Fabrication of an Organofunctionalized Talc-like Magnesium Phyllosilicate for the Electrochemical Sensing of Lead Ions in Water Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Material Characterization
2.2.1. Preparation of the Organofunctionalized Talc-like Magnesium Phyllosilicate
2.2.2. Preparation of the Working Electrode
3. Results and Discussion
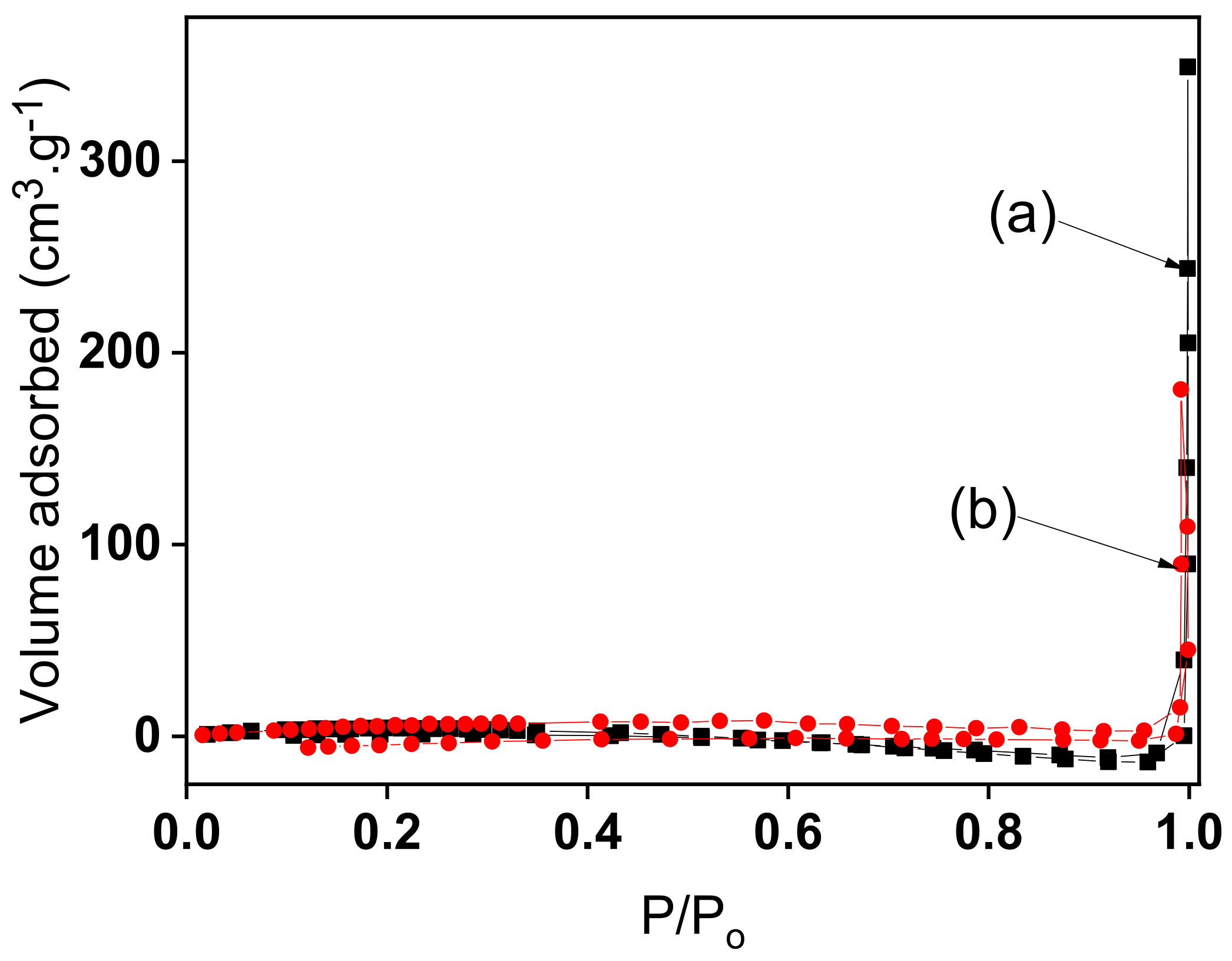
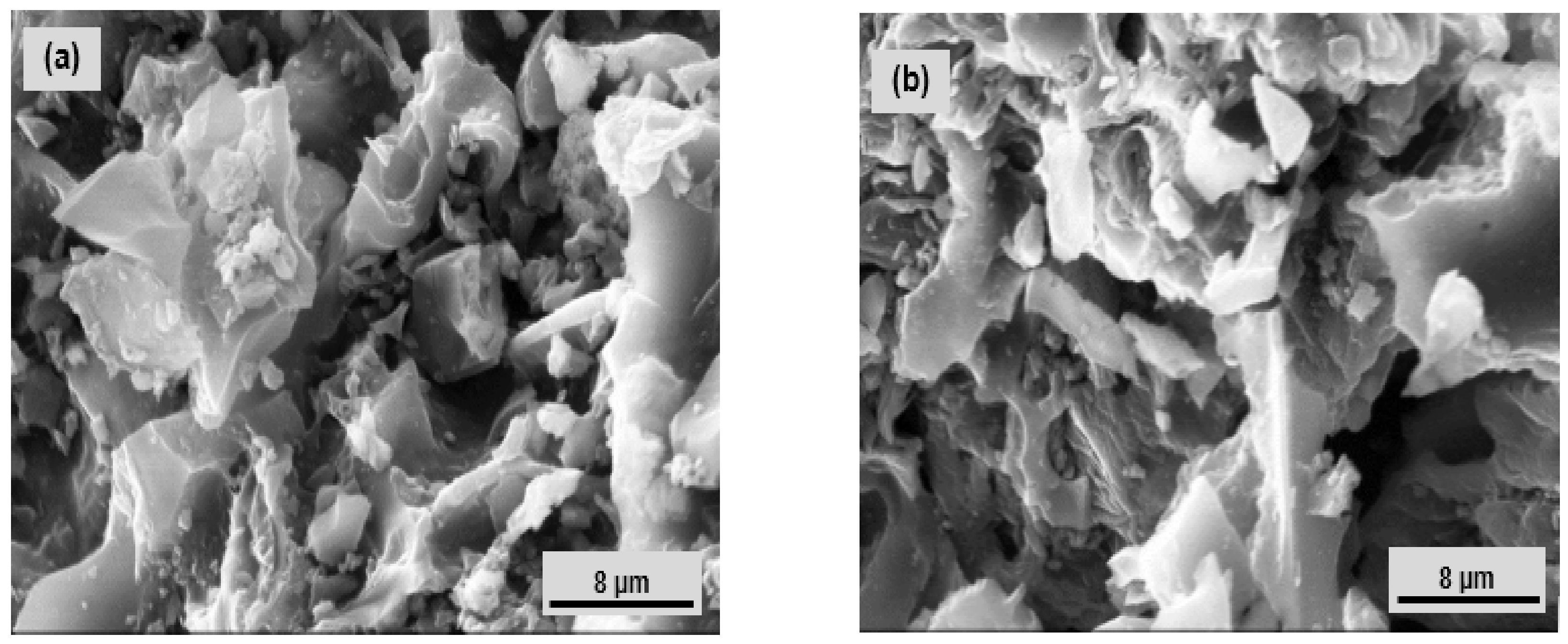
3.1. Physicochemical Characterization of Organofunctionalized Clay Material
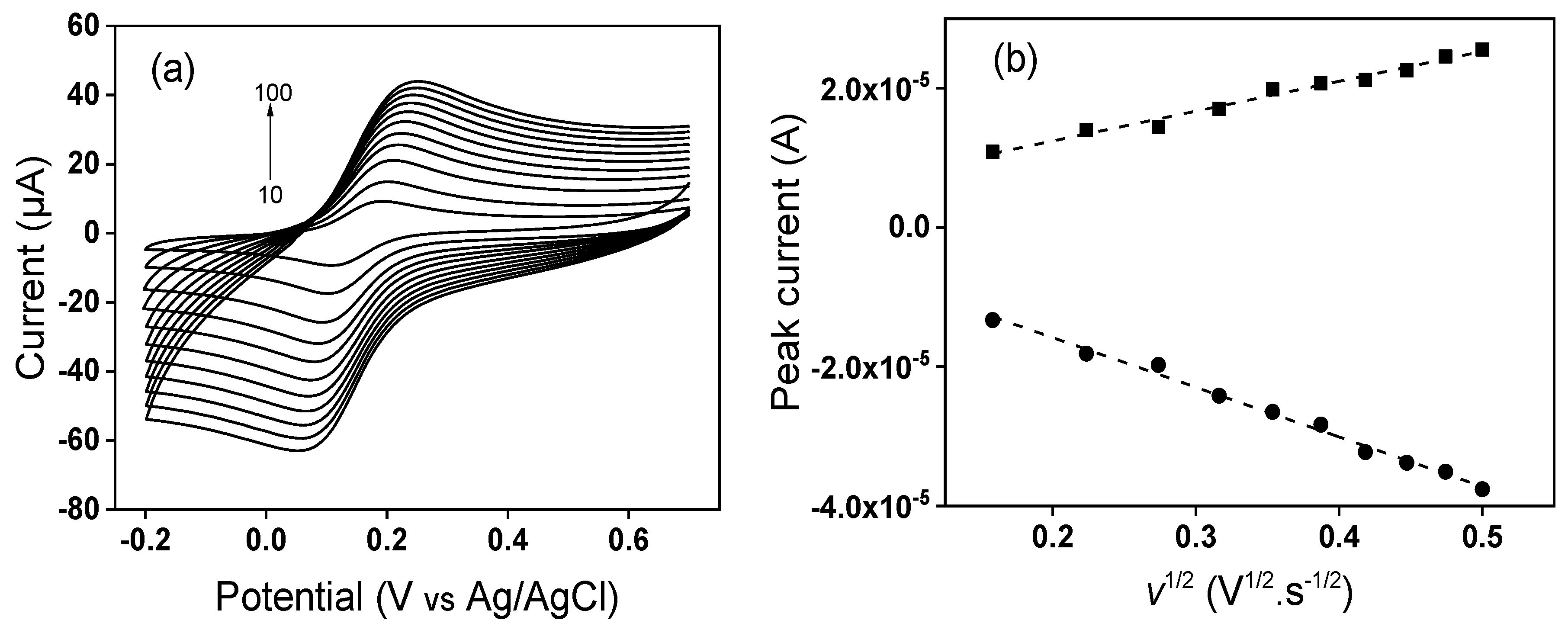
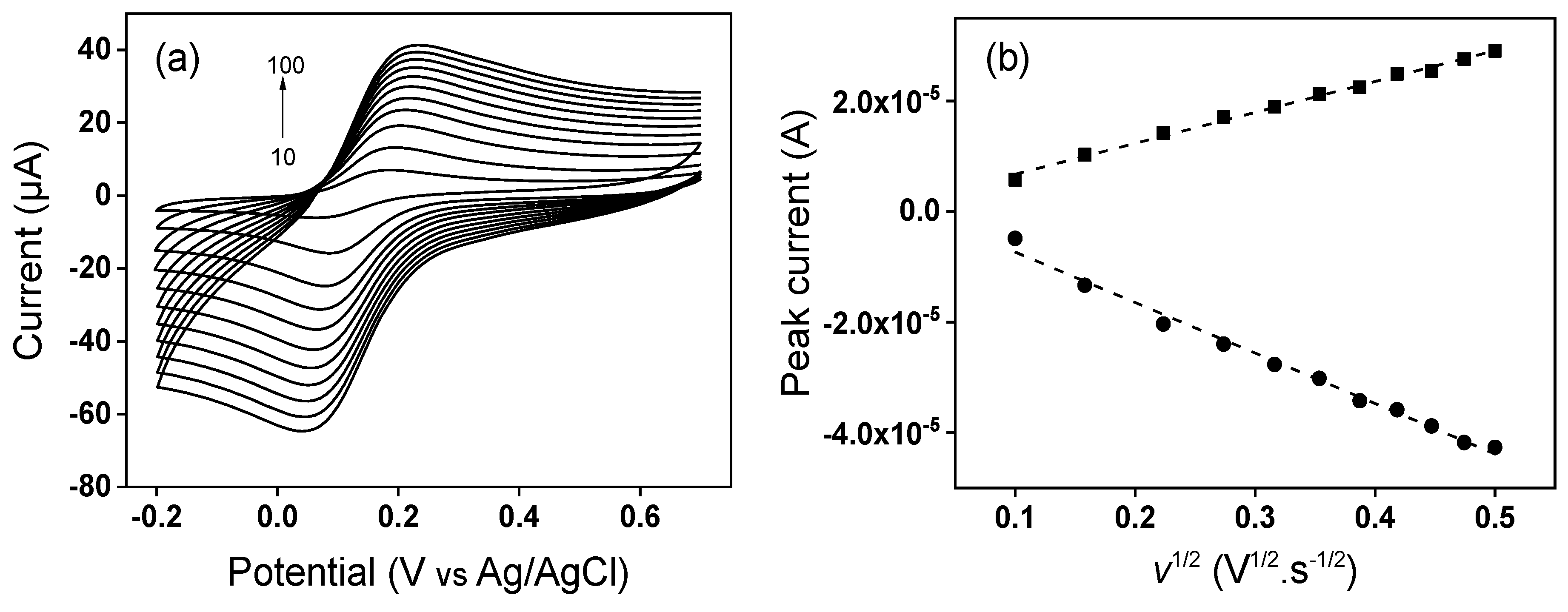
3.2. Electrochemical Characterization of Modified GCE by Cyclic Voltammetry
3.3. Determination of Electroactive Surface Area
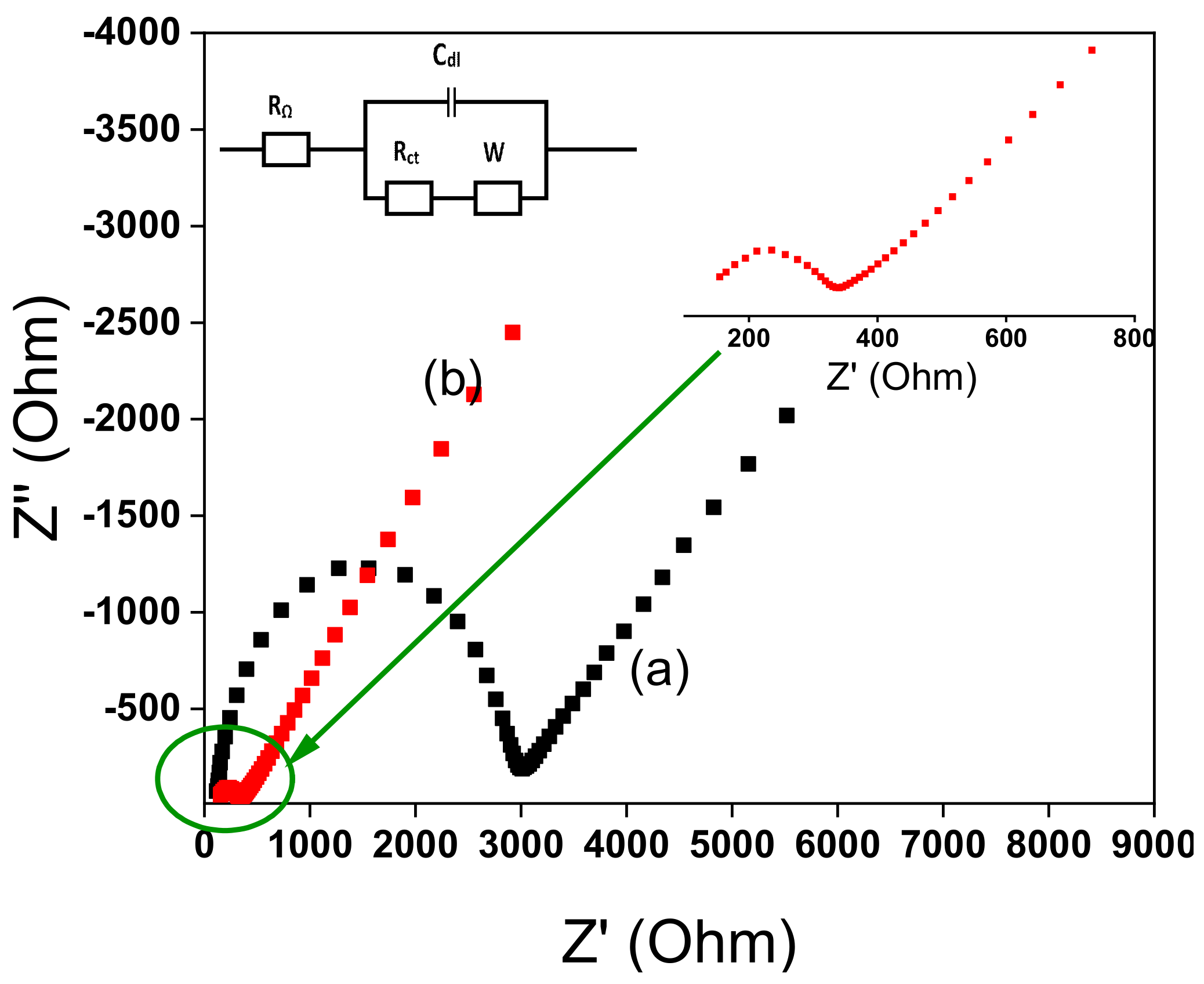
3.4. Impedance Characterization
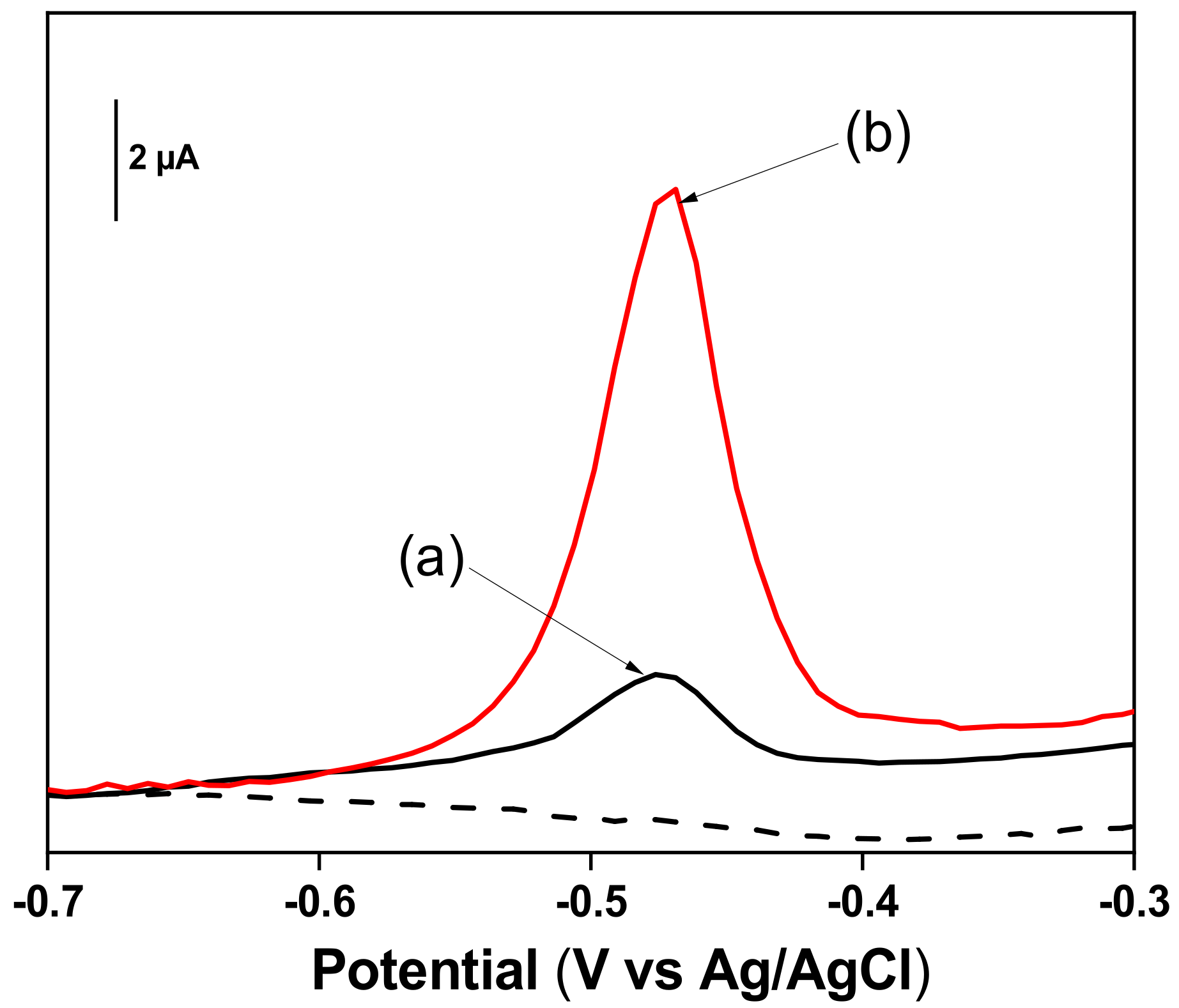
3.5. Electrochemical Behavior of Pb2+ Ions at TalcNH2/GCE
3.6. Optimization of the Experimental Conditions for the Detection of Lead Ions atTalcNH2/GCE
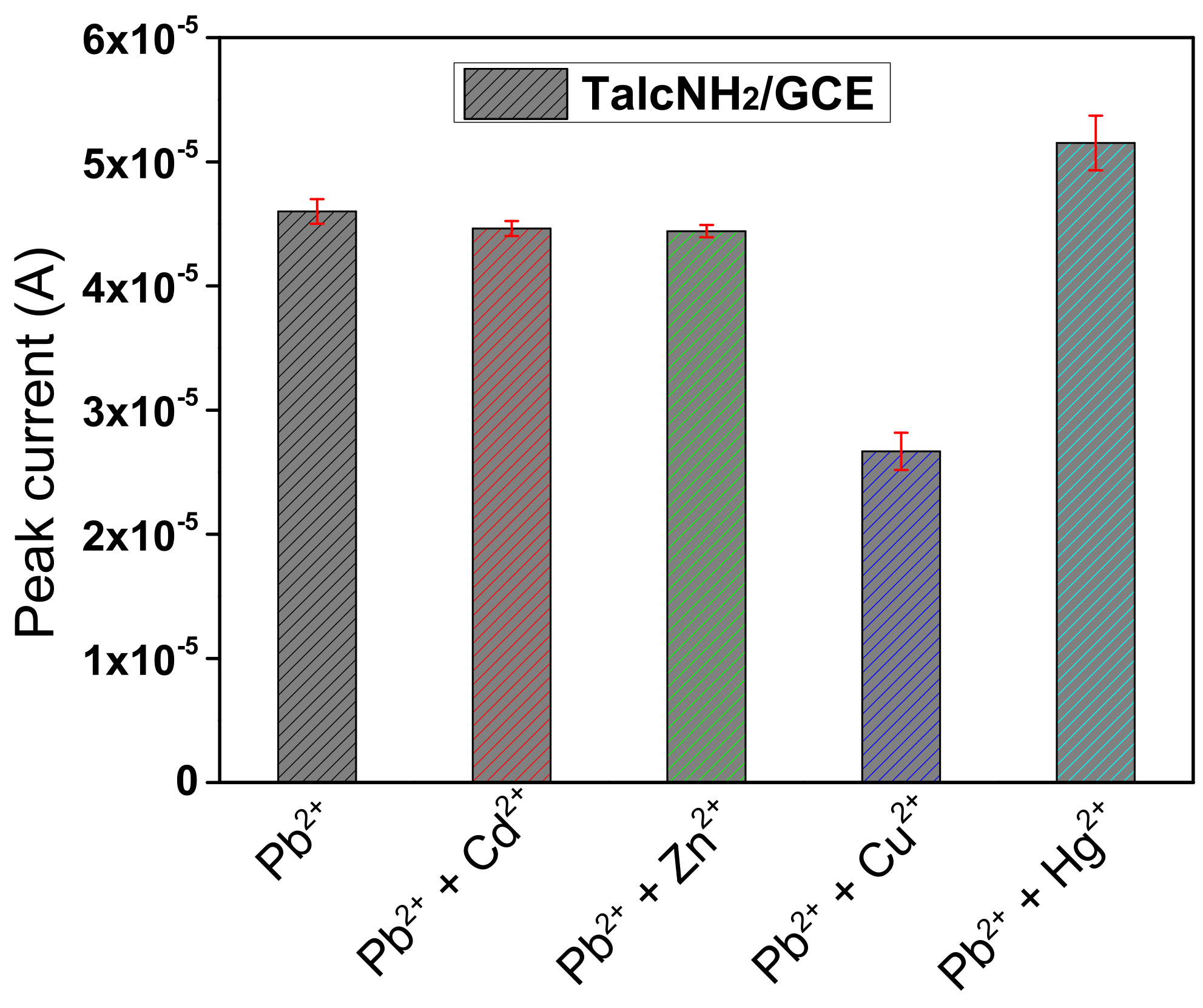
3.7. Detection Limit, Interference Study and Analytical Application of the Developed Sensor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guggenheim, S.; Adams, J.M.; Bain, D.C.; Bergaya, F.; Brigatti, M.F.; Drits, V.A.; Formoso, M.L.L.; Galán, E.; Kogure, T.; Stanjek, H. Summary of recommendations of nomenclature committees relevant to clay mineralogy: Report of the Association Internationale pour l’Etude des Argiles (AIPEA) Nomenclature Committee for 2006. Clays Clay Miner. 2006, 54, 761–772. [Google Scholar] [CrossRef]
- Wang, J.; Somasundaran, P. Adsorption and conformation of carboxymethyl cellulose at solid–liquid interfaces using spectroscopic, AFM and allied techniques. J. Colloid Interface Sci. 2005, 291, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Lugwisha, E.H.J. Properties of fired bodies made from Tanzanian talc-clay mixes for ceramic applications. Tanzan. J. Sci. 2009, 32, 69–79. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Ibrahim, G.A.; Hassan, M.M.A. Improvement of Egyptian talc quality for industrial uses by flotation process and leaching. Int. J. Miner. Process. 2007, 83, 132–145. [Google Scholar] [CrossRef]
- Temuujin, J.; Okada, K.; Jadambaa, T.; Mackenzie, K.J.D.; Amarsanaa, J. Effect of grinding on the preparation of porous material from talc by selective leaching. J. Mater. Sci. Lett. 2002, 21, 1607–1609. [Google Scholar] [CrossRef]
- Sanchez-Soto, P.J.; Wiewióra, A.; Avilés, M.A.; Justo, A.; Pérez-Maqueda, L.A.; Pérez-Rodríguez, J.L.; Bylina, P. Talc from Puebla de Lillo, Spain. II. Effect of dry grinding on particle size and shape. Appl. Clay Sci. 1997, 12, 297–312. [Google Scholar] [CrossRef]
- Wallqvist, V.; Claesson, P.M.; Swerin, A.; Schoelkopf, J.; Gane, P.A.C. Influence of Wetting and Dispersing Agents on the Interaction between Talc and Hydrophobic Particles. Langmuir 2009, 25, 6909–6915. [Google Scholar] [CrossRef]
- Da Fonseca, M.G.; Airoldi, C. New amino-inorganic hybrids from talc silylation and copper adsorption properties. Mater. Res. Bull. 2001, 36, 277–287. [Google Scholar] [CrossRef]
- Wesołowski, M. Thermal decomposition of talc: A review. Thermochim. Acta 1984, 78, 395–421. [Google Scholar] [CrossRef]
- Feng, B.; Peng, J.; Guo, W.; Zhang, W.; Ai, G.; Wang, H. The effect of changes in pH on the depression of talc by chitosan and the associated mechanisms. Powder Technol. 2018, 325, 58–63. [Google Scholar] [CrossRef]
- Yi, H.; Zhao, Y.; Rao, F.; Song, S. Hydrophobic agglomeration of talc fines in aqueous suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 327–332. [Google Scholar] [CrossRef]
- Boyd, S.A.; Lee, J.-F.; Mortland, M.M. Attenuating organic contaminant mobility by soil modification. Nature 1988, 333, 345–347. [Google Scholar] [CrossRef]
- Da Fonseca, M.G.; Silva, C.R.; Airoldi, C. Aminated Phyllosilicates Synthesized via a Sol–Gel Process. Langmuir 1999, 15, 5048–5055. [Google Scholar] [CrossRef]
- Fukushima, Y.; Tani, M. An organic/inorganic hybrid layered polymer: Methacrylate–magnesium(nickel) phyllosilicate. J. Chem. Soc. Chem. Commun. 1995, 63, 241–242. [Google Scholar] [CrossRef]
- Burkett, S.L.; Press, A.; Mann, S. Synthesis, Characterization, and Reactivity of Layered Inorganic–Organic Nanocomposites Based on 2:1 Trioctahedral Phyllosilicates. Chem. Mater. 1997, 9, 1071–1073. [Google Scholar] [CrossRef]
- Fonseca, M.G.; Airoldi, C. Mercaptopropyl magnesium phyllosilicate—Thermodynamic data on the interaction with divalent cations in aqueous solution. Thermochim. Acta 2000, 359, 1–9. [Google Scholar] [CrossRef]
- Fukushima, Y.; Tani, M. Synthesis of 2:1 Type 3-(Methacryloxy) propyl Magnesium (Nickel) Phyllosilicate. Bull. Chem. Soc. Jpn. 1996, 69, 3667–3671. [Google Scholar] [CrossRef]
- Silva, C.R.; Fonseca, M.G.; Barone, J.S.; Airoldi, C. Layered Inorganic–Organic Talc-like Nanocomposites. Chem. Mater. 2002, 14, 175–179. [Google Scholar] [CrossRef]
- Da Fonseca, M.G.; Airoldi, C. New layered inorganic–organic nanocomposites containing n-propylmercapto copper phyllosilicates. J. Mater. Chem. 2000, 10, 1457–1463. [Google Scholar] [CrossRef]
- Da Fonseca, M.G.; da Silva Filho, E.C.; Machado Junior, R.S.A.; Arakaki, L.N.H.; Espinola, J.G.P.; Airoldi, C. Zinc phyllosilicates containing amino pendant groups. J. Solid State Chem. 2004, 177, 2316–2322. [Google Scholar] [CrossRef]
- Minet, J.; Abramson, S.; Bresson, B.; Sanchez, C.; Montouillout, V.; Lequeux, N. New Layered Calcium Organosilicate Hybrids with Covalently Linked Organic Functionalities. Chem. Mater. 2004, 16, 3955–3962. [Google Scholar] [CrossRef]
- Mizutani, T.; Fukushima, Y.; Okada, A.; Kamigaito, O. Synthesis of nickel and magnesium phyllosilicates with 1:1 and 2:1 layer structures. Bull. Chem. Soc. Jpn. 1990, 63, 2094–2098. [Google Scholar] [CrossRef]
- Sasai, R.; Itoh, H.; Shindachi, I.; Shichi, T.; Takagi, K. Photochromism of Clay–Diarylethene Hybrid Materials in Optically Transparent Gelatin Films. Chem. Mater. 2001, 13, 2012–2016. [Google Scholar] [CrossRef]
- Da Fonseca, M.G.; Silva, C.R.; Barone, J.S.; Airoldi, C. Layered hybrid nickel phyllosilicates and reactivity of the gallery space. J. Mater. Chem. 2000, 10, 789–795. [Google Scholar] [CrossRef]
- Toeri, J.; Osorio-Madrazo, A.; Laborie, M.-P. Preparation and Chemical/Microstructural Characterization of Azacrown Ether-Crosslinked Chitosan Films. Materials 2017, 10, 400. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Elliott, P.; Arnold, R.; Barltrop, D.; Thornton, I.; House, I.M.; Henry, J.A. Clinical lead poisoning in England: An analysis of routine sources of data. Occup. Environ. Med. 1999, 56, 820–824. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Ioannou, K.-I.G. Development of a sequential injection dispersive liquid–liquid microextraction system for electrothermal atomic absorption spectrometry by using a hydrophobic sorbent material: Determination of lead and cadmium in natural waters. Anal. Chim. Acta 2010, 668, 35–40. [Google Scholar] [CrossRef]
- Wan, Z.; Xu, Z.; Wang, J. Flow injection on-line solid phase extraction for ultra-trace lead screening with hydride generation atomic fluorescence spectrometry. Analyst 2006, 131, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-F.; Chang, W.-S.; Hsieh, Y.-K.; Wang, C.-F. Lead determination in whole blood by laser ablation coupled with inductively coupled plasma mass spectrometry. Talanta 2009, 79, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.A.; Meyerhoff, M.E. Recent Advances in the Development and Analytical Applications of Biosensing Probes. C R C Crit. Rev. Anal. Chem. 1988, 20, 149–196. [Google Scholar] [CrossRef]
- Ngouoko, J.J.K.; Tajeu, K.Y.; Temgoua, R.C.T.; Doungmo, G.; Doench, I.; Tamo, A.K.; Kamgaing, T.; Osorio-Madrazo, A.; Tonle, I.K. Hydroxyapatite/L-Lysine Composite Coating as Glassy Carbon Electrode Modifier for the Analysis and Detection of Nile Blue A. Materials 2022, 15, 4262. [Google Scholar] [CrossRef]
- Sun, D.; Sun, Z. Electrochemical determination of Pb2+ using a carbon nanotube/Nafion composite film-modified electrode. J. Appl. Electrochem. 2008, 38, 1223–1227. [Google Scholar] [CrossRef]
- Yantasee, W.; Lin, Y.; Hongsirikarn, K.; Fryxell, G.E.; Addleman, R.; Timchalk, C. Electrochemical Sensors for the Detection of Lead and Other Toxic Heavy Metals: The Next Generation of Personal Exposure Biomonitors. Environ. Health Perspect. 2007, 115, 1683–1690. [Google Scholar] [CrossRef]
- Tonlé, I.K.; Letaief, S.; Ngameni, E.; Walcarius, A.; Detellier, C. Square Wave Voltammetric Determination of Lead(II) Ions Using a Carbon Paste Electrode Modified by a Thiol-Functionalized Kaolinite. Electroanalysis 2011, 23, 245–252. [Google Scholar] [CrossRef]
- Ngassa, G.B.P.; Tonlé, I.K.; Walcarius, A.; Ngameni, E. One-step co-intercalation of cetyltrimethylammonium and thiourea in smectite and application of the organoclay to the sensitive electrochemical detection of Pb(II). Appl. Clay Sci. 2014, 99, 297–305. [Google Scholar] [CrossRef]
- Jiokeng, S.L.Z.; Dongmo, L.M.; Ymélé, E.; Ngameni, E.; Tonlé, I.K. Sensitive stripping voltammetry detection of Pb(II) at a glassy carbon electrode modified with an amino-functionalized attapulgite. Sens. Actuators B Chem. 2017, 242, 1027–1034. [Google Scholar] [CrossRef]
- Guenang, L.S.; Dongmo, L.M.; Jiokeng, S.L.Z.; Kamdem, A.T.; Doungmo, G.; Tonlé, I.K.; Bassetto, V.C.; Jović, M.; Lesch, A.; Girault, H. Montmorillonite clay-modified disposable ink-jet-printed graphene electrode as a sensitive voltammetric sensor for the determination of cadmium(II) and lead(II). SN Appl. Sci. 2020, 2, 443. [Google Scholar] [CrossRef]
- Sales, J.A.A.; Airoldi, C. Calorimetric investigation of metal ion adsorption on 3-glycidoxypropyltrimethylsiloxane + propane-1,3-diamine immobilized on silica gel. Thermochim. Acta 2005, 427, 77–83. [Google Scholar] [CrossRef]
- Tanev, P.T.; Pinnavaia, T.J. A Neutral Templating Route to Mesoporous Molecular Sieves. Science 1995, 267, 865–867. [Google Scholar] [CrossRef]
- Sales, J.A.A.; Petrucelli, G.C.; Oliveira, F.J.V.E.; Airoldi, C. Some features associated with organosilane groups grafted by the sol–gel process onto synthetic talc-like phyllosilicate. J. Colloid Interface Sci. 2006, 297, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ukrainczyk, L.; Bellman, R.A.; Anderson, A.B. Template Synthesis and Characterization of Layered Al- and Mg-Silsesquioxanes. J. Phys. Chem. B 1997, 101, 531–539. [Google Scholar] [CrossRef]
- Whilton, N.T.; Burkett, S.L.; Mann, S. Hybrid lamellar nanocomposites based on organically functionalized magnesium phyllosilicate clays with interlayer reactivity. J. Mater. Chem. 1998, 8, 1927–1932. [Google Scholar] [CrossRef]
- Kamdem Tamo, A.; Doench, I.; Morales Helguera, A.; Hoenders, D.; Walther, A.; Madrazo, A.O. Biodegradation of Crystalline Cellulose Nanofibers by Means of Enzyme Immobilized-Alginate Beads and Microparticles. Polymers 2020, 12, 1522. [Google Scholar] [CrossRef]
- Marquez-Bravo, S.; Doench, I.; Molina, P.; Bentley, F.E.; Tamo, A.K.; Passieux, R.; Lossada, F.; David, L.; Osorio-Madrazo, A. Functional Bionanocomposite Fibers of Chitosan Filled with Cellulose Nanofibers Obtained by Gel Spinning. Polymers 2021, 13, 1563. [Google Scholar] [CrossRef]
- Lall, A.; Kamdem Tamo, A.; Doench, I.; David, L.; Nunes de Oliveira, P.; Gorzelanny, C.; Osorio-Madrazo, A. Nanoparticles and Colloidal Hydrogels of Chitosan–Caseinate Polyelectrolyte Complexes for Drug-Controlled Release Applications. Int. J. Mol. Sci. 2020, 21, 5602. [Google Scholar] [CrossRef]
- Djouonkep, L.D.W.; Tamo, A.K.; Doench, I.; Selabi, N.B.S.; Ilunga, E.M.; Lenwoue, A.R.K.; Gauthier, M.; Cheng, Z.; Osorio-Madrazo, A. Synthesis of High Performance Thiophene–Aromatic Polyesters from Bio-Sourced Organic Acids and Polysaccharide-Derived Diol: Characterization and Degradability Studies. Molecules 2022, 27, 325. [Google Scholar] [CrossRef]
- Deussi Ngaha, M.C.; Kougoum Tchieda, V.; Kamdem Tamo, A.; Doungmo, G.; Njanja, E.; Kenfack Tonle, I. Aminoalcohol-functionalization of Alkali Palm Oil Fiber and Application as Electrochemical Sensor for 2-nitrophenol Determination. Electroanalysis 2022, 9, 5222. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; David, L.; Peniche-Covas, C.; Rochas, C.; Putaux, J.-L.; Trombotto, S.; Alcouffe, P.; Domard, A. Fine microstructure of processed chitosan nanofibril networks preserving directional packing and high molecular weight. Carbohydr. Polym. 2015, 131, 1–8. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: Hoboken, NJ, USA, 2015; ISBN 978-0-470-61637-6. [Google Scholar]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy: A Guide for Students of Organic Chemistry; Saunders College: Philadelphia, PA, USA, 1979; ISBN 9780721671192. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; ISBN 9780470405840. [Google Scholar]
- Wilson, M.J. Clay Mineralogy: Spectroscopic and Chemical Determinative Methods; Springer: Dordrecht, The Netherlands, 1994; ISBN 978-94-011-0727-3. [Google Scholar]
- Fritsch, E.; Balan, E.; Petit, S.; Juillot, F. Structural, textural, and chemical controls on the OH stretching vibrations in serpentine-group minerals. Eur. J. Miner. 2021, 33, 447–462. [Google Scholar] [CrossRef]
- Dongmo, L.M.; Guenang, L.S.; Jiokeng, S.L.Z.; Kamdem, A.T.; Doungmo, G.; Victor, B.C.; Jović, M.; Lesch, A.; Tonlé, I.K.; Girault, H. A new sensor based on an amino-montmorillonite-modified inkjet-printed graphene electrode for the voltammetric determination of gentisic acid. Mikrochim. Acta 2021, 188, 36. [Google Scholar] [CrossRef]
- Ferrage, E.; Martin, F.; Petit, S.; Pejo-soucaille, S.; Micoud, P.; Fourty, G.; Ferret, J.; Salvi, S.; de Parseval, P.; Fortune, J.P. Evaluation of talc morphology using FTIR and H/D substitution. Clay Miner. 2003, 38, 141–150. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; Laborie, M.-P. Morphological and Thermal Investigations of Cellulosic Bionanocomposites. In Biopolymer Nanocomposites; Dufresne, A., Thomas, S., Pothen, L.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 411–436. ISBN 9781118609958. [Google Scholar]
- Von Palubitzki, L.; Wang, Y.; Hoffmann, S.; Vidal-Y-Sy, S.; Zobiak, B.; Failla, A.V.; Schmage, P.; John, A.; Osorio-Madrazo, A.; Bauer, A.T.; et al. Differences of the tumour cell glycocalyx affect binding of capsaicin-loaded chitosan nanocapsules. Sci. Rep. 2020, 10, 22443. [Google Scholar] [CrossRef]
- Samyn, P.; Osorio-Madrazo, A. Native Crystalline Polysaccharide Nanofibers: Processing and Properties. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–36. ISBN 978-3-319-42789-8. [Google Scholar]
- Lizundia, E.; Costa, C.M.; Alves, R.; Lanceros-Méndez, S. Cellulose and its derivatives for lithium ion battery separators: A review on the processing methods and properties. Carbohydr. Polym. Technol. Appl. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Abushammala, H.; Pontes, J.F.; Gomes, G.H.; Osorio-Madrazo, A.; Thiré, R.M.S.M.; Pereira, F.V.; Laborie, M.-P.G. Swelling, viscoelastic, and anatomical studies on ionic liquid-swollen Norway spruce as a screening tool toward ionosolv pulping. Holzforschung 2015, 69, 1059–1067. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, Y.; Lv, Y.; Wang, J.; Jia, C.; Liu, J.; Zhang, X.; Sun, J.; Shao, Z. Carboxymethyl Cellulose Nanofibrils with a Treelike Matrix: Preparation and Behavior of Pickering Emulsions Stabilization. ACS Sustain. Chem. Eng. 2019, 7, 12887–12896. [Google Scholar] [CrossRef]
- Bentley, F.E.; Passieux, R.; David, L.; Osorio-Madrazo, A. Pure Chitosan Biomedical Textile Fibers from Mixtures of Low- and High-Molecular Weight Bidisperse Polymer Solutions: Processing and Understanding of Microstructure–Mechanical Properties’ Relationship. Int. J. Mol. Sci. 2022, 23, 4767. [Google Scholar] [CrossRef]
- Amine, S.; Montembault, A.; Fumagalli, M.; Osorio-Madrazo, A.; David, L. Controlled Polyelectrolyte Association of Chitosan and Carboxylated Nano-Fibrillated Cellulose by Desalting. Polymers 2021, 13, 2023. [Google Scholar] [CrossRef]
- Doench, I.; Torres-Ramos, M.E.W.; Montembault, A.; Nunes de Oliveira, P.; Halimi, C.; Viguier, E.; Heux, L.; Siadous, R.; Thiré, R.M.S.M.; Osorio-Madrazo, A. Injectable and Gellable Chitosan Formulations Filled with Cellulose Nanofibers for Intervertebral Disc Tissue Engineering. Polymers 2018, 10, 1202. [Google Scholar] [CrossRef]
- Kamdem Tamo, A.; Doench, I.; Walter, L.; Montembault, A.; Sudre, G.; David, L.; Morales-Helguera, A.; Selig, M.; Rolauffs, B.; Bernstein, A.; et al. Development of Bioinspired Functional Chitosan/Cellulose Nanofiber 3D Hydrogel Constructs by 3D Printing for Application in the Engineering of Mechanically Demanding Tissues. Polymers 2021, 13, 1663. [Google Scholar] [CrossRef]
- Opallo, M.; Lesniewski, A. A review on electrodes modified with ionic liquids. J. Electroanal. Chem. 2011, 656, 2–16. [Google Scholar] [CrossRef]
- Goyal, R.N.; Gupta, V.K.; Chatterjee, S. Voltammetric biosensors for the determination of paracetamol at carbon nanotube modified pyrolytic graphite electrode. Sens. Actuators B Chem. 2010, 149, 252–258. [Google Scholar] [CrossRef]
- Martin Santos, A.; Wong, A.; Araújo Almeida, A.; Fatibello-Filho, O. Simultaneous determination of paracetamol and ciprofloxacin in biological fluid samples using a glassy carbon electrode modified with graphene oxide and nickel oxide nanoparticles. Talanta 2017, 174, 610–618. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Huang, W.; Zhang, T.; Hu, X.; Perman, J.A.; Ma, S. A metal–organic framework and conducting polymer based electrochemical sensor for high performance cadmium ion detection. J. Mater. Chem. A 2017, 5, 8385–8393. [Google Scholar] [CrossRef]
- Bouwe, R.G.B.; Tonle, I.K.; Letaief, S.; Ngameni, E.; Detellier, C. Structural characterisation of 1,10-phenanthroline–montmorillonite intercalation compounds and their application as low-cost electrochemical sensors for Pb(II) detection at the sub-nanomolar level. Appl. Clay Sci. 2011, 52, 258–265. [Google Scholar] [CrossRef]
- Wei, Y.; Gao, C.; Meng, F.-L.; Li, H.-H.; Wang, L.; Liu, J.-H.; Huang, X.-J. SnO2/Reduced Graphene Oxide Nanocomposite for the Simultaneous Electrochemical Detection of Cadmium(II), Lead(II), Copper(II), and Mercury(II): An Interesting Favorable Mutual Interference. J. Phys. Chem. C 2012, 116, 1034–1041. [Google Scholar] [CrossRef]
- Ghoneim, M.M.; Hassanein, A.M.; Hammam, E.; Beltagi, A.M. Simultaneous determination of Cd, Pb, Cu, Sb, Bi, Se, Zn, Mn, Ni, Co and Fe in water samples by differential pulse stripping voltammetry at a hanging mercury drop electrode. Anal. Bioanal. Chem. 2000, 367, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Ebunang, D.V.T.; Tajeu, K.Y.; Pecheu, C.N.; Jiokeng, S.L.Z.; Tamo, A.K.; Doench, I.; Osorio-Madrazo, A.; Tonle, I.K.; Ngameni, E. Amino-Functionalized Laponite Clay Material as a Sensor Modifier for the Electrochemical Detection of Quercetin. Sensors 2022, 22, 6173. [Google Scholar] [CrossRef]
- El Mhammedi, M.A.; Achak, M.; Chtaini, A. Ca10(PO4)6(OH)2-modified carbon-paste electrode for the determination of trace lead(II) by square-wave voltammetry. J. Hazard. Mater. 2009, 161, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Wang, Y.; Chen, Z.; Lou, T.; Qin, W. Nanomaterial/Ionophore-Based Electrode for Anodic Stripping Voltammetric Determination of Lead: An Electrochemical Sensing Platform toward Heavy Metals. Anal. Chem. 2009, 81, 5088–5094. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, H.; Fu, C.; Wang, F.; Ding, Y.; Kuang, Y. A novel design of engineered multi-walled carbon nanotubes material and its improved performance in simultaneous detection of Cd(II) and Pb(II) by square wave anodic stripping voltammetry. Sens. Actuators B Chem. 2016, 236, 144–152. [Google Scholar] [CrossRef]
- Oliveira, V.H.B.; Rechotnek, F.; da Silva, E.P.; Marques, V.d.S.; Rubira, A.F.; Silva, R.; Lourenço, S.A.; Muniz, E.C. A sensitive electrochemical sensor for Pb2+ ions based on ZnO nanofibers functionalized by L-cysteine. J. Mol. Liq. 2020, 309, 113041. [Google Scholar] [CrossRef]
- Mohammadi, S.; Taher, M.A.; Beitollahi, H. Synthesis and application of a natural-based nanocomposite with carbon nanotubes for sensitive voltammetric determination of lead (II) ions. Int. J. Environ. Anal. Chem. 2020, 100, 65–81. [Google Scholar] [CrossRef]







| Molar Ratios | |||||||
|---|---|---|---|---|---|---|---|
| Reaction Mixture | Product | Weight (%) | |||||
| Si/Mg | C/N | Si/Mg | C/N | C | H | N | |
| Nat-Talc | - | - | 1.33 | - | 0.357 | 0.469 | 0 |
| TalcNH2 | 1.33 | 3.00 | 0.50 | 3.33 | 14.90 (14.57 *) | 4.83 (4.89 *) | 4.42 (5.10 *) |
| Electrode | DL (µM) | Linear Range (µM) | Reference |
|---|---|---|---|
| Ca10(PO4)6(OH)2 modified CPE * | 0.0768 | 0.002–0.24 | [75] |
| Nanosized HA nafion modified GCE | 0.001 | 0.005–0.8 | [76] |
| GCE covered by engineered MWCNT ** | 0.0188 | 0.4–80 | [77] |
| GCE covered by zinc oxide nanofibers functionalized by L-cysteine | 0.0012 | 0.03–0.42 | [78] |
| CPE modified with magnetic eggshell nanocomposite and MWCNT | 0.452 | 1.5–600 | [79] |
| TalcNH2/GCE | 0.0745 | 0.8–2.5 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecheu, C.N.; Jiokeng, S.L.Z.; Tamo, A.K.; Doungmo, G.; Doench, I.; Osorio-Madrazo, A.; Tonle, I.K.; Ngameni, E. Fabrication of an Organofunctionalized Talc-like Magnesium Phyllosilicate for the Electrochemical Sensing of Lead Ions in Water Samples. Nanomaterials 2022, 12, 2928. https://doi.org/10.3390/nano12172928
Pecheu CN, Jiokeng SLZ, Tamo AK, Doungmo G, Doench I, Osorio-Madrazo A, Tonle IK, Ngameni E. Fabrication of an Organofunctionalized Talc-like Magnesium Phyllosilicate for the Electrochemical Sensing of Lead Ions in Water Samples. Nanomaterials. 2022; 12(17):2928. https://doi.org/10.3390/nano12172928
Chicago/Turabian StylePecheu, Chancellin Nkepdep, Sherman Lesly Zambou Jiokeng, Arnaud Kamdem Tamo, Giscard Doungmo, Ingo Doench, Anayancy Osorio-Madrazo, Ignas Kenfack Tonle, and Emmanuel Ngameni. 2022. "Fabrication of an Organofunctionalized Talc-like Magnesium Phyllosilicate for the Electrochemical Sensing of Lead Ions in Water Samples" Nanomaterials 12, no. 17: 2928. https://doi.org/10.3390/nano12172928






