Anchoring NiO Nanosheet on the Surface of CNT to Enhance the Performance of a Li-O2 Battery
Abstract
:1. Introduction
2. Experimental Section
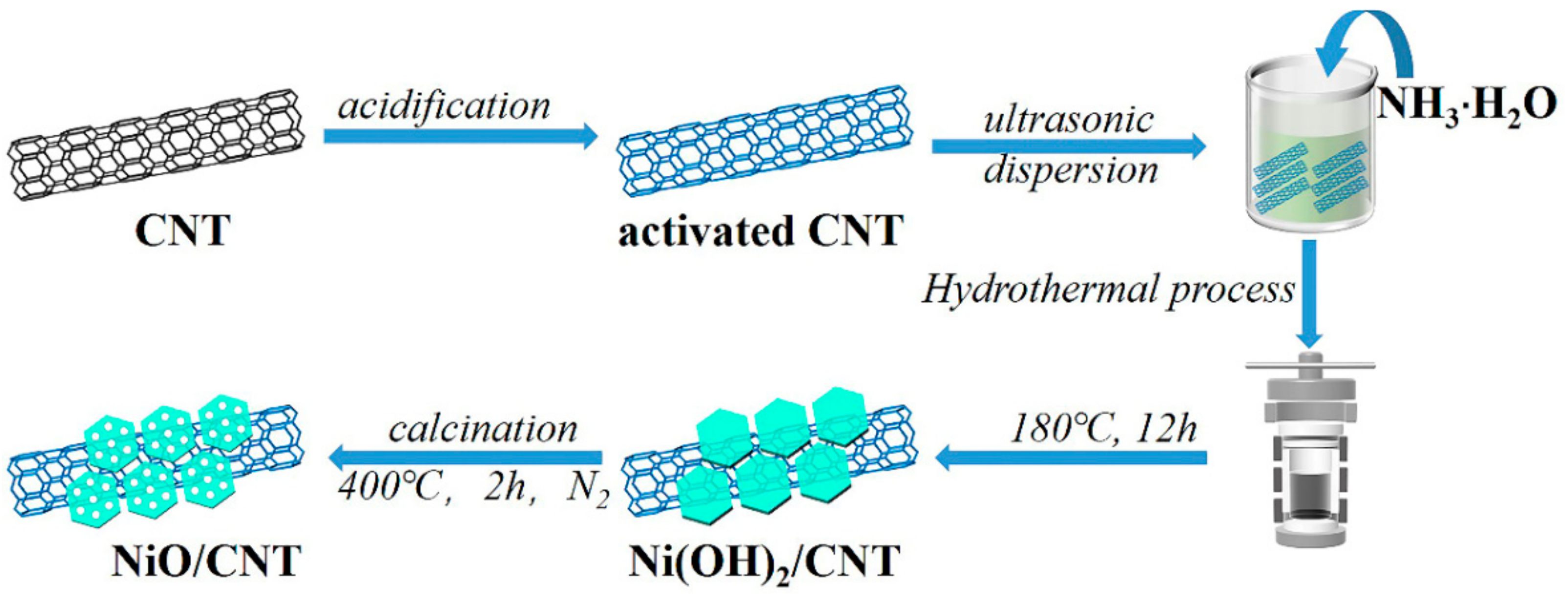
2.1. Acidification of CNT
2.2. Preparation of NiO/CNT
2.3. Material Characterization
2.4. Electrochemical Tests
3. Results and Discussion
3.1. Characterizations of Materials’ Morphology and Structure
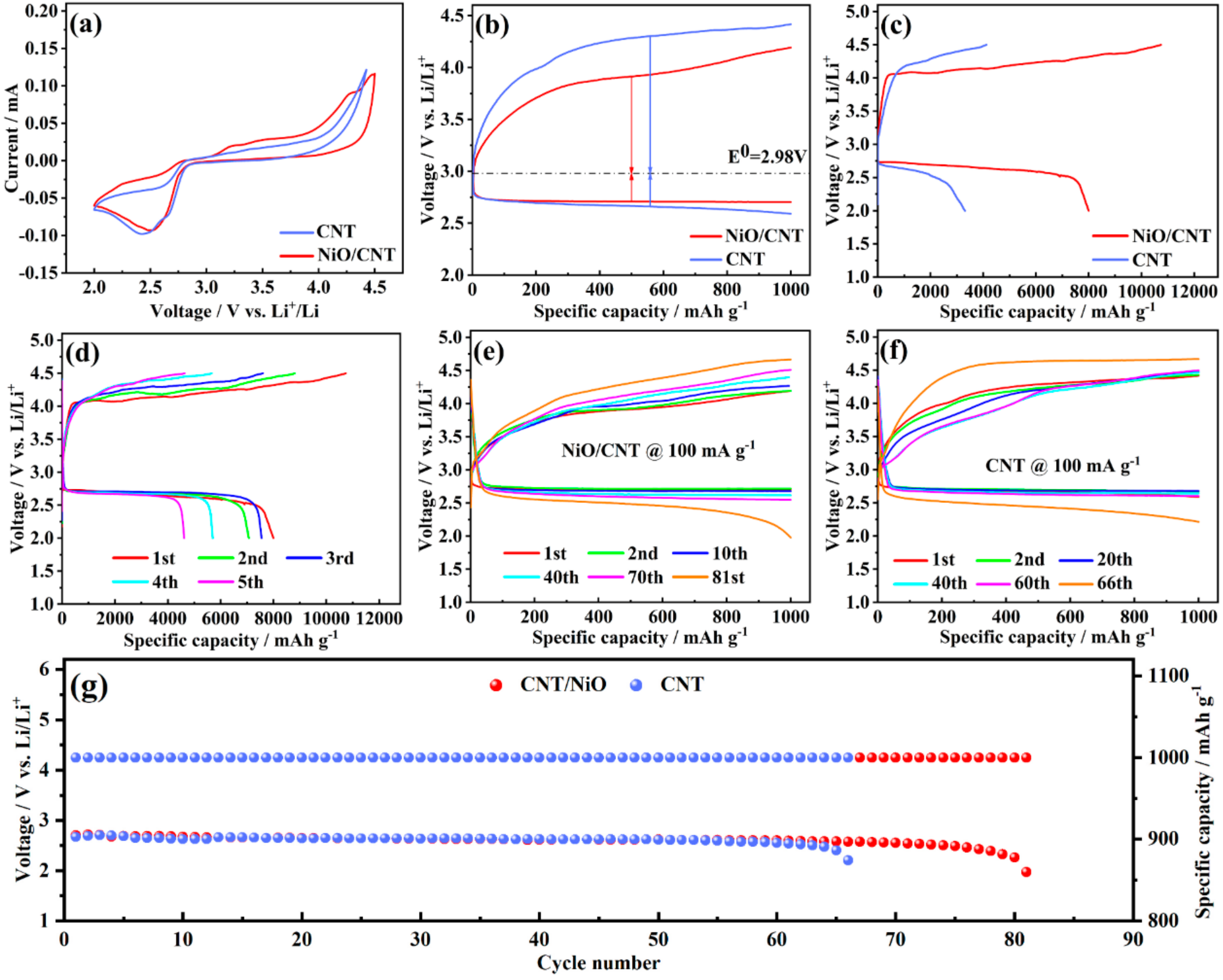
3.2. Electrochemical Performance Test
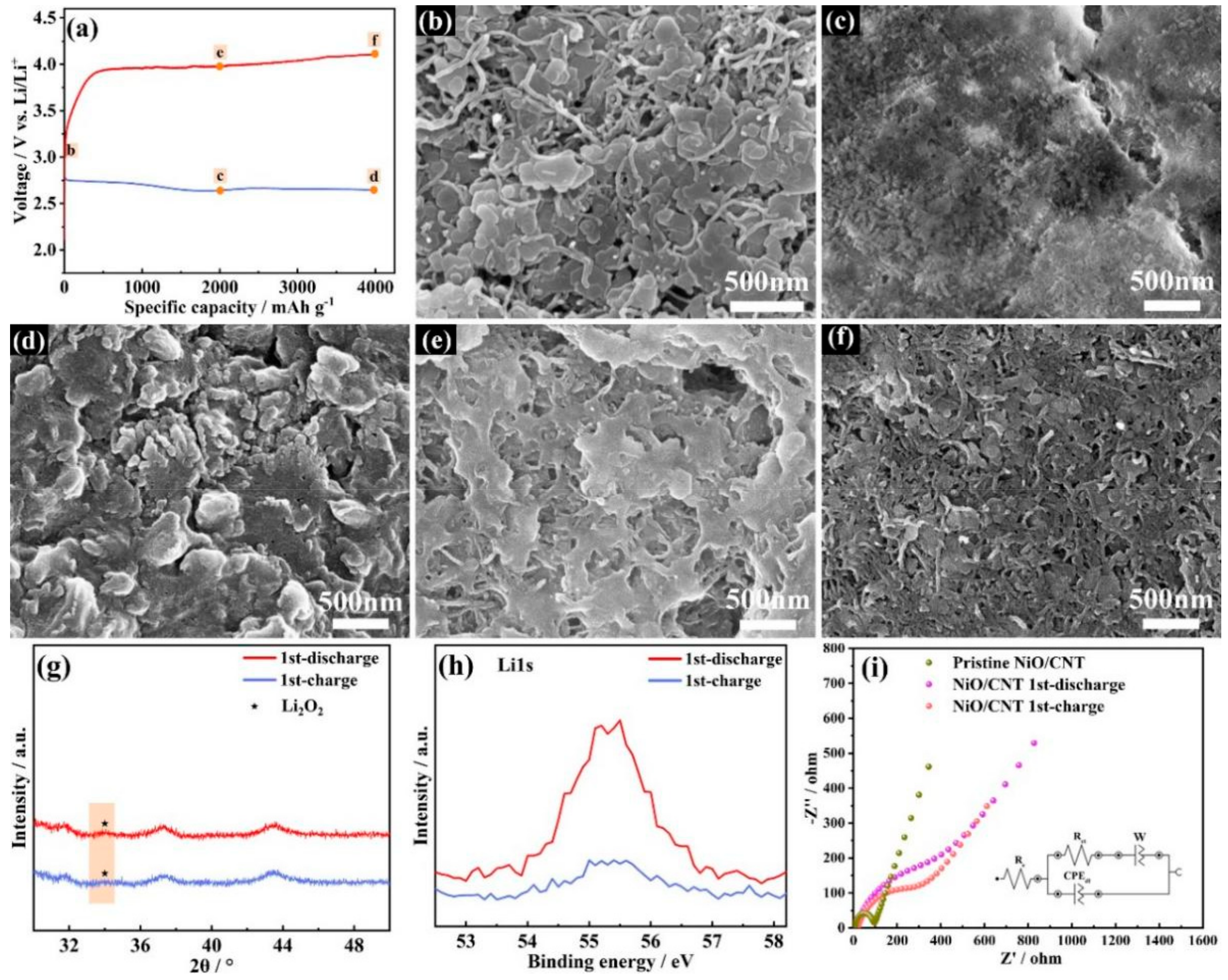
3.3. Characterizations of Discharge Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Wang, X.; Li, F.; Xu, J. Fundamental understanding and construction of solid-state Li-air batteries. Small Sci. 2022, 2, 2200005. [Google Scholar] [CrossRef]
- Kang, J.; Lee, J.; Jung, J.; Park, J.; Jang, T.; Kim, H.; Nam, J.; Lim, H.; Yoon, K.; Ryu, W.; et al. Lithium-air batteries: Air-breathing challenges and perspective. ACS Nano 2020, 14, 14549–14578. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Lin, Z.; Zhang, J.; Lei, Y.; Meng, Y.; Chen, X.; Zhao, S.; Hong, B.; Wang, J.; Li, D.; et al. A novel approach to facile synthesis of boron and nitrogen co-doped carbon and its application in lithium oxygen batteries. Energy Storage Mater. 2021, 41, 61–68. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ding, M.; Li, X.; Li, C.; Li, Z.; Yin, L. Challenges and Strategy on Parasitic Reaction for High-Performance Nonaqueous Li-O2 Batteries. Adv. Energy Mater. 2020, 10, 2001789–2001830. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y. Nonaqueous lithium–oxygen batteries: Reaction mechanism and critical open questions. Energy Storage Mater. 2020, 28, 235–246. [Google Scholar] [CrossRef]
- Wang, H.; Yin, F.; Liu, N.; Kou, R.; He, X.; Sun, C.; Chen, B.; Liu, D.; Yin, H. Engineering Fe-Fe3C@Fe-N-C active sites and hybrid structures from dual Metal-Organic Frameworks for oxygen reduction reaction in H2-O2 fuel cell and Li-O2 battery. Adv. Funct. Mater. 2019, 29, 1901531–1901541. [Google Scholar] [CrossRef]
