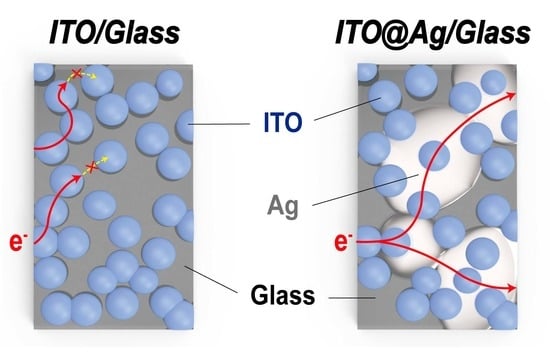
Electrically Tunable Solution-Processed Transparent Conductive Thin Films Based on Colloidally Dispersed ITO@Ag Composite Ink
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of ITO@Ag Colloidal Sol
2.3. ITO@Ag Transparent Electrode Fabrication
2.4. Characterization
3. Results and Discussion
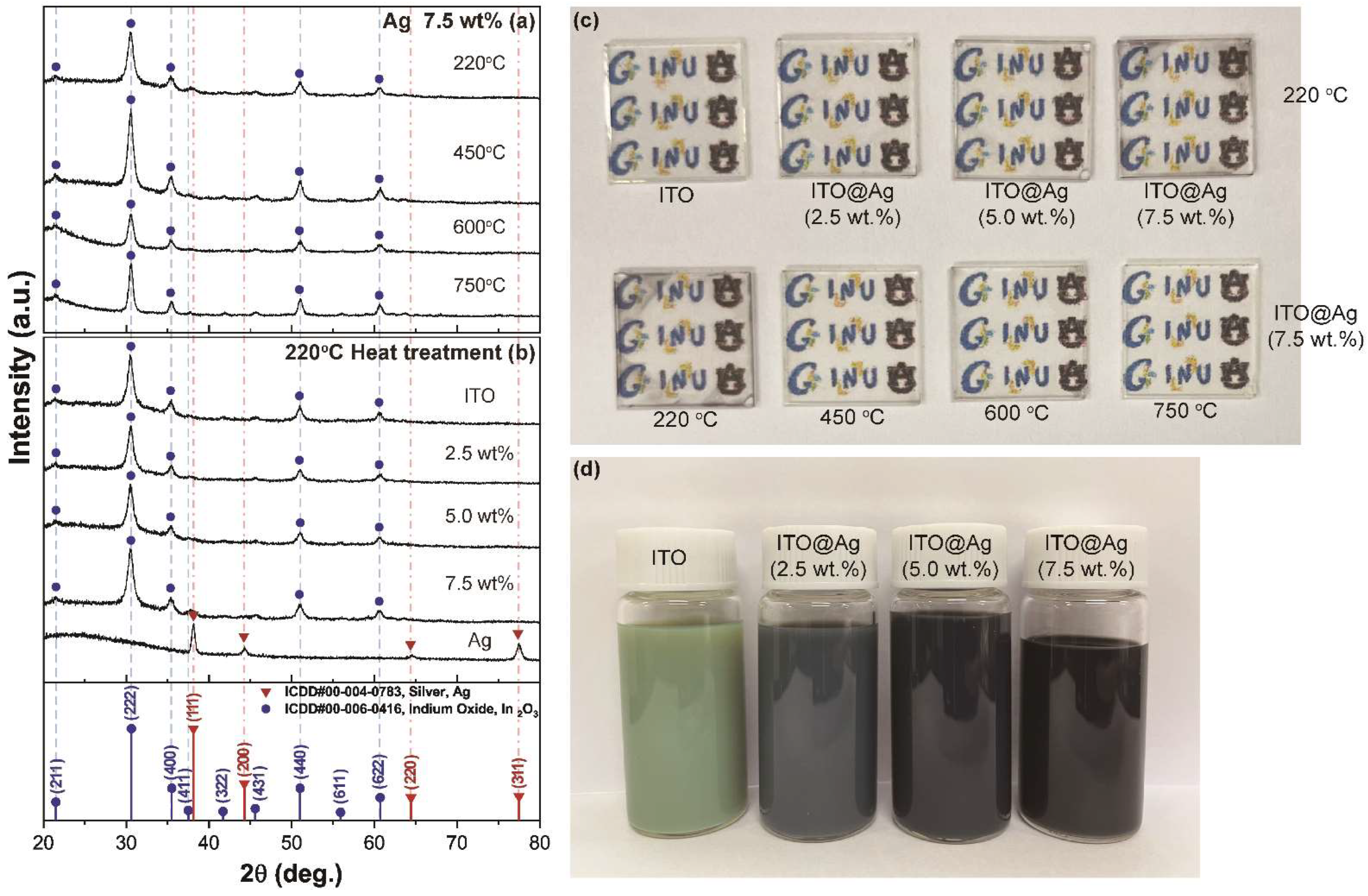
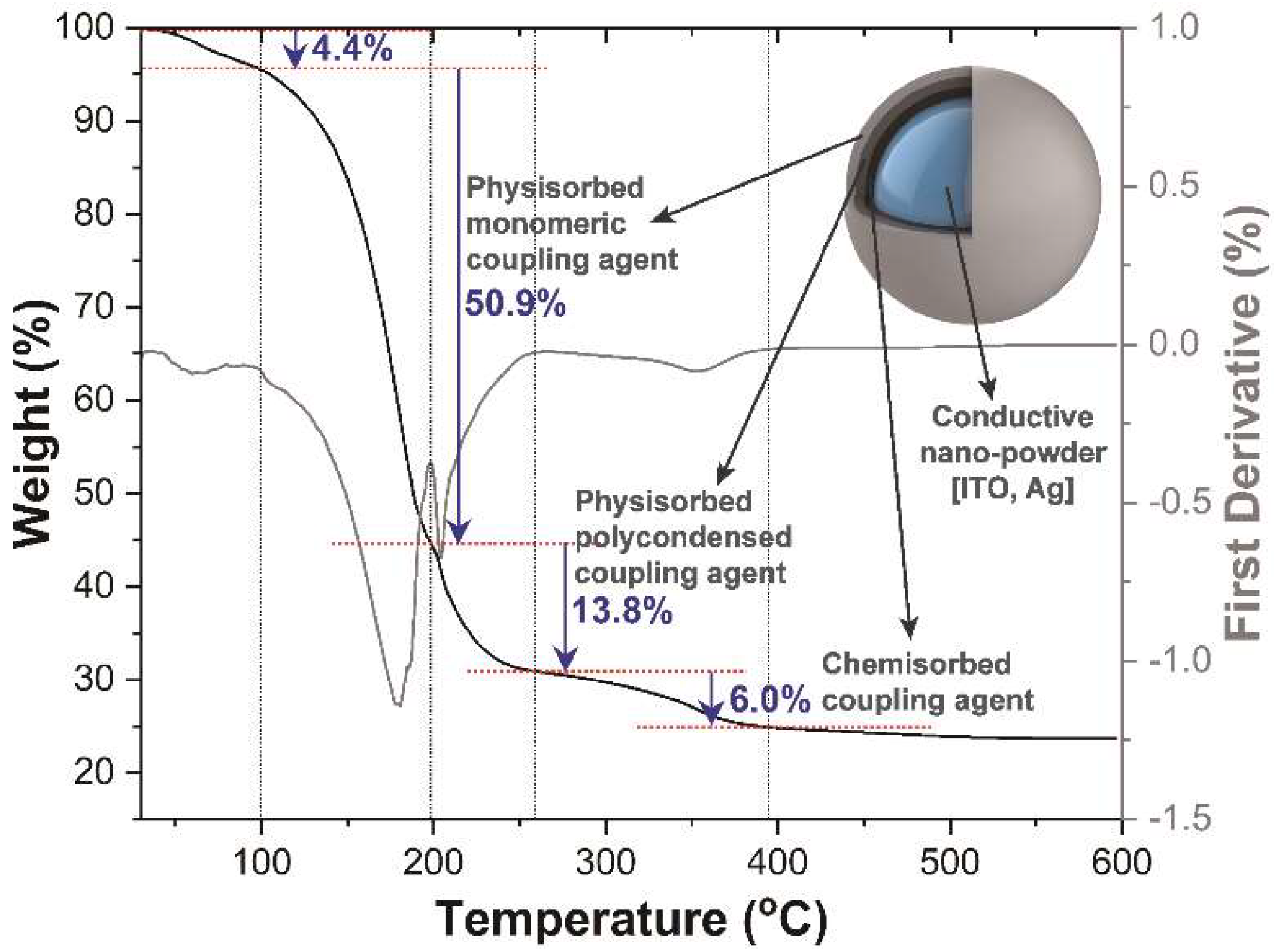
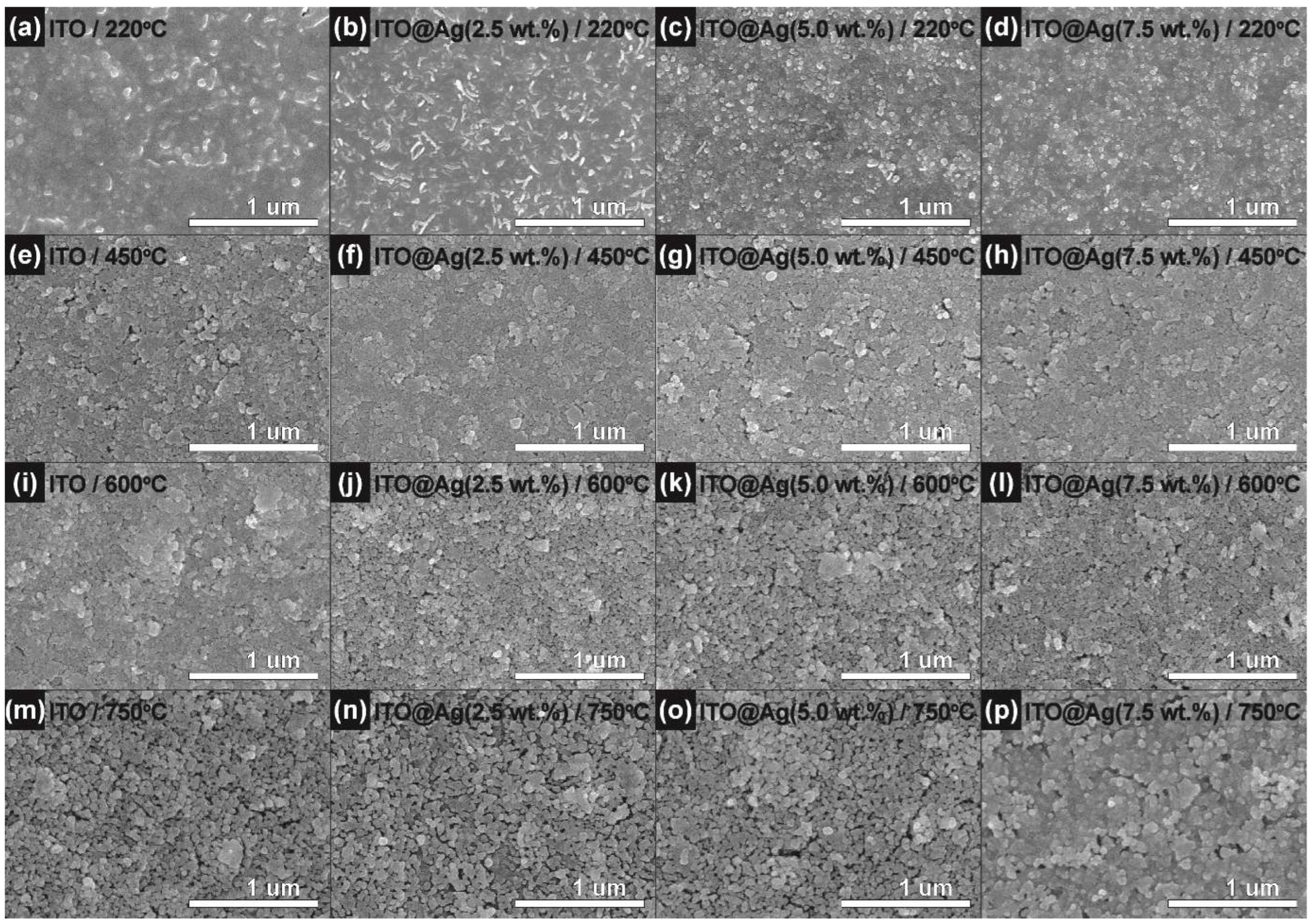
3.1. Structural, Morphological, and Compositional Properties of ITO@Ag Thin Film
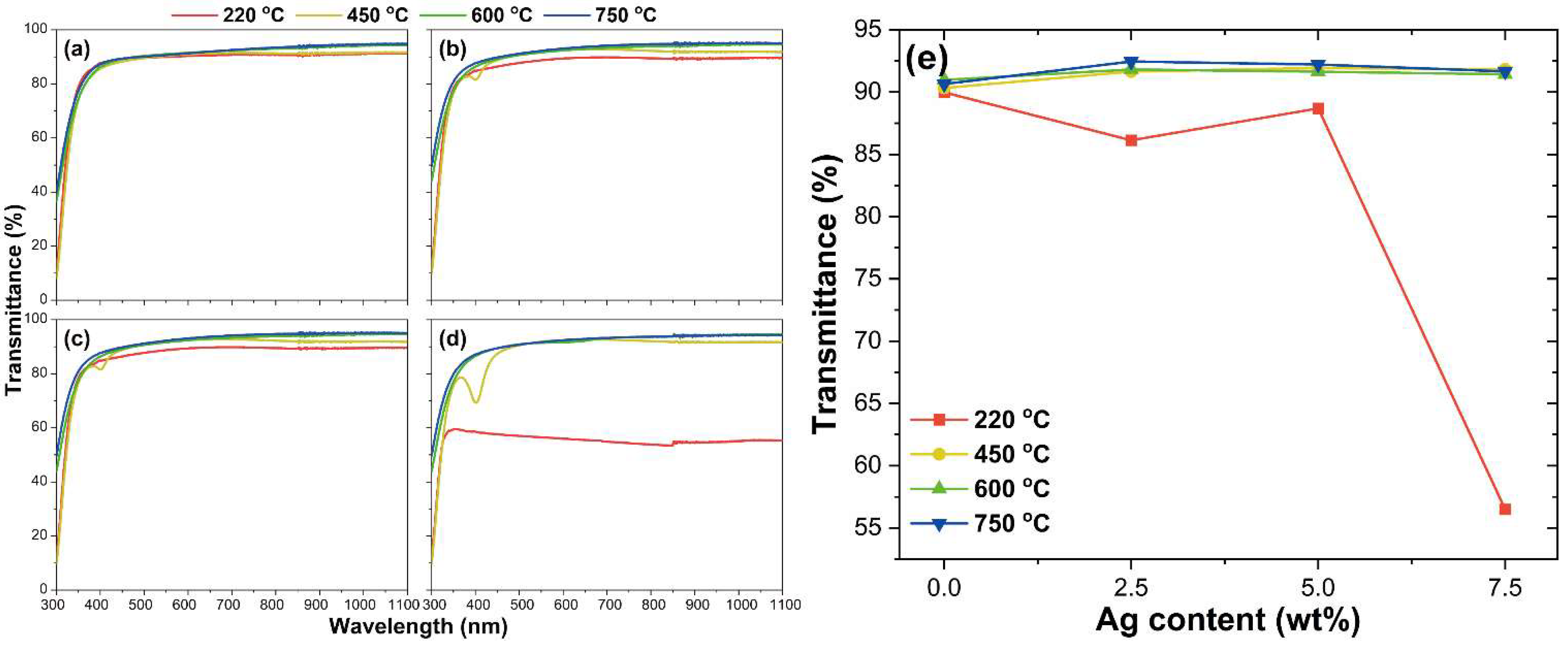
3.2. Optical Properties of ITO@Ag Thin Film
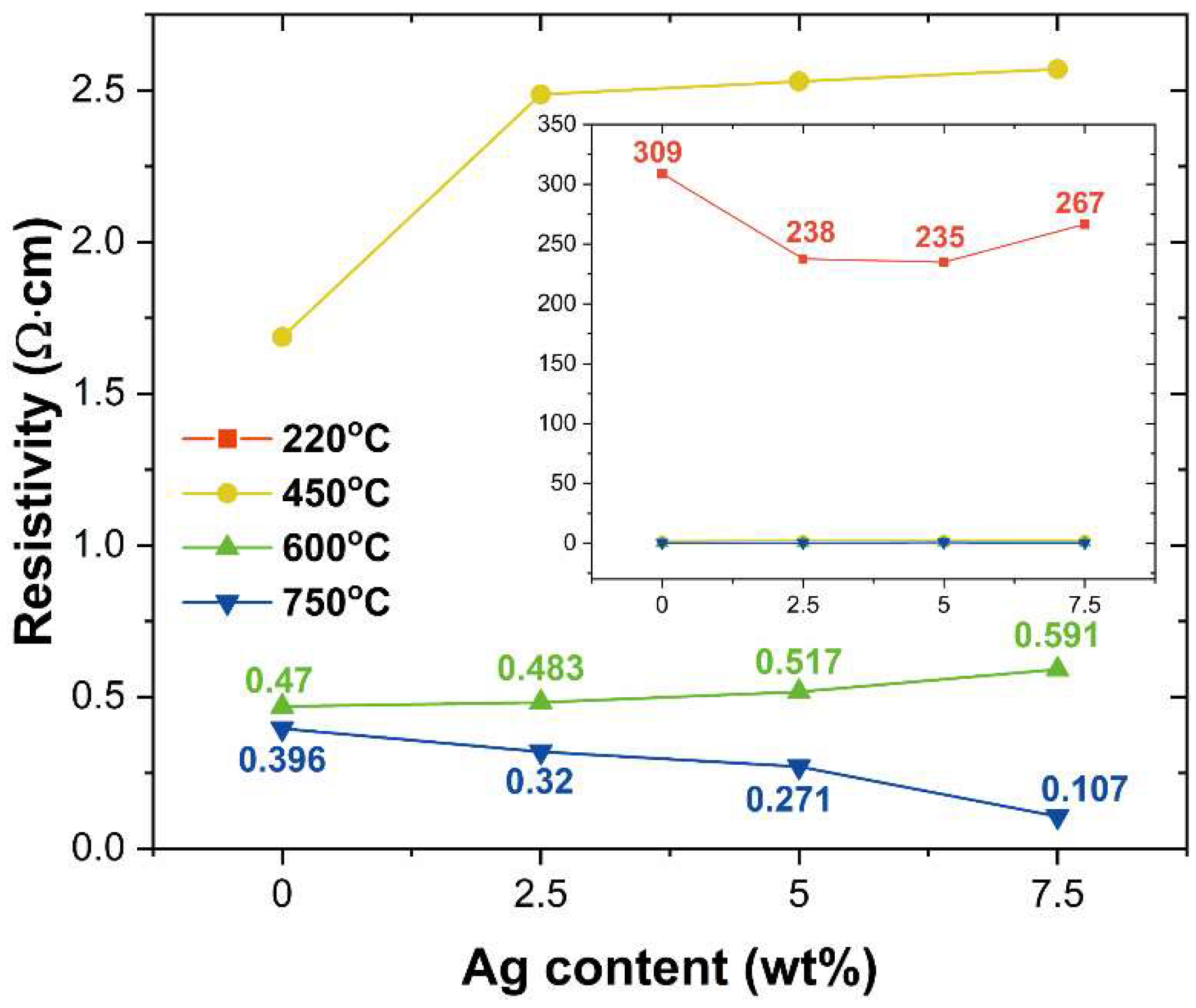
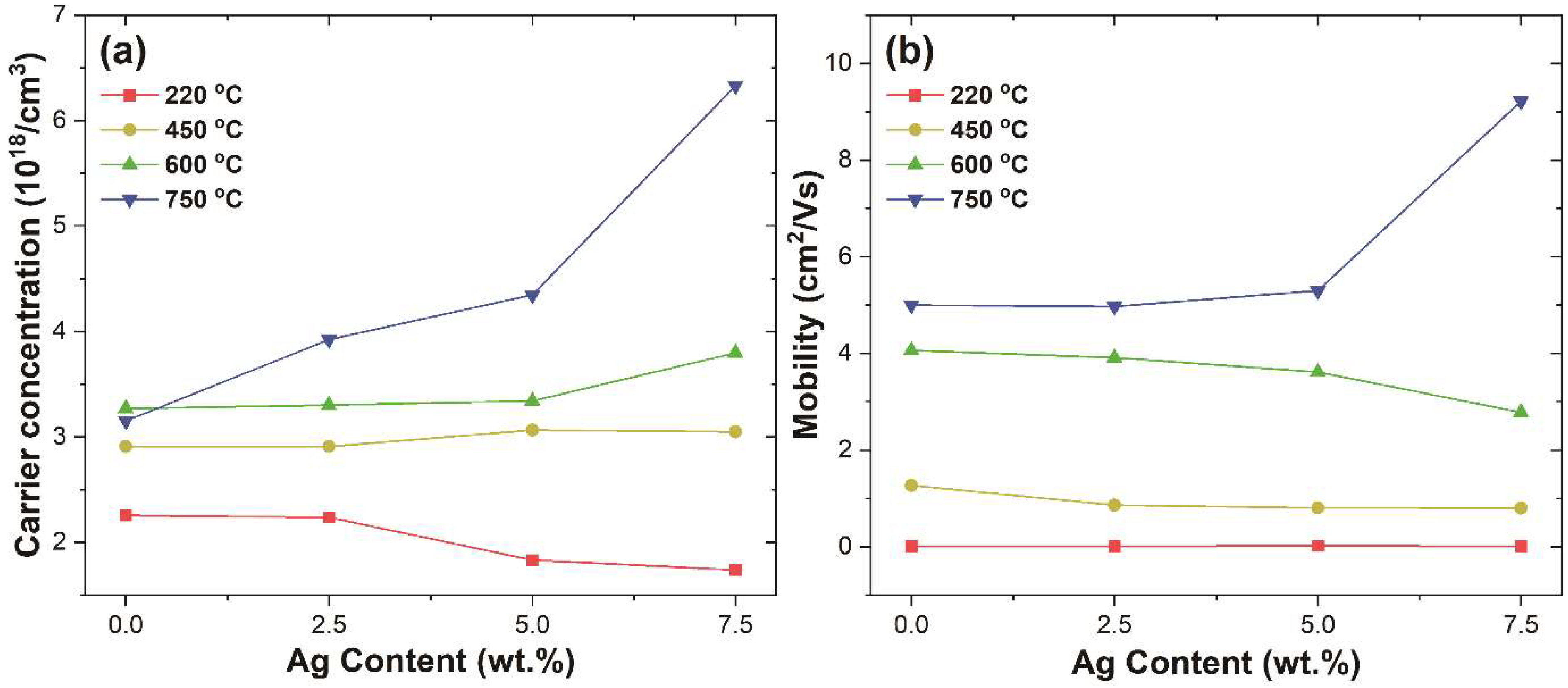
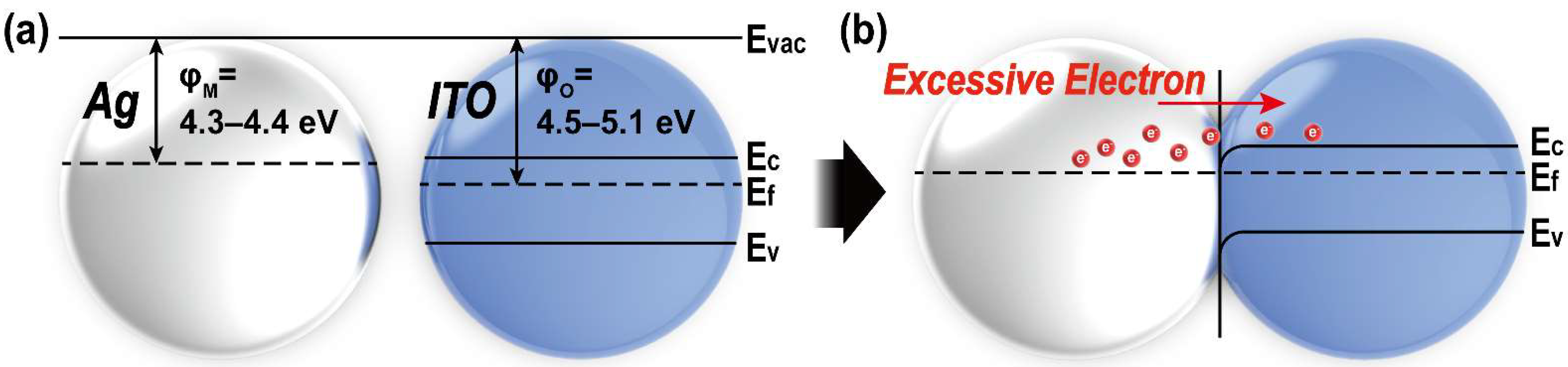
3.3. Electrical Properties of ITO@Ag Thin Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ginley, D.S.; Bright, C. Transparent Conducting Oxides. MRS Bull. 2000, 25, 15–18. [Google Scholar] [CrossRef]
- Ellmer, K. Past achievements and future challenges in the development of optically transparent electrodes. Nat. Photonics 2012, 6, 809–817. [Google Scholar] [CrossRef]
- Granqvist, C.G. Transparent conductors as solar energy materials: A panoramic review. Sol. Energy Mater. Sol. Cells 2007, 91, 1529–1598. [Google Scholar] [CrossRef]
- Kim, B.H.; Staller, C.M.; Cho, S.H.; Heo, S.; Garrison, C.E.; Kim, J.; Milliron, D.J. High Mobility in Nanocrystal-Based Transparent Conducting Oxide Thin Films. ACS Nano 2018, 12, 3200–3208. [Google Scholar] [CrossRef] [PubMed]
- Runnerstrom, E.L.; Llordés, A.; Lounis, S.D.; Milliron, D.J. Nanostructured electrochromic smart windows: Traditional materials and NIR-selective plasmonic nanocrystals. Chem. Commun. 2014, 50, 10555–10572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llordés, A.; Garcia, G.; Gazquez, J.; Milliron, D.J. Tunable near-infrared and visible-light transmittance in nanocrystal-in-glass composites. Nature 2013, 500, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, J.; Wang, Z.; Gao, W.; An, Q.; Zhang, M.; Ma, X.; Wang, J.; Miao, J.; Yang, C.; et al. Semitransparent ternary nonfullerene polymer solar cells exhibiting 9.40% efficiency and 24.6% average visible transmittance. Nano Energy 2019, 55, 424–432. [Google Scholar] [CrossRef]
