating Co@Nitrogen-doping graphene boosts overall water splitting under alkaline condition. J. Electroanal. Chem. 2022, 904, 115882. [Google Scholar] [CrossRef]
- Ullah, N.; Xie, M.; Chen, L.; Yaseen, W.; Zhao, W.; Yang, S.; Xu, Y.; Xie, J. Novel 3D graphene ornamented with CoO nanoparticles as an efficient bifunctional electrocatalyst for oxygen and hydrogen evolution reactions. Mater. Chem. Phys. 2021, 261, 124237. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Ola, O.; Zhao, J.; Yang, Z.; Tiwari, S.K.; Wang, N.; Zhu, Y. Recent Progress in Graphene-Based Electrocatalysts for Hydrogen Evolution Reaction. Nanomaterials 2022, 12, 1806. https://doi.org/10.3390/nano12111806
Qin X, Ola O, Zhao J, Yang Z, Tiwari SK, Wang N, Zhu Y. Recent Progress in Graphene-Based Electrocatalysts for Hydrogen Evolution Reaction. Nanomaterials. 2022; 12(11):1806. https://doi.org/10.3390/nano12111806
Chicago/Turabian StyleQin, Xupeng, Oluwafunmilola Ola, Jianyong Zhao, Zanhe Yang, Santosh K. Tiwari, Nannan Wang, and Yanqiu Zhu. 2022. "Recent Progress in Graphene-Based Electrocatalysts for Hydrogen Evolution Reaction" Nanomaterials 12, no. 11: 1806. https://doi.org/10.3390/nano12111806