- Adpakpang, K.; Oh, S.; Agyeman, D.; Jin, X.; Jarulertwathana, N.; Kim, I.; Sarakonsri, T.; Kang, Y.; Hwang, S. Holey 2D nanosheets of low-valent manganese oxides with an excellent oxygen catalytic activity and a high functionality as a catalyst for Li-O2 batteries. Adv. Funct. Mater. 2018, 28, 1707106–1707116. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; Jung, H.; Aurbach, D.; Sun, Y. Redox Mediators for Li-O2 batteries: Status and perspectives. Adv. Mater. 2018, 30, 1704162–1704174. [Google Scholar] [CrossRef]
- Li, D.; Liang, J.; Robertson, S.J.; Chen, Y.; Wang, N.; Shao, M.; Shi, Z. Heterogeneous bimetallic organic coordination polymer-derived Co/Fe@NC bifunctional catalysts for rechargeable Li-O2 batteries. ACS Appl. Mater. Interfaces 2022, 14, 5459–5467. [Google Scholar] [CrossRef]
- Xia, Q.; Li, D.; Zhao, L.; Wang, J.; Long, Y.; Han, X.; Zhou, Z.; Liu, Y.; Zhang, Y.; Li, Y.; et al. Recent advances in heterostructured cathodic electrocatalysts for non-aqueous Li-O2 batteries. Chem. Sci. 2022, 13, 2841–2856. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yuan, M.; Guo, D.; Wen, C.; Li, X.; Huang, X.; Li, H.; Sun, G. Theoretical design and structural modulation of as surface-functionalized Ti3C2Tx mxene-based heterojunction electrocatalyst for a Li-oxygen battery. ACS Nano 2022, 16, 4487–4499. [Google Scholar] [CrossRef]
- Dong, H.; Tang, P.; Li, X.; Wang, Y.; Wang, D.; Wang, H.; Liu, S.; Yang, C.; Wu, C. Pt/NiO microsphere composite as efficient multifunctional catalysts for nonaqueous lithium-oxygen batteries and alkaline fuel cells: The synergistic effect of Pt and Ni. ACS Appl. Mater. Interfaces 2019, 11, 39789–39797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-F.; Sheng, T.; Zhou, Y.; Wu, Y.-J.; Xiang, C.-C.; Lin, J.-X.; Li, Y.-Y.; Li, J.-T.; Huang, L.; Sun, S.-G. Li-CO2/O2 battery operating at ultra-low overpotential and low O2 content on Pt/CNT catalyst. Chem. Eng. J. 2022, 448, 137541. [Google Scholar] [CrossRef]