- Xu, C.; Jin, K.; Xiao, Z.; Zhao, Z.; Ma, X.; Wang, X.; Li, J.; Xu, W.; Zhang, S.; Ding, L.; et al. Wide Bandgap Polymer with Narrow Photon Harvesting in Visible Light Range Enables Efficient Semitransparent Organic Photovoltaics. Adv. Funct. Mater. 2021, 31, 2107934. [Google Scholar] [CrossRef]
- Xu, C.; Jin, K.; Xiao, Z.; Zhao, Z.; Yan, Y.; Zhu, X.; Li, X.; Zhou, Z.; Jeong, S.Y.; Ding, L.; et al. Efficient Semitransparent Layer-by-Layer Organic Photovoltaics via Optimizing Wide Bandgap and Narrow Absorption Polymer Layer Thickness. Solar RRL 2022, 2200308. [Google Scholar] [CrossRef]
- Kotliarenko, A.; Azzolini, O.; Keppel, G.; Pira, C.; Esposito, J. Investigation of a Possible Material-Saving Approach of Sputtering Techniques for Radiopharmaceutical Target Production. Appl. Sci. 2021, 11, 9219. [Google Scholar] [CrossRef]
- Chou, C.-S.; Chou, F.-C.; Kang, J.-Y. Preparation of ZnO-coated TiO2 electrodes using dip coating and their applications in dye-sensitized solar cells. Powder Technol. 2012, 215–216, 38–45. [Google Scholar] [CrossRef]
- Moon, B.-H.; Sung, Y.-M.; Han, C.-H. Titanium oxide Films Prepared by Sputtering, Sol Gel and Dip Coating Methods for Photovoltaic Application. Energy Procedia 2013, 34, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Kymakis, E.; Stratakis, E.; Stylianakis, M.M.; Koudoumas, E.; Fotakis, C. Spin coated graphene films as the transparent electrode in organic photovoltaic devices. Thin Solid Film. 2011, 520, 1238–1241. [Google Scholar] [CrossRef]
- Kymakis, E.; Savva, K.; Stylianakis, M.M.; Fotakis, C.; Stratakis, E. Flexible Organic Photovoltaic Cells with In Situ Nonthermal Photoreduction of Spin-Coated Graphene Oxide Electrodes. Adv. Funct. Mater. 2013, 23, 2742–2749. [Google Scholar] [CrossRef]
- Kumar, S.; Kim, H.; Kim, D.K.; Iyer, S.S.K. Spin and doctor-blade coated PEDOT:PSS back electrodes in inverted organic solar cells. Sol. Energy 2020, 204, 64–70. [Google Scholar] [CrossRef]
