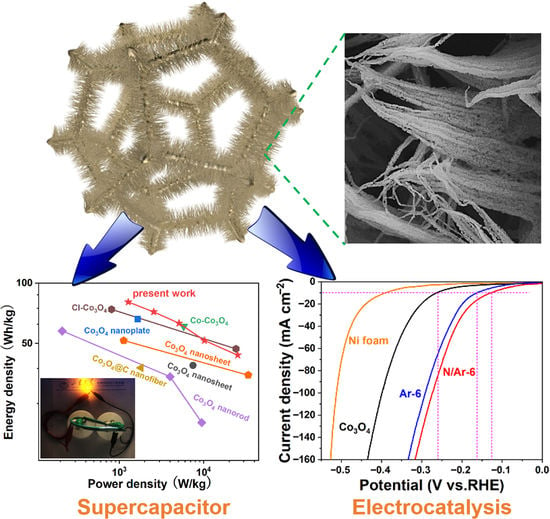
Plasma-Engineered N-CoOx Nanowire Array as a Bifunctional Electrode for Supercapacitor and Electrocatalysis
Abstract
:1. Introduction
2. Materials and Methods
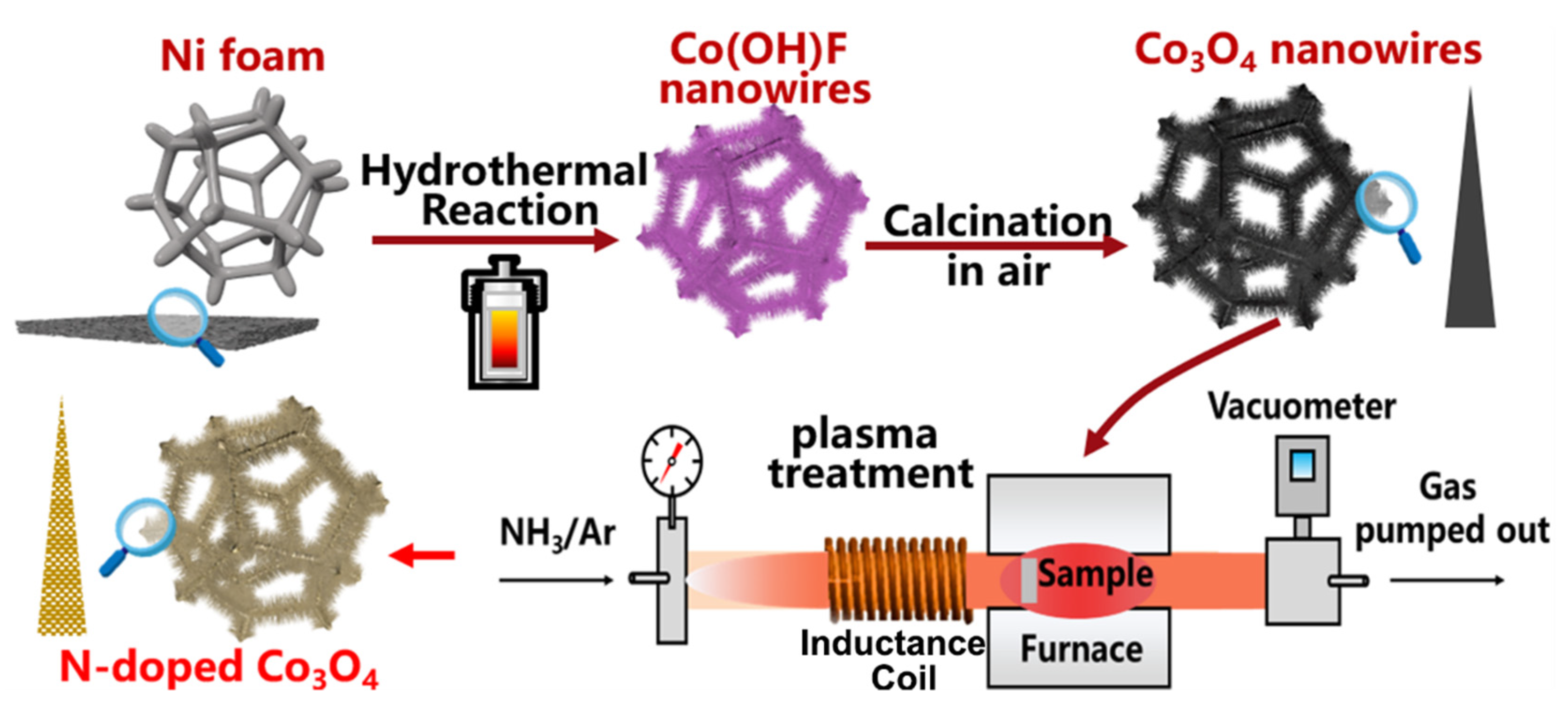
2.1. Direct Growth of Co3O4 Nanowires on Ni Foam
2.2. Plasma-Induced Fabrication of N-Doped Co3O4