- Hu, X.; Luo, G.; Zhao, Q.; Wu, D.; Yang, T.; Wen, J.; Wang, R.; Xu, C.; Hu, N. Ru single atoms on N-Doped carbon by spatial confinement and ionic substitution strategies for high-performance Li-O2 batteries. J. Am. Chem. Soc. 2020, 142, 16776–16786. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wei, C.; Zheng, X.; Zeng, K.; Chen, X.; Rummeli, M.H.; Strasser, P.; Yang, R. Ru clusters anchored on Magnéli phase Ti4O7 nanofibers enables flexible and highly efficient Li–O2 batteries. Energy Storage Mater. 2022, 50, 355–364. [Google Scholar] [CrossRef]
- Song, S.; Xu, W.; Zheng, J.; Luo, L.; Engelhard, M.; Bowden, M.; Liu, B.; Wang, C.; Zhang, J. Complete decomposition of Li2CO3 in Li-O2 batteries using Ir/B4C as noncarbon-based oxygen electrode. Nano Lett. 2017, 17, 1417–1424. [Google Scholar] [CrossRef]
- Wang, G.; Tu, F.; Xie, J.; Du, G.; Zhang, S.; Cao, G.; Zhao, X. High-performance Li-O2 batteries with controlled Li2O2 growth in graphene/Au-nanoparticles/Au-nanosheets sandwich. Adv. Sci. 2016, 3, 1500339–1500345. [Google Scholar] [CrossRef]
- Zhang, Y.; Jie, M.; Yuan, M.; Li, Y.; Shen, R.; Cheong, W.C.; Han, T.; Sun, G.; Chen, C.; Nan, C. Design of Hollow PdO-Co3O4 Nano-Dodecahedrons with Moderate Catalytic Activity for Li-O2 Batteries. Chem. Commun. 2019, 55, 12683–12686. [Google Scholar] [CrossRef]
- Dai, L.; Sun, Q.; Guo, J.; Cheng, J.; Xu, X.; Guo, H.; Li, D.; Chen, L.; Si, P.; Lou, J.; et al. Mesoporous Mn2O3 rods as a highly efficient catalyst for Li-O2 battery. J. Power Sources 2019, 435, 226833–226840. [Google Scholar] [CrossRef]
- Lian, Z.; Lu, Y.; Ma, S.; Li, Z.; Liu, Q. Metal atom-doped Co3O4 nanosheets for Li-O2 battery catalyst: Study on the difference of catalytic activity. Chem. Eng. J. 2022, 445, 136852. [Google Scholar] [CrossRef]
- Li, D.; Kang, Z.; Sun, H.; Wang, Y.; Xie, H.; Liu, J.; Zhu, J. A bifunctional MnxCo3-xO4-decorated separator for efficient Li-LiI-O2 batteries: A novel strategy to promote redox coupling and inhibit redox shuttling. Chem. Eng. J. 2022, 428, 131105. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, G.; Yu, Z.; Zhou, T.; Shi, W.; Luo, C.; Zhou, H.; Chen, L.; Sheng, W.; Zhou, M.; et al. High rate and long cycle life in Li-O2 batteries with highly efficient catalytic cathode configured with Co3O4 nanoflower. Nano Energy 2019, 64, 103896–103906. [Google Scholar] [CrossRef]
- Sun, Z.; Cao, X.; Tian, M.; Zeng, K.; Jiang, Y.; Rummeli, M.; Strasser, P.; Yang, R. Synergized multimetal oxides with amorphous/crystalline heterostructure as efficient electrocatalysts for lithium–oxygen batteries. Adv. Energy Mater. 2021, 11, 2100110. [Google Scholar] [CrossRef]
- Wang, T.; Lu, L.; Sun, C. A long life solid-state lithium–oxygen battery enabled by a durable oxygen deficient flower-like CeO2 microsphere based solid electrolyte. Inorg. Chem. Front. 2022, 9, 2508–2516. [Google Scholar] [CrossRef]
- Cui, X.; Luo, Y.; Zhou, Y.; Dong, W.; Chen, W. Application of functionalized graphene in Li-O2 batteries. Nanotechnology 2021, 32, 132003–132019. [Google Scholar] [CrossRef]
- He, L.; Shi, K.; Pan, Y.; Wu, J.; Li, X.; Xin, X. Dual-functional 3D carbon fibers decorated with Co nanoparticles and Co-Nx sites for rechargeable aprotic Li–O2 batteries. New J. Chem. 2022, 46, 11570–11578. [Google Scholar] [CrossRef]
- Xiao, X.; Li, X.; Wang, J.; Yan, G.; Wang, Z.; Guo, H.; Peng, W. Three-dimensionally mesoporous dual (Co, Fe) metal oxide/CNTs composite as electrocatalysts for air cathodes in Li-O2 batteries. Ceram. Int. 2018, 44, 21942–21949. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Dong, S.; Ge, X.; Zhang, P.; Miao, X.; Zhang, Z.; Wang, C.; Yin, L. One-step route synthesized Co2P/Ru/N-doped carbon nanotube hybrids as bifunctional electrocatalysts for high-performance Li-O2 batteries. Small 2019, 15, e1900001. [Google Scholar] [CrossRef]
- Hu, A.; Long, J.; Shu, C.; Xu, C.; Yang, T.; Liang, R.; Li, J. NiCo2S4 nanorod arrays supported on carbon textile as a free-standing electrode for stable and long-life Lithium-Oxygen batteries. ChemElectroChem 2019, 6, 349–358. [Google Scholar] [CrossRef]
- Bie, S.; Du, M.; He, W.; Zhang, H.; Yu, Z.; Liu, J.; Liu, M.; Yan, W.; Zhou, L.; Zou, Z. Carbon nanotube@RuO2 as a high performance catalyst for Li-CO2 batteries. ACS Appl. Mater. Interfaces 2019, 11, 5146–5151. [Google Scholar] [CrossRef]
- Chen, H.; Ye, Y.; Chen, X.; Zhang, L.; Liu, G.; Wang, S.; Ding, L.-X. N-doped porous carbon nanofibers inlaid with hollow Co3O4 nanoparticles as an efficient bifunctional catalyst for rechargeable Li-O2 batteries. Chin. J. Catal. 2022, 43, 1511–1519. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J.; Sheng, T.; Lu, Y.; Yin, Z.; Li, Y.; Peng, X.; Zhou, Y.; Li, J.; Wu, Y.; et al. Synergetic effect of Ru and NiO in the electrocatalytic decomposition of Li2CO3 to enhance the performance of a Li-CO2/O2 battery. ACS Catal. 2020, 10, 1640–1651. [Google Scholar] [CrossRef]
- Song, K.; Ai, W.; Zhang, Y.; Zeng, Y.; Yu, Y.; Qiao, H.; Liu, Z.; Shen, X.; Hu, X.; Hu, X. Three-dimensional self-supported CuCo2O4 nanowires@NiO nanosheets core/shell arrays as an oxygen electrode catalyst for Li-O2 batteries. J. Mater. Chem. A 2021, 9, 3007–3017. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Huang, J.; Zhou, X.; Wu, Q.; Luo, Z.; Wang, F. Hierarchical mesoporous/macroporous Co-doped NiO nanosheet arrays as free-standing electrode materials for rechargeable Li-O2 batteries. ACS Appl. Mater. Interfaces 2019, 11, 44556–44565. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Dong, S.; Ge, X.; Zhang, P.; Miao, X.; Wang, R.; Zhang, Z.; Yin, L. Hierarchical NiCo2S4@NiO core-shell heterostructures as catalytic cathode for long-life Li-O2 batteries. Adv. Energy Mater. 2019, 9, 1900788–1900801. [Google Scholar] [CrossRef]
- Wang, H.; Fan, B.; Luo, Z.; Wu, Q.; Zhou, X.; Wang, F. A unique hierarchical structure: NiCo2O4 nanowire decorated NiO nanosheets as a carbon-free cathode for Li-O2 battery. Catal. Sci. Technol. 2021, 11, 7632–7639. [Google Scholar] [CrossRef]
- Zhao, G.; Li, Z.; Tong, P.; Sun, K. Preparation of NiO/multiwalled carbon nanotube nanocomposite for use as the oxygen cathode catalyst in rechargeable Li–O2 batteries. J. Solid State Electrochem. 2013, 17, 1759–1764. [Google Scholar] [CrossRef]
- Hoa, N.; El-Safty, S. Synthesis of mesoporous NiO nanosheets for the detection of toxic NO2 gas. Chem. Eur. J. 2011, 17, 12896–12901. [Google Scholar] [CrossRef]
- Price, B.; Hudson, J.; Tour, J. Green chemical functionalization of single-walled carbon nanotubes in ionic liquids. J. Am. Chem. Soc. 2005, 127, 14867–14870. [Google Scholar] [CrossRef]
- Tian, N.; Zhang, Y.; Li, X.; Xiao, K.; Du, X.; Dong, F.; Waterhouse, G.I.N.; Zhang, T.; Huang, H. Precursor-reforming protocol to 3D mesoporous g-C3N4 established by ultrathin self-doped nanosheets for superior hydrogen evolution. Nano Energy 2017, 38, 72–81. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, B.; Jiang, S.; Liu, H.; Dou, M. Construction of three-dimensional cobalt sulfide/multi-heteroatom co-doped porous carbon as an efficient trifunctional electrocatalyst. Nanoscale 2022. [Google Scholar] [CrossRef]
- Zhang, P.F.; Lu, Y.Q.; Wu, Y.J.; Yin, Z.W.; Li, J.T.; Zhou, Y.; Hong, Y.H.; Li, Y.Y.; Huang, L.; Sun, S.G. High-Performance Rechargeable Li-CO2/O2 Battery with Ru/N-Doped CNT Catalyst. Chem. Eng. J. 2019, 363, 224–233. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wang, S.; Dong, Y.; Du, H.; Zhao, J.; Zhang, P. Anchoring NiO Nanosheet on the Surface of CNT to Enhance the Performance of a Li-O2 Battery. Nanomaterials 2022, 12, 2386. https://doi.org/10.3390/nano12142386
Chen S, Wang S, Dong Y, Du H, Zhao J, Zhang P. Anchoring NiO Nanosheet on the Surface of CNT to Enhance the Performance of a Li-O2 Battery. Nanomaterials. 2022; 12(14):2386. https://doi.org/10.3390/nano12142386
Chicago/Turabian StyleChen, Shuang, Shukun Wang, Yunyun Dong, Hongmei Du, Jinsheng Zhao, and Pengfang Zhang. 2022. "Anchoring NiO Nanosheet on the Surface of CNT to Enhance the Performance of a Li-O2 Battery" Nanomaterials 12, no. 14: 2386. https://doi.org/10.3390/nano12142386