- Ji, G.; Wang, Y.; Luo, Q.; Han, K.; Xie, M.; Zhang, L.; Wu, N.; Lin, J.; Xiao, S.; Li, L.-Q.; et al. Fully Coated Semitransparent Organic Solar Cells with a Doctor-Blade-Coated Composite Anode Buffer Layer of Phosphomolybdic Acid and PEDOT:PSS and a Spray-Coated Silver Nanowire Top Electrode. ACS Appl. Mater. Interfaces 2018, 10, 943–954. [Google Scholar] [CrossRef]
- Khim, D.; Han, H.; Baeg, K.J.; Kim, J.; Kwak, S.W.; Kim, D.Y.; Noh, Y.Y. Simple Bar-Coating Process for Large-Area, High-Performance Organic Field-Effect Transistors and Ambipolar Complementary Integrated Circuits. Adv. Mater. 2013, 25, 4302–4308. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Q.; Payne, G.F.; Sun, R.; Wang, X. A highly conductive, pliable and foldable Cu/cellulose paper electrode enabled by controlled deposition of copper nanoparticles. Nanoscale 2019, 11, 725–732. [Google Scholar] [CrossRef]
- Chaturvedi, N.; Gasparini, N.; Corzo, D.; Bertrandie, J.; Wehbe, N.; Troughton, J.; Baran, D. All Slot-Die Coated Non-Fullerene Organic Solar Cells with PCE 11%. Adv. Funct. Mater. 2021, 31, 2009996. [Google Scholar] [CrossRef]
- Krebs, F.C. Polymer solar cell modules prepared using roll-to-roll methods: Knife-over-edge coating, slot-die coating and screen printing. Sol. Energy Mater. Sol. Cells 2009, 93, 465–475. [Google Scholar] [CrossRef]
- Joshi, S.M.; Gerhardt, R.A. Effect of annealing atmosphere (Ar vs. air) and temperature on the electrical and optical properties of spin-coated colloidal indium tin oxide films. J. Mater. Sci. 2013, 48, 1465–1473. [Google Scholar] [CrossRef]
- Choi, S.I.; Nam, K.M.; Park, B.K.; Seo, W.S.; Park, J.T. Preparation and Optical Properties of Colloidal, Monodisperse, and Highly Crystalline ITO Nanoparticles. Chem. Mater. 2008, 20, 2609–2611. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Li, G.; Petruska, M.A.; Paine, D.C.; Sun, S. A Facile Solution-Phase Approach to Transparent and Conducting ITO Nanocrystal Assemblies. J. Am. Chem. Soc. 2012, 134, 13410–13414. [Google Scholar] [CrossRef]
- Kőrösi, L.; Scarpellini, A.; Petrik, P.; Papp, S.; Dékány, I. Sol–gel synthesis of nanostructured indium tin oxide with controlled morphology and porosity. Appl. Surf. Sci. 2014, 320, 725–731. [Google Scholar] [CrossRef]
- Crockett, B.M.; Jansons, A.W.; Koskela, K.M.; Sharps, M.C.; Johnson, D.W.; Hutchison, J.E. Influence of Nanocrystal Size on the Optoelectronic Properties of Thin, Solution-Cast Sn-Doped In2O3 Films. Chem. Mater. 2019, 31, 3370–3380. [Google Scholar] [CrossRef]
- Kruis, F.E.; Kusters, K.A.; Pratsinis, S.E.; Scarlett, B. A Simple Model for the Evolution of the Characteristics of Aggregate Particles Undergoing Coagulation and Sintering. Aerosol Sci. Technol. 1993, 19, 514–526. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.S.; Lu, G.Q.; Whittaker, A.K.; Millar, G.J.; Zhu, H.Y. Comprehensive Study of Surface Chemistry of MCM-41 Using 29Si CP/MAS NMR, FTIR, Pyridine-TPD, and TGA. J. Phys. Chem. B 1997, 101, 6525–6531. [Google Scholar] [CrossRef]
- Yamazaki, R.; Karyu, N.; Noda, M.; Fujii, S.; Nakamura, Y. Quantitative measurement of physisorbed silane on a silica particle surface treated with silane coupling agents by thermogravimetric analysis. J. Appl. Polym. Sci. 2016, 133, 43256. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. A review of current coupling agents for modification of metal oxide nanoparticles. Prog. Org. Coat. 2015, 86, 194–207. [Google Scholar] [CrossRef]
- Šetka, M.; Calavia, R.; Vojkůvka, L.; Llobet, E.; Drbohlavova, J.; Vallejos, S. Raman and XPS studies of ammonia sensitive polypyrrole nanorods and nanoparticles. Sci. Rep. 2019, 9, 8465. [Google Scholar] [CrossRef]
- Gao, Z.; Yang, H.; Fu, X.; Jin, Q.; Wu, Q.; Kang, L.; Wu, J. Efficient photoreduction of Cr(VI) on TiO2/functionalized activated carbon (TiO2/AC-AEMP): Improved adsorption of Cr(VI) and induced transfer of electrons. Environ. Sci. Pollut. Res. 2020, 27, 17446–17457. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, H.; Nishimura, T.; Ayame, A. Supported silver catalysts for the oxidation of ethylene: The effects of thermal treatment of alumina as catalyst support. J. Catal. 1979, 57, 372–379. [Google Scholar] [CrossRef]
- You, X.; Chen, F.; Zhang, J.; Anpo, M. A novel deposition precipitation method for preparation of Ag-loaded titanium dioxide. Catal. Lett. 2005, 102, 247–250. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Z.; Ueno, K.; Tatara, R.; Dokko, K.; Watanabe, M. One-step, template-free synthesis of highly porous nitrogen/sulfur-codoped carbons from a single protic salt and their application to CO2 capture. J. Mater. Chem. A 2015, 3, 17849–17857. [Google Scholar] [CrossRef]
- Yang, H.; Tian, J.; Li, T.; Cui, H. Synthesis of novel Ag/Ag2O heterostructures with solar full spectrum (UV, visible and near-infrared) light-driven photocatalytic activity and enhanced photoelectrochemical performance. Catal. Commun. 2016, 87, 82–85. [Google Scholar] [CrossRef]
- Shahcheraghi, S.H.; Khayati, G.R. Kinetics analysis of non-isothermal decomposition of Ag2O-graphite mixture. Trans. Nonferrous Met. Soc. China 2014, 24, 2991–3000. [Google Scholar] [CrossRef]
- Tom, T.; Ros, E.; López-Pintó, N.; Miguel Asensi, J.; Andreu, J.; Bertomeu, J.; Puigdollers, J.; Voz, C. Influence of Co-Sputtered Ag:Al Ultra-Thin Layers in Transparent V2O5/Ag:Al/AZO Hole-Selective Electrodes for Silicon Solar Cells. Materials 2020, 13, 4905. [Google Scholar] [CrossRef]
- Xu, W.-F.; Chin, C.C.; Hung, D.W.; Wei, P.K. Transparent electrode for organic solar cells using multilayer structures with nanoporous silver film. Sol. Energy Mater. Sol. Cells 2013, 118, 81–89. [Google Scholar] [CrossRef]
- Irfan, M.; Polonskyi, O.; Hinz, A.; Mollea, C.; Bosco, F.; Strunskus, T.; Balagna, C.; Perero, S.; Faupel, F.; Ferraris, M. Antibacterial, highly hydrophobic and semi transparent Ag/plasma polymer nanocomposite coating on cotton fabric obtained by plasma based co-deposition. Cellulose 2019, 26, 8877–8894. [Google Scholar] [CrossRef]
- Wang, T.; Daiber, B.; Frost, J.M.; Mann, S.A.; Garnett, E.C.; Walsh, A.; Ehrler, B. Indirect to direct bandgap transition in methylammonium lead halide perovskite. Energy Environ. Sci. 2017, 10, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Viezbicke, B.D.; Patel, S.; Davis, B.E.; Birnie III, D.P. Evaluation of the Tauc method for optical absorption edge determination: ZnO thin films as a model system. Phys. Status Solidi (B) 2015, 252, 1700–1710. [Google Scholar] [CrossRef]
- Hamberg, I.; Granqvist, C.G.; Berggren, K.F.; Sernelius, B.E.; Engström, L. Band-gap widening in heavily Sn-doped In2O3. Phys. Rev. B 1984, 30, 3240–3249. [Google Scholar] [CrossRef] [Green Version]
- Shinde, S.K.; Dubal, D.P.; Ghodake, G.S.; Fulari, V.J. Morphological modulation of Mn:CdSe thin film and its enhanced electrochemical properties. J. Electroanal. Chem. 2014, 727, 179–183. [Google Scholar] [CrossRef]
- Nie, J.C.; Yang, J.Y.; Piao, Y.; Li, H.; Sun, Y.; Xue, Q.M.; Xiong, C.M.; Dou, R.F.; Tu, Q.Y. Quantum confinement effect in ZnO thin films grown by pulsed laser deposition. Appl. Phys. Lett. 2008, 93, 173104. [Google Scholar] [CrossRef]
- Gâlcă, A.C.; Secu, M.; Vlad, A.; Pedarnig, J.D. Optical properties of zinc oxide thin films doped with aluminum and lithium. Thin Solid Film. 2010, 518, 4603–4606. [Google Scholar] [CrossRef]
- Dong, B.Z.; Fang, G.J.; Wang, J.F.; Guan, W.J.; Zhao, X.Z. Effect of thickness on structural, electrical, and optical properties of ZnO: Al films deposited by pulsed laser deposition. J. Appl. Phys. 2007, 101, 033713. [Google Scholar] [CrossRef]
- Lu, J.G.; Fujita, S.; Kawaharamura, T.; Nishinaka, H.; Kamada, Y.; Ohshima, T.; Ye, Z.Z.; Zheng, Y.J.; Zhang, L.P. Carrier concentration dependence of band gap shift in n-type ZnO:Al films. J. Appl. Phys. 2007, 101, 083705. [Google Scholar] [CrossRef]
- Barik, U.; Aryasomayajula, S. Electrical and Optical Properties of Silver Oxide (Ag2O) Thin Films Prepared by Reactive Electron Beam Evaporation. In Proceedings-Spie The International Society For Optical Engineering, New Delhi, India, 11–15 December 2001; Volume 2, pp. 1271–1274. [Google Scholar]
- Mayadas, A.; Shatzkes, M.; Janak, J. Electrical resistivity model for polycrystalline films: The case of specular reflection at external surfaces. Appl. Phys. Lett. 1969, 14, 345–347. [Google Scholar] [CrossRef]
- Yang, X.; Gao, P.; Yang, Z.; Zhu, J.; Huang, F.; Ye, J. Optimizing ultrathin Ag films for high performance oxide-metal-oxide flexible transparent electrodes through surface energy modulation and template-stripping procedures. Sci. Rep. 2017, 7, 44576. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Diao, X.; Wang, C.; Hao, W.; Wang, T. Effects of Heat Treatment on Properties of ITO Films Prepared by RF Magnetron Sputtering. Vacuum 2004, 75, 183–188. [Google Scholar] [CrossRef]
- Simo, A.; Polte, J.; Pfander, N.; Vainio, U.; Emmerling, F.; Rademann, K. Formation Mechanism of Silver Nanoparticles Stabilized in Glassy Matrices. J. Am. Chem. Soc. 2012, 134, 18824–18833. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, G.I.N.; Bowmaker, G.A.; Metson, J.B. The thermal decomposition of silver (I, III) oxide: A combined XRD, FT-IR and Raman spectroscopic study. Phys. Chem. Chem. Phys. 2001, 3, 3838–3845. [Google Scholar] [CrossRef]
- Klöppel, A.; Kriegseis, W.; Meyer, B.K.; Scharmann, A.; Daube, C.; Stollenwerk, J.; Trube, J. Dependence of the electrical and optical behaviour of ITO–silver–ITO multilayers on the silver properties. Thin Solid Film. 2000, 365, 139–146. [Google Scholar] [CrossRef]
- Sugiyama, K.; Ishii, H.; Ouchi, Y.; Seki, K. Dependence of indium–tin–oxide work function on surface cleaning method as studied by ultraviolet and x-ray photoemission spectroscopies. J. Appl. Phys. 1999, 87, 295–298. [Google Scholar] [CrossRef]
- Uda, M.; Nakamura, A.; Yamamoto, T.; Fujimoto, Y. Work function of polycrystalline Ag, Au and Al. J. Electron Spectrosc. Relat. Phenom. 1998, 88–91, 643–648. [Google Scholar] [CrossRef]
- Haacke, G. New figure of merit for transparent conductors. J. Appl. Phys. 1976, 47, 4086–4089. [Google Scholar] [CrossRef]
- Cho, Y.S.; Yi, G.R.; Hong, J.J.; Jang, S.H.; Yang, S.M. Colloidal indium tin oxide nanoparticles for transparent and conductive films. Thin Solid Film. 2006, 515, 1864–1871. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kim, H.M.; Hong, J.J.; Yi, G.R.; Jang, S.H.; Yang, S.M. Dispersion stabilization of conductive transparent oxide nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2009, 336, 88–98. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Gauvin, M.; Decorde, N.; Delpech, F.; Fazzini, P.F.; Viallet, B.; Viau, G.; Grisolia, J.; Ressier, L. A transparent flexible z-axis sensitive multi-touch panel based on colloidal ITO nanocrystals. Nanoscale 2015, 7, 12631–12640. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, H.; Yang, S.; Fan, L. Transparent and conductive indium tin oxide/polyimide films prepared by high-temperature radio-frequency magnetron sputtering. J. Appl. Polym. Sci. 2015, 132, 42753. [Google Scholar] [CrossRef]
- Amalathas, A.P.; Alkaisi, M.M. Effects of film thickness and sputtering power on properties of ITO thin films deposited by RF magnetron sputtering without oxygen. J. Mater. Sci. Mater. Electron. 2016, 27, 11064–11071. [Google Scholar] [CrossRef]
- Dong, L.; Zhu, G.S.; Xu, H.R.; Jiang, X.P.; Zhang, X.Y.; Zhao, Y.Y.; Yan, D.L.; Yu, A.B. Preparation of indium tin oxide (ITO) thin film with (400) preferred orientation by sol–gel spin coating method. J. Mater. Sci. Mater. Electron. 2019, 30, 8047–8054. [Google Scholar] [CrossRef]
- Gan, Y.; Liu, J.; Zeng, S. Transparent conductive indium tin oxide film fabricated by dip-coating technique from colloid precursor. Surf. Coat. Technol. 2006, 201, 25–29. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, Y.L.; Jo, J.-H.; Kim, D.-J.; Kim, S.H. Electrically Tunable Solution-Processed Transparent Conductive Thin Films Based on Colloidally Dispersed ITO@Ag Composite Ink. Nanomaterials 2022, 12, 2060. https://doi.org/10.3390/nano12122060
Cha YL, Jo J-H, Kim D-J, Kim SH. Electrically Tunable Solution-Processed Transparent Conductive Thin Films Based on Colloidally Dispersed ITO@Ag Composite Ink. Nanomaterials. 2022; 12(12):2060. https://doi.org/10.3390/nano12122060
Chicago/Turabian StyleCha, Yoo Lim, Jeong-Hye Jo, Dong-Joo Kim, and Sun Hee Kim. 2022. "Electrically Tunable Solution-Processed Transparent Conductive Thin Films Based on Colloidally Dispersed ITO@Ag Composite Ink" Nanomaterials 12, no. 12: 2060. https://doi.org/10.3390/nano12122060