2.3. Characterization
2.4. Electrochemical Measurements
2.4.1. Electrochemical Evaluation of Supercapacitor Performance
2.4.2. Electrochemical Performance of Electrocatalytic Hydrogen Evolution
2.5. Calculations
3. Results and Discussion
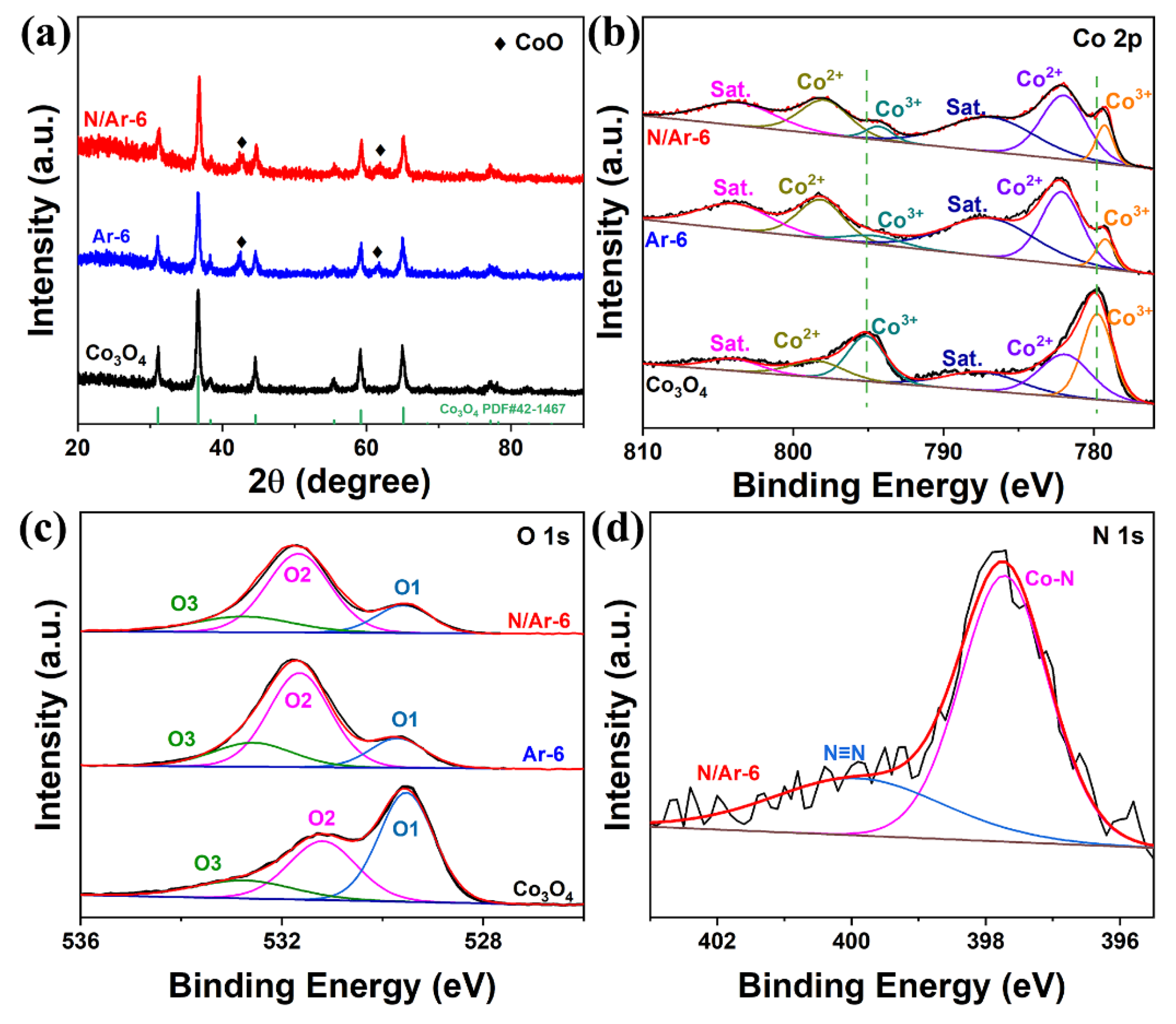
3.1. Structural Characterization
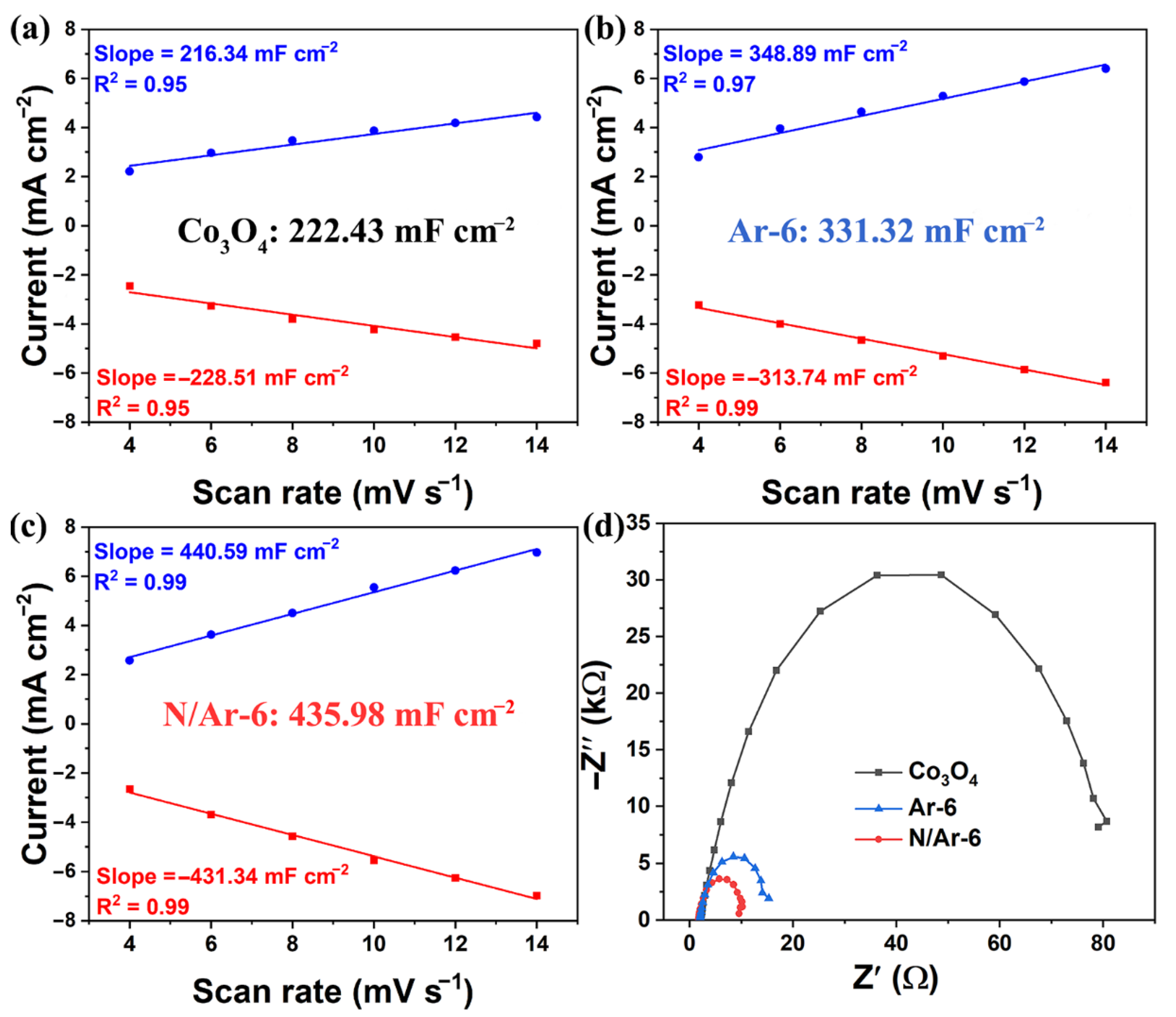
3.2. Half Cell Studies
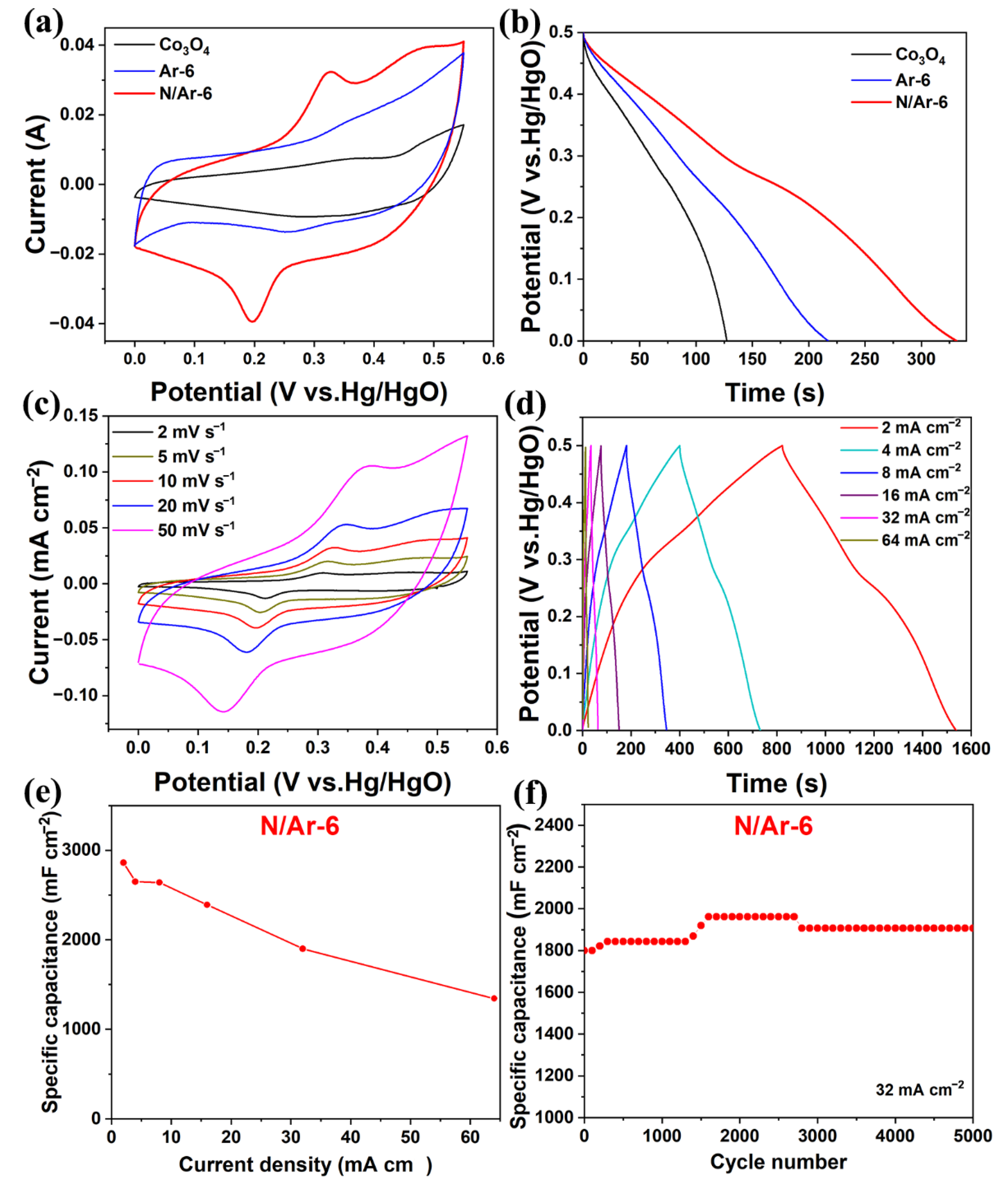
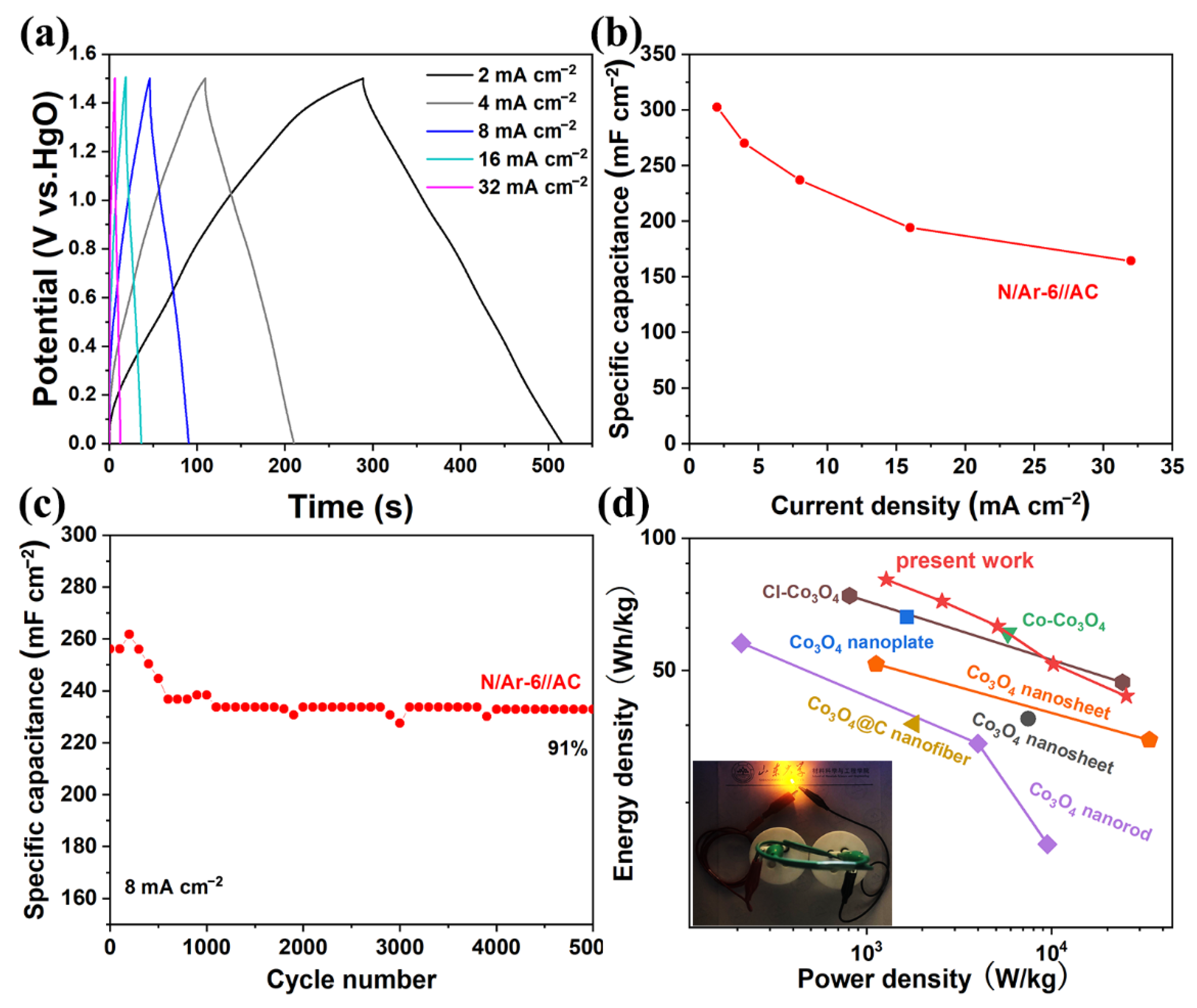
3.3. Asymmetric Supercapacitor Devices Performance
3.4. HER Performance
3.5. Mechanisam Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brosemer, K.; Schelly, C.; Gagnon, V.; Arola, K.L.; Pearce, J.M.; Bessette, D.; Olabisi, L.S. The energy crises revealed by COVID: Intersections of Indigeneity, inequity, and health. Energy Res. Soc. Sci. 2020, 68, 101661. [Google Scholar] [CrossRef]
- Fan, W.; Hao, Y. An empirical research on the relationship amongst renewable energy consumption, economic growth and foreign direct investment in China. Renew. Energy 2020, 146, 598–609. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S. Preventing carbon emission retaliatory rebound post-COVID-19 requires expanding free trade and improving energy efficiency. Sci. Total Environ. 2020, 746, 141158. [Google Scholar] [CrossRef]
- Dell’Anna, F. Green jobs and energy efficