Sputtering-Assisted Synthesis of Copper Oxide–Titanium Oxide Nanorods and Their Photoactive Performances
Abstract
:1. Introduction
2. Experiments
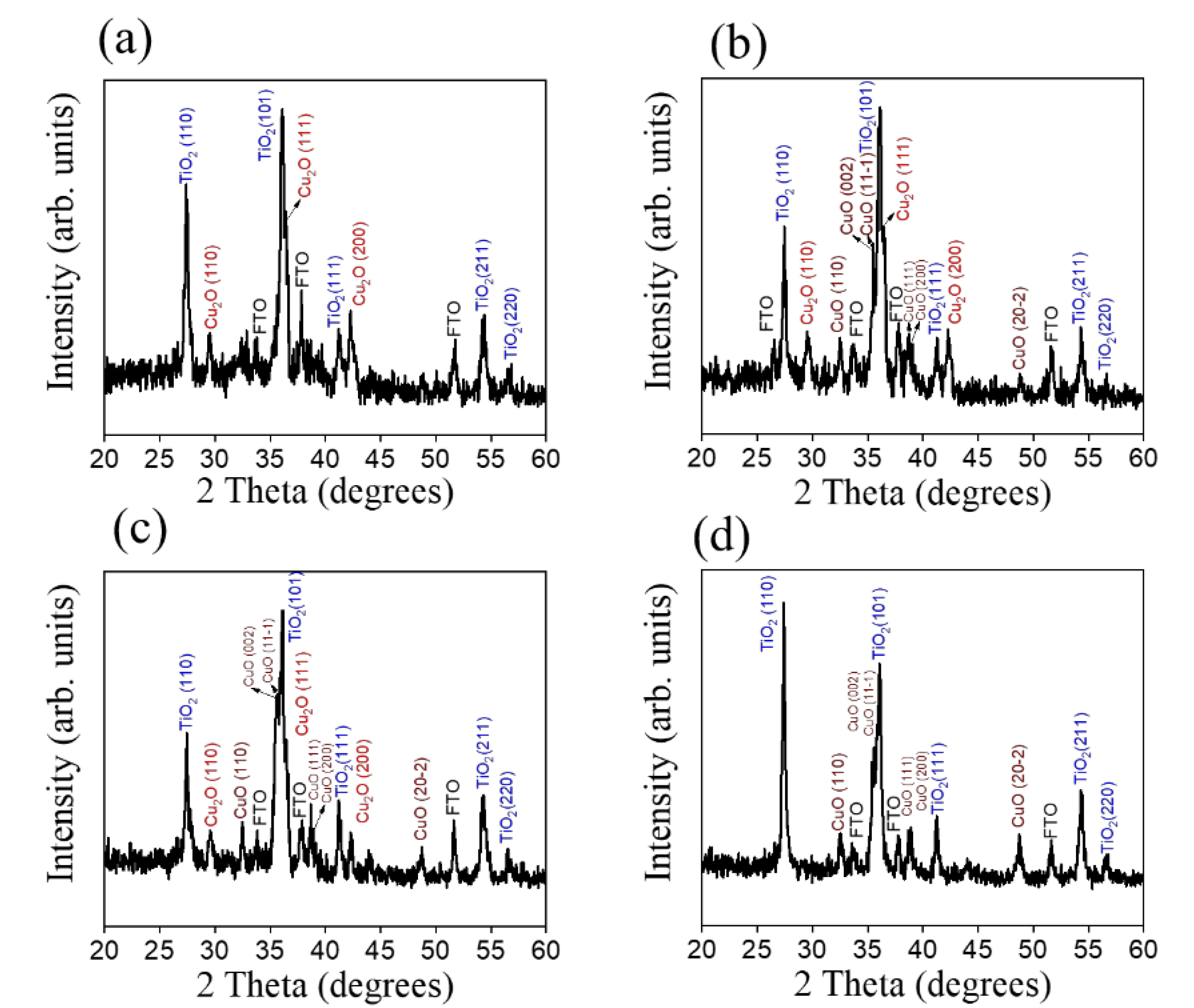
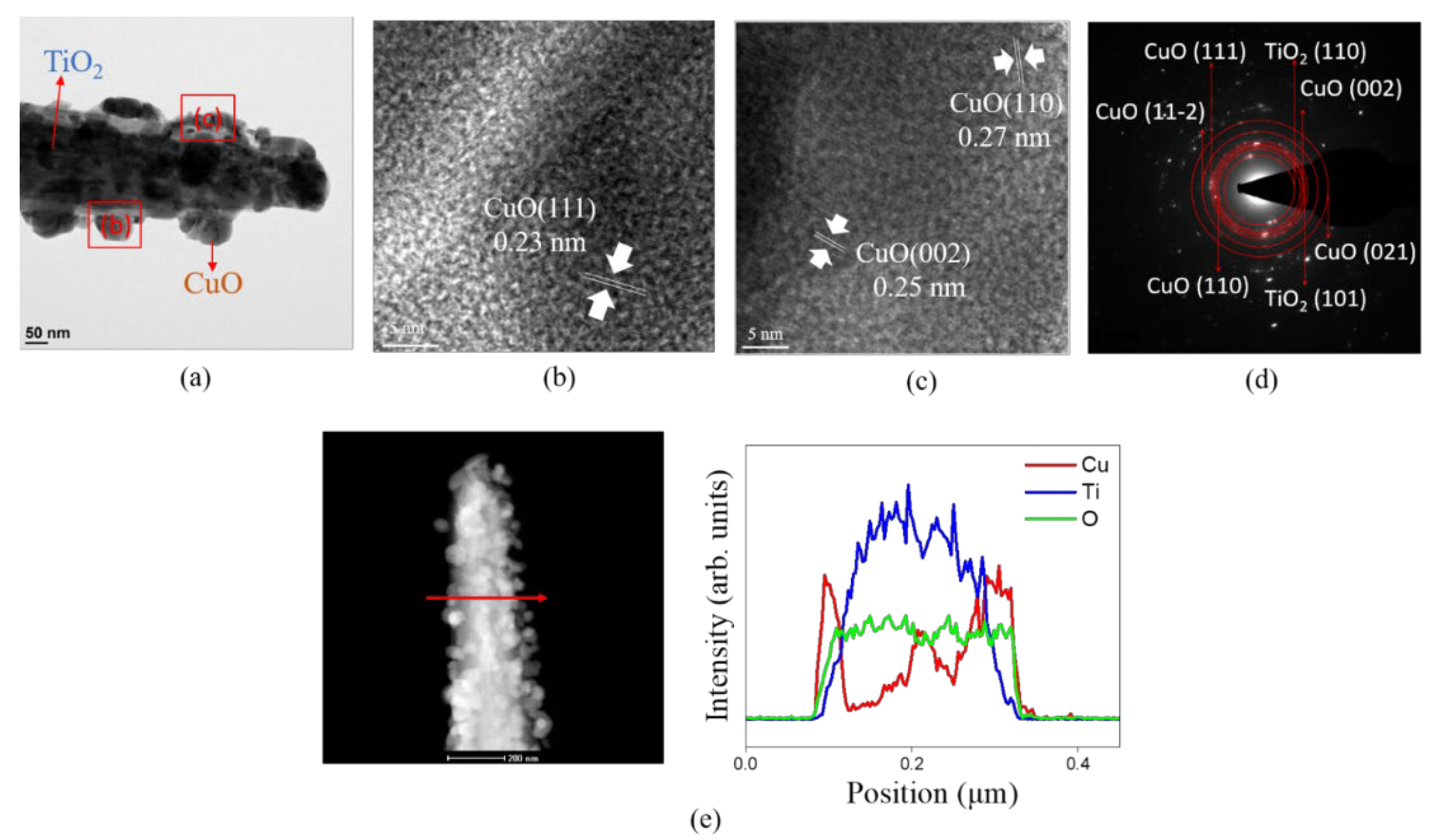
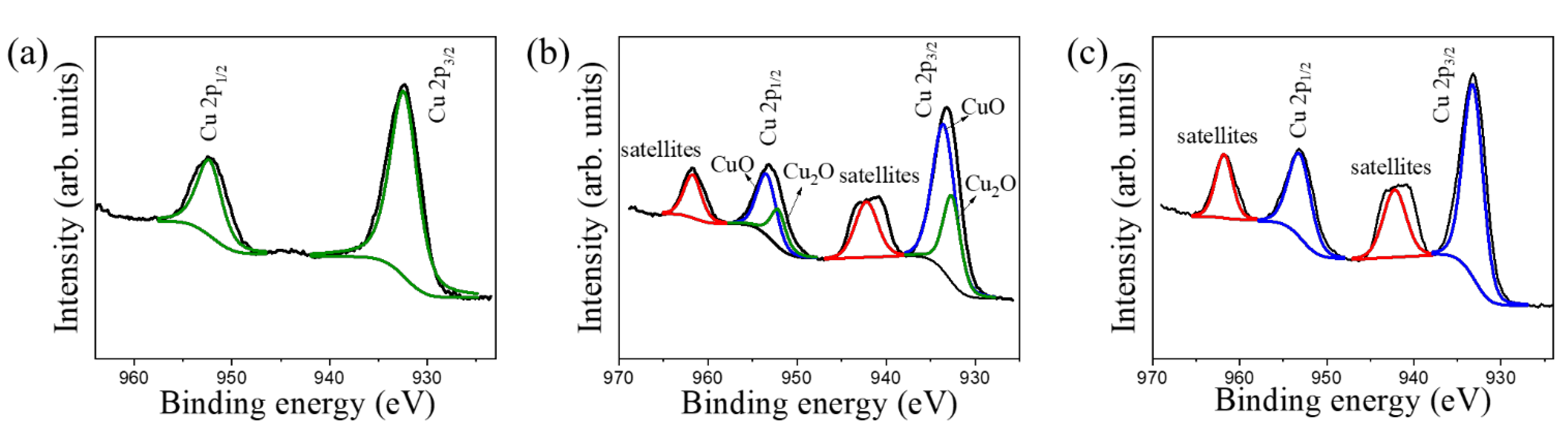
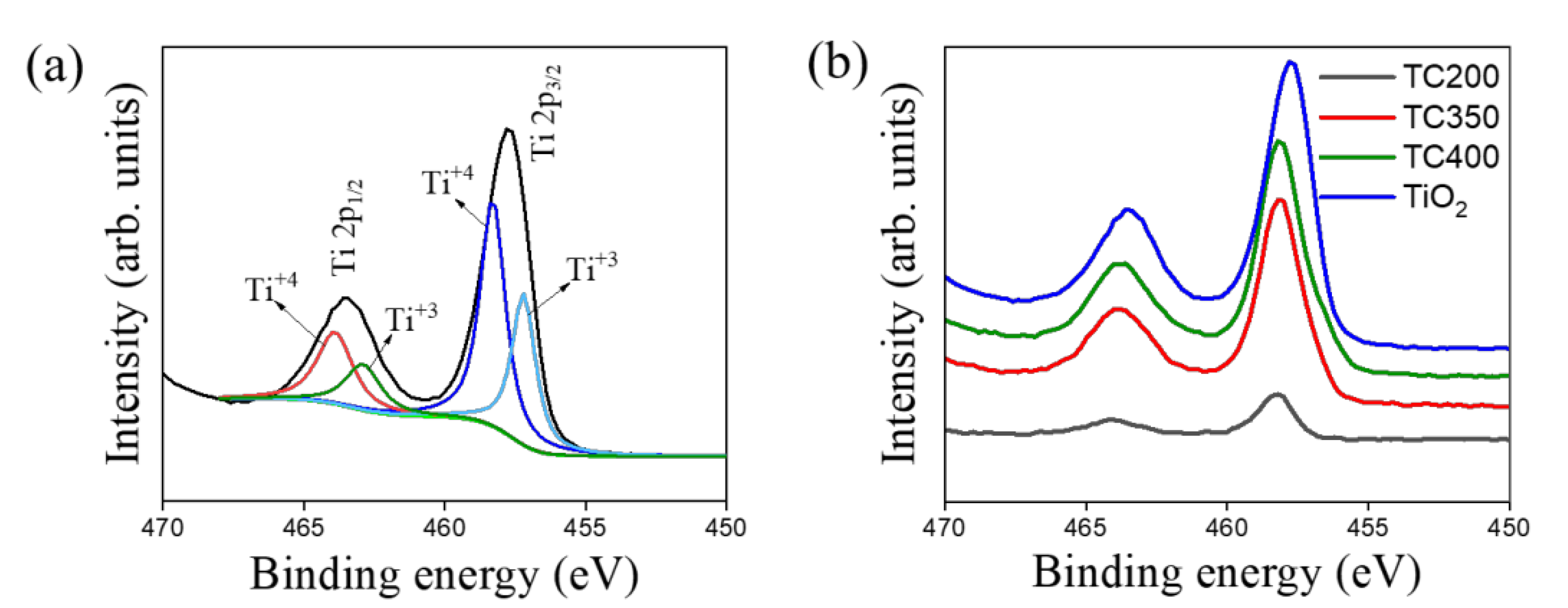
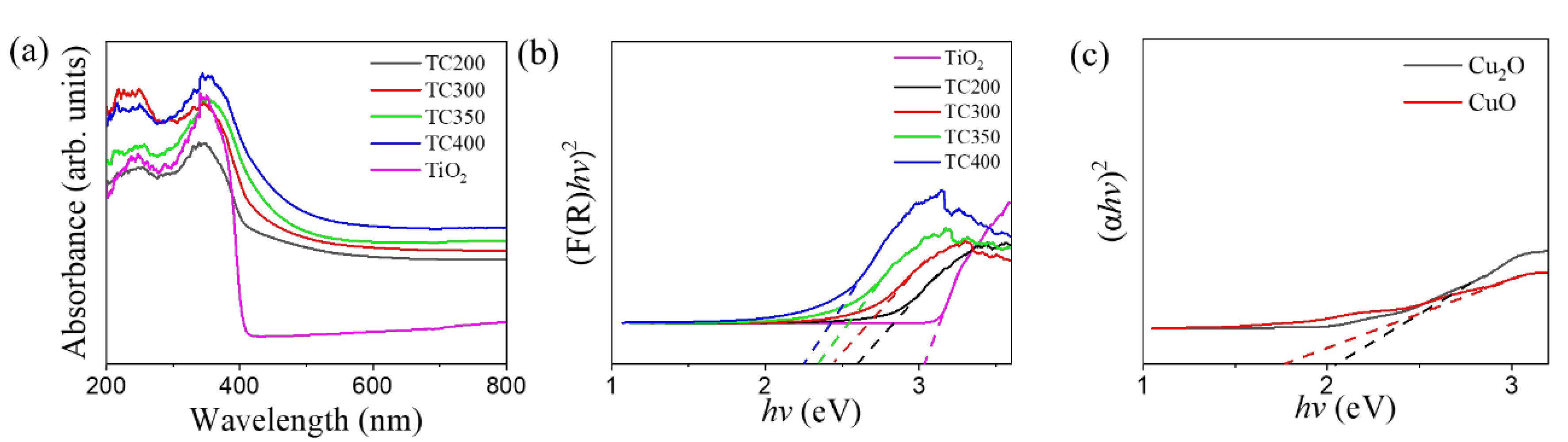
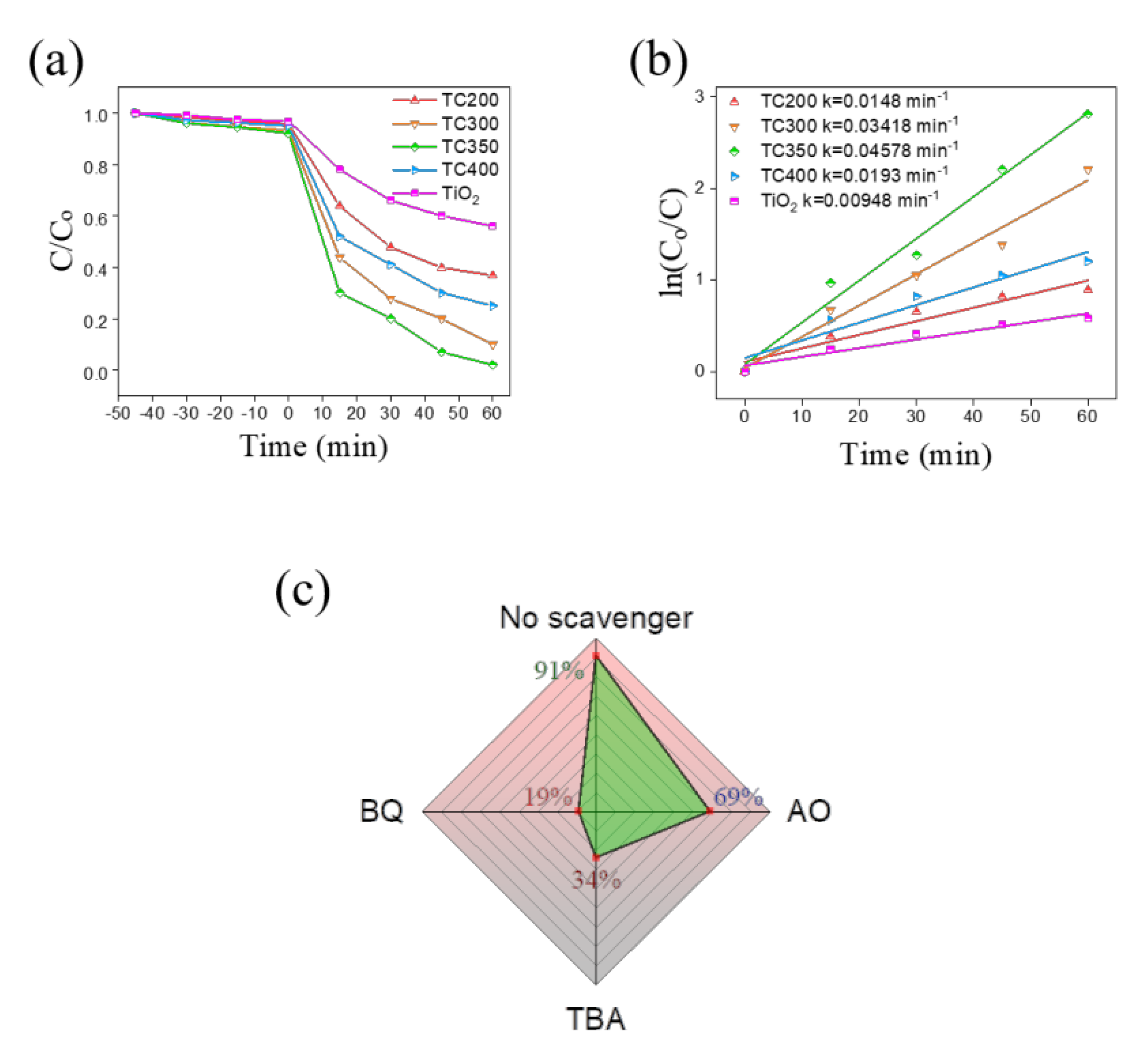
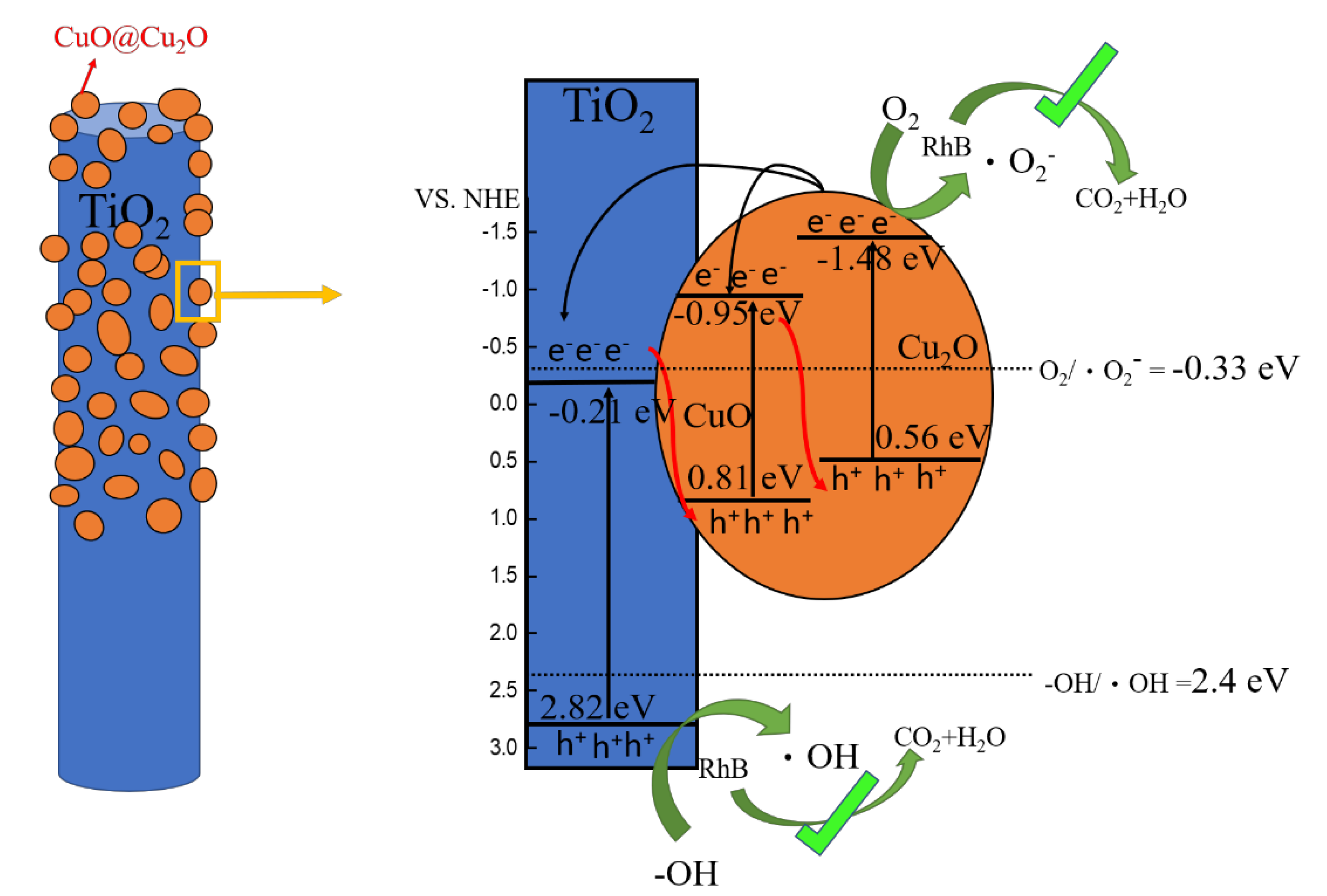
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liang, Y.-C.; Zhao, W.-C. Morphology-dependent photocatalytic and gas-sensing functions of three-dimensional TiO2–ZnO nanoarchitectures. CrystEngComm 2020, 22, 7575–7589. [Google Scholar] [CrossRef]
- Wei, N.; Cui, H.; Wang, C.; Zhang, G.; Song, Q.; Sun, W.; Song, X.; Sun, M.; Tian, J. Bi2O3 nanoparticles incorporated porous TiO2 films as an effective p-n junction with enhanced photocatalytic activity. J. Am. Ceram. Soc. 2017, 100, 1339–1349. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Chiang, K.-J. Coverage Layer Phase Composition-Dependent Photoactivity of One-Dimensional TiO2–Bi2O3 Composites. Nanomaterials 2020, 10, 1005. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, T.N.; Vaz, M.D.O.; Teixeira, S.R. The effects of surfactant in the sol–gel synthesis of CuO/TiO2 nanocomposites on its photocatalytic activities under UV-visible and visible light illuminations. New J. Chem. 2020, 44, 1888–1904. [Google Scholar] [CrossRef]
- Wang, M.; Sun, L.; Lin, Z.; Cai, J.; Xie, K.; Lin, C. p–n Heterojunction photoelectrodes composed of Cu2O-loaded TiO2 nanotube arrays with enhanced photoelectrochemical and photoelectrocatalytic activities. Energy Environ. Sci. 2013, 6, 1211–1220. [Google Scholar] [CrossRef]
- Liu, H.; Gao, L. Preparation and Properties of Nanocrystalline α-Fe2O3-Sensitized TiO2 Nanosheets as a Visible Light Photocatalyst. J. Am. Ceram. Soc. 2006, 89, 370–373. [Google Scholar] [CrossRef]
- Ye, M.; Pan, J.; Guo, Z.; Liu, X.; Chen, Y. Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite. Nanotechnol. Rev. 2020, 9, 558–567. [Google Scholar] [CrossRef]
- Lutz, T.; MacLachlan, A.; Sudlow, A.; Nelson, J.; Hill, M.S.; Molloy, K.C.; Haque, S.A. Thermal decomposition of solution processable metal xanthates on mesoporous titanium dioxide films: A new route to quantum-dot sensitised heterojunctions. Phys. Chem. Chem. Phys. 2012, 14, 16192–16196. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Li, S.; Zhang, S.; Deng, S.; Huang, J.; Li, Q.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. Enhanced photocatalytic performances of n-TiO2 nanotubes by uniform creation of p–n heterojunctions with p-Bi2O3 quantum dots. Nanoscale 2015, 7, 11552–11560. [Google Scholar] [CrossRef]
- Shi, W.; Wang, J.C.; Chen, A.; Xu, X.; Wang, S.; Li, R.; Zhang, W.; Hou, Y. Cu Nanoparticles Modified Step-Scheme Cu2O/WO3 Heterojunction Nanoflakes for Visible-Light-Driven Conversion of CO2 to CH4. Nanomaterials 2022, 12, 2284. [Google Scholar] [CrossRef]
- Mizuno, K.; Izaki, M.; Murase, K.; Shinagawa, T.; Chigane, M.; Inaba, M.; Tasaka, A.; Awakura, Y. Structural and Electrical Characterizations of Electrodeposited p-Type Semiconductor Cu2O Films. J. Electrochem. Soc. 2005, 152, C179. [Google Scholar] [CrossRef]
- Wu, F.; Myung, Y.; Banerjee, P. Unravelling transient phases during thermal oxidation of copper for dense CuO nanowire growth. CrystEngComm 2014, 16, 3264–3267. [Google Scholar] [CrossRef]
- Wang, J.; Ji, G.; Liu, Y.; Gondal, M.; Chang, X. Cu2O/TiO2 heterostructure nanotube arrays prepared by an electrodeposition method exhibiting enhanced photocatalytic activity for CO2 reduction to methanol. Catal. Commun. 2014, 46, 17–21. [Google Scholar] [CrossRef]
- Shi, Q.; Ping, G.; Wang, X.; Xu, H.; Li, J.; Cui, J.; Abroshan, H.; Ding, H.; Li, G. CuO/TiO2 heterojunction composites: An efficient photocatalyst for selective oxidation of methanol to methyl formate. J. Mater. Chem. A 2019, 7, 2253–2260. [Google Scholar] [CrossRef]
- Park, S.M.; Razzaq, A.; Park, Y.H.; Sorcar, S.; Park, Y.; Grimes, C.A.; In, S.I. Hybrid CuxO–TiO2 Heterostructured Composites for Photocatalytic CO2 Reduction into Methane Using Solar Irradiation: Sunlight into Fuel. ACS Omega 2016, 1, 868–875. [Google Scholar] [CrossRef] [Green Version]
- Diachenko, O.; Kováč, J., Jr.; Dobrozhan, O.; Novák, P.; Kováč, J.; Skriniarova, J.; Opanasyuk, A. Structural and Optical Properties of CuO Thin Films Synthesized Using Spray Pyrolysis Method. Coatings 2021, 11, 1392. [Google Scholar] [CrossRef]
- Dai, M.-J.; Lin, S.-S.; Shi, Q.; Liu, F.; Wang, W.-X.; Chen, S.-C.; Kuo, T.-Y.; Sun, H. Transparent Conductive p-Type Cuprous Oxide Films in Vis-NIR Region Prepared by Ion-Beam Assisted DC Reactive Sputtering. Coatings 2020, 10, 473. [Google Scholar] [CrossRef]
- Nair, M.; Guerrero, L.; Arenas, O.L.; Nair, P. Chemically deposited copper oxide thin films: Structural, optical and electrical characteristics. Appl. Surf. Sci. 1999, 150, 143–151. [Google Scholar] [CrossRef]
- Mahendra, G.; Malathi, R.; Kedhareswara, S.P.; LakshmiNarayana, A.; Dhananjaya, M.; Guruprakash, N.; Hussain, O.M.; Mauger, A.; Julien, C.M. RF Sputter-Deposited Nanostructured CuO Films for Micro-Supercapacitors. Appl. Nano 2021, 2, 46–66. [Google Scholar] [CrossRef]
- Valladares, L.D.L.S.; Salinas, D.H.; Dominguez, A.B.; Najarro, D.A.; Khondaker, S.I.; Mitrelias, T.; Barnes, C.H.W.; Aguiar, J.A.; Majima, Y. Crystallization and electrical resistivity of Cu2O and CuO obtained by thermal oxidation of Cu thin films on SiO2/Si substrates. Thin Solid Film. 2012, 520, 6368–6374. [Google Scholar] [CrossRef]
- Serin, N.; Serin, T.; Horzum, Ş.; Celik, Y. Annealing effects on the properties of copper oxide thin films prepared by chemical deposition. Semicond. Sci. Technol. 2005, 20, 398–401. [Google Scholar] [CrossRef]
- Khojier, K.; Behju, A. Annealing Temperature Effect On Nanostructure And Phase Transition Of Copper Oxide Thin Films. Int. J. Nano Dimens. 2012, 2, 185–190. [Google Scholar]
- Liang, Y.-C.; Xu, N.-C.; Chiang, K.-J. Surface Morphology-Dependent Functionality of Titanium Dioxide–Nickel Oxide Nanocomposite Semiconductors. Nanomaterials 2019, 9, 1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidyasagar, C.C.; Naik, Y.A.; Venkatesha, T.G.; Viswanatha, R. Solid-State Synthesis and Effect of Temperature on Optical Properties of CuO Nanoparticles. Nano-Micro Lett. 2012, 4, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Siqingaowa, Z.; Yao, H. Preparation and characterization of nanocrystalline ZnO by direct precipitation method. Front. Chem. China 2006, 1, 277–280. [Google Scholar] [CrossRef]
- Neumann, J.P.; Zhong, T.; Chang, Y.A. The Cu−O (Copper-Oxygen) system. Bull. Alloy. Phase Diagr. 1984, 5, 136–140. [Google Scholar] [CrossRef]
- Adilov, S.; Afanaciev, V.P.; Kashkul, I.N.; Kumekov, S.; Mukhin, N.V.; Terukov, E.I. Studying the Composition and Structure of Films Obtained by Thermal Oxidation of Copper. Glass Phys. Chem. 2017, 43, 272–275. [Google Scholar] [CrossRef]
- Akkari, F.C.; Kanzari, M.; Rezig, B. Preparation and characterization of obliquely deposited copper oxide thin films. Eur. Phys. J. Appl. Phys. 2007, 40, 49–54. [Google Scholar] [CrossRef]
- Johan, M.R.; Suan, M.S.M.; Hawari, N.L.; Ching, H.A. Annealing Effects on the Properties of Copper Oxide Thin Films Prepared by Chemical Deposition. Int. J. Electrochem. Sci. 2011, 6, 6094–6104. [Google Scholar]
- Dong, K.; He, J.; Liu, J.; Li, F.; Yu, L.; Zhang, Y.; Zhou, X.; Ma, H. Photocatalytic performance of Cu2O-loaded TiO2/rGO nanoheterojunctions obtained by UV reduction. J. Mater. Sci. 2017, 52, 6754–6766. [Google Scholar] [CrossRef]
- Datye, A.K.; Xu, Q.; Kharas, K.C.; McCarty, J.M. Particle size distributions in heterogeneous catalysts: What do they tell us about the sintering mechanism? Catal. Today 2006, 111, 59–67. [Google Scholar] [CrossRef]
- Lim, Y.F.; Chua, C.S.; Lee, C.J.J.; Chi, D. Sol–gel deposited Cu2O and CuO thin films for photocatalytic water splitting. Phys. Chem. Chem. Phys. 2014, 16, 25928–25934. [Google Scholar] [CrossRef]
- Wang, Y.; Lü, Y.; Zhan, W.; Xie, Z.; Kuang, Q.; Zheng, L. Synthesis of Porous Cu2O/CuO Cages using Cu-based Metal-Organic-Framework as Templates and their Gas-sensing Properties. J. Mater. Chem. A 2015, 3, 12796–12803. [Google Scholar] [CrossRef]
- Khan, M.A.; Nayan, N.; Ahmad, M.K.; Soon, C.F. Surface Study of CuO Nanopetals by Advanced Nanocharacterization Techniques with Enhanced Optical and Catalytic Properties. Nanomaterials 2020, 10, 1298. [Google Scholar] [CrossRef]
- Dubale, A.A.; Pan, C.-J.; Tamirat, A.G.; Chen, H.-M.; Su, W.-N.; Chen, C.-H.; Rick, J.; Ayele, D.W.; Aragaw, B.A.; Lee, J.-F.; et al. Heterostructured Cu2O/CuO decorated with nickel as a highly efficient photocathode for photoelectrochemical water reduction. J. Mater. Chem. A 2015, 3, 12482–12499. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Liu, Y.-C. Design of Nanoscaled Surface Morphology of TiO2–Ag2O Composite Nanorods through Sputtering Decoration Process and Their Low-Concentration NO2 Gas-Sensing Behaviors. Nanomaterials 2019, 9, 1150. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.-C.; Wang, C.-C. Hydrothermally derived zinc sulfide sphere-decorated titanium dioxide flower-like composites and their enhanced ethanol gas-sensing performance. J. Alloy. Compd. 2018, 730, 333–341. [Google Scholar] [CrossRef]
- Hamad, H.; Elsenety, M.M.; Sadik, W.; El-Demerdash, A.G.; Nashed, A.; Mostafa, A.; Elyamny, S. The superior photocatalytic performance and DFT insights of S-scheme CuO@TiO2 heterojunction composites for simultaneous degradation of organics. Sci. Rep. 2022, 12, 2217. [Google Scholar] [CrossRef]
- Wang, S.; Huo, R.; Zhang, R.; Zheng, Y.; Li, C.; Pan, L. Synthesis of core–shell N-TiO2@CuOx with enhanced visible light photocatalytic performance. RSC Adv. 2018, 8, 24866–24872. [Google Scholar] [CrossRef] [Green Version]
- Musa, A.; Akomolafe, T.; Carter, M. Production of cuprous oxide, a solar cell material, by thermal oxidation and a study of its physical and electrical properties. Sol. Energy Mater. Sol. Cells 1998, 51, 305–316. [Google Scholar] [CrossRef]
- Deng, X.; Wang, C.; Shao, M.; Xu, X.; Huang, J. Low-temperature solution synthesis of CuO/Cu2O nanostructures for enhanced photocatalytic activity with added H2O2: Synergistic effect and mechanism insight. RSC Adv. 2017, 7, 4329–4338. [Google Scholar] [CrossRef] [Green Version]
- Nakatani, K.; Himoto, K.; Kono, Y.; Nakahashi, Y.; Anma, H.; Okubo, T.; Maekawa, M.; Kuroda-Sowa, T. Synthesis, Crystal Structure, and Electroconducting Properties of a 1D Mixed-Valence Cu(I)–Cu(II) Coordination Polymer with a Dicyclohexyl Dithiocarbamate Ligand. Crystals 2015, 5, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Balık, M.; Bulut, V.; Erdogan, I.Y. Optical, structural and phase transition properties of Cu2O, CuO and Cu2O/CuO: Their photoelectrochemical sensor applications. Int. J. Hydrog. Energy 2019, 44, 18744–18755. [Google Scholar] [CrossRef]
- Hsu, Y.-K.; Yu, C.-H.; Chen, Y.-C.; Lin, Y.-G. Synthesis of novel Cu2O micro/nanostructural photocathode for solar water splitting. Electrochim. Acta 2013, 105, 62–68. [Google Scholar] [CrossRef]
- Masudy-Panah, S.; Radhakrishnan, K.; Tan, H.R.; Yi, R.; Wong, T.I.; Dalapati, G.K. Ten It Wong, Goutam Kumar Dalapati, Titanium doped cupric oxide for photovoltaic application. Sol. Energy Mater. Sol. Cells 2015, 140, 266–274. [Google Scholar] [CrossRef]
- Dong, Y.; Tao, F.; Wang, L.; Lan, M.; Zhang, J.; Hong, T. One-pot preparation of hierarchical Cu2O hollow spheres for improved visible-light photocatalytic properties. RSC Adv. 2020, 10, 22387–22396. [Google Scholar] [CrossRef]
- Jeong, D.; Jo, W.; Jeong, J.; Kim, T.; Han, S.; Son, M.-K.; Jung, H. Characterization of Cu2O/CuO heterostructure photocathode by tailoring CuO thickness for photoelectrochemical water splitting. RSC Adv. 2022, 12, 2632–2640. [Google Scholar] [CrossRef]
- Liang, Y.C.; Chou, Y.H. Matrix phase induced boosting photoactive performance of ZnO nanowire turf-coated Bi2O3 plate composites. J. Am. Ceram. Soc. 2021, 104, 5432–5444. [Google Scholar] [CrossRef]
- Kmentova, H.; Henrotte, O.; Yalavarthi, R.; Haensch, M.; Heinemann, C.; Zbořil, R.; Schmuki, P.; Kment, Š.; Naldoni, A. Nanoscale Assembly of BiVO4/CdS/CoOx Core–Shell Heterojunction for Enhanced Photoelectrochemical Water Splitting. Catalysts 2021, 11, 682. [Google Scholar] [CrossRef]
- Huang, W.; Li, W.-X. Surface and interface design for heterogeneous catalysis. Phys. Chem. Chem. Phys. 2019, 21, 523–536. [Google Scholar] [CrossRef]
- Zheng, D.; Yu, L.; Liu, W.; Dai, X.; Niu, X.; Fu, W.; Shi, W.; Wu, F.; Cao, X. Structural advantages and enhancement strategies of heterostructure water-splitting electrocatalysts. Cell Rep. Phys. Sci. 2021, 2, 100443. [Google Scholar] [CrossRef]
- Cong, Y.; Ge, Y.; Zhang, T.; Wang, Q.; Shao, M.; Zhang, Y. Fabrication of Z-Scheme Fe2O3–MoS2–Cu2O Ternary Nanofilm with Significantly Enhanced Photoelectrocatalytic Performance. Nd. Eng. Chem. Res. 2018, 57, 881–890. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Wang, Y.-P. Optimizing crystal characterization of WO3–ZnO composites for boosting photoactive performance via manipulating crystal formation conditions. CrystEngComm 2021, 23, 3498–3509. [Google Scholar] [CrossRef]
- Bengas, R.; Lahmar, H.; Redha, K.M.; Mentar, L.; Azizi, A.; Schmerber, G.; Dinia, A. Electrochemical synthesis of n-type ZnS layers on p-Cu2O/n-ZnO heterojunctions with different deposition temperatures. RSC Adv. 2019, 9, 29056–29069. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yi, Z.; Wu, G.; Shen, Q. Novel Y doped BiVO4 thin film electrodes for enhanced photoelectric and photocatalytic performance. J. Photochem. Photobiol. A Chem. 2016, 327, 25–32. [Google Scholar] [CrossRef]
- Jang, J.S.; Kim, H.G.; Lee, J.S. Heterojunction semiconductors: A strategy to develop efficient photocatalytic materials for visible light water splitting. Catal. Today 2012, 185, 270–277. [Google Scholar] [CrossRef]
- Chen, H.; Leng, W.; Xu, Y. Enhanced Visible-Light Photoactivity of CuWO4 through a Surface-Deposited CuO. J. Phys. Chem. C 2014, 118, 9982–9989. [Google Scholar] [CrossRef]
- Peng, B.; Zhang, S.; Yang, S.; Chen, H.; Wang, H.; Yu, H.; Zhang, S.; Peng, F. Synthesis and characterization of g-C3N4/Cu2O composite catalyst with enhanced photocatalytic activity under visible light irradiation. Mater. Res. Bull. 2014, 56, 19–24. [Google Scholar] [CrossRef]
- Peng, B.; Zhang, S.; Yang, S.; Wang, H.; Yu, H.; Zhang, S.; Peng, F. The facile hydrothermal synthesis of CuO@ZnO heterojunction nanostructures for enhanced photocatalytic hydrogen evolution. New J. Chem. 2019, 43, 6794–6805. [Google Scholar]
- Aguilera-Ruiz, E.; De La Garza-Galván, M.; Zambrano-Robledo, P.; Ballesteros-Pacheco, J.C.; Vazquez-Arenas, J.; Peral, J.; García-Pérez, U.M. Facile synthesis of visible-light-driven Cu2O/BiVO4 composites for the photomineralization of recalcitrant pesticides. RSC Adv. 2017, 7, 45885–45895. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.C.; Chiang, K.J. Design and tuning functionality of rod-like titanium dioxide–nickel oxide composites via a combinational methodology. Nanotechnology 2020, 31, 195709. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lai, S.; Xu, W.; Fang, J.; Chen, X.; Beiyuan, J.; Zhou, X.; Lin, K.; Liu, J.; Guan, G. Novel ternary BiOI/g-C3N4/CeO2 catalysts for enhanced photocatalytic degradation of tetracycline under visible-light radiation via double charge transfer process. J. Alloy. Compd. 2019, 809, 151804. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 heterostructures for CO2 reduction through a direct Z-scheme: Protecting Cu2O from photocorrosion. Appl. Catal. B Environ. Vol. 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Dasineh Khiavi, N.; Katal, R.; Kholghi Eshkalak, S.; Masudy-Panah, S.; Ramakrishna, S.; Jiangyong, H. Visible Light Driven Heterojunction Photocatalyst of CuO–Cu2O Thin Films for Photocatalytic Degradation of Organic Pollutants. Nanomaterials 2019, 9, 1011. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Sun, Y.; Fan, Z.; Zhao, D.; Xiong, S.; Zhang, B.; Zhou, S.; Liu, G. Mechanisms for ·O−2 and OH Production on Flowerlike BiVO4 Photocatalysis Based on Electron Spin Resonance. Front. Chem. 2018, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Yoo, H.; Kahng, S.; Kim, J.H. Z-scheme assisted ZnO/Cu2O-CuO photocatalysts to increase photoactive electrons in hydrogen evolution by water splitting. Sol. Energy Mater. Sol. Cells 2020, 204, 110211. [Google Scholar] [CrossRef]
- Wei, T.; Zhu, Y.N.; An, X.; Liu, L.M.; Cao, X.; Liu, H.; Qu, J. Defect Modulation of Z-Scheme TiO2/Cu2O Photocatalysts for Durable Water Splitting. ACS Catal. 2019, 9, 8346–8354. [Google Scholar] [CrossRef]
- Huang, L.; Peng, F.; Yu, H.; Wang, H. Preparation of cuprous oxides with different sizes and their behaviors of adsorption, visible-light driven photocatalysis and photocorrosion. Solid State Sci. 2009, 11, 129–138. [Google Scholar] [CrossRef]
- Wang, P.; Wen, X.; Amal, R.; Ng, Y.H. Introducing a protective interlayer of TiO2 in Cu2O–CuO heterojunction thin film as a highly stable visible light photocathode. RSC Adv. 2015, 5, 5231–5236. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.-C.; Li, T.-H. Sputtering-Assisted Synthesis of Copper Oxide–Titanium Oxide Nanorods and Their Photoactive Performances. Nanomaterials 2022, 12, 2634. https://doi.org/10.3390/nano12152634
Liang Y-C, Li T-H. Sputtering-Assisted Synthesis of Copper Oxide–Titanium Oxide Nanorods and Their Photoactive Performances. Nanomaterials. 2022; 12(15):2634. https://doi.org/10.3390/nano12152634
Chicago/Turabian StyleLiang, Yuan-Chang, and Tsun-Hsuan Li. 2022. "Sputtering-Assisted Synthesis of Copper Oxide–Titanium Oxide Nanorods and Their Photoactive Performances" Nanomaterials 12, no. 15: 2634. https://doi.org/10.3390/nano12152634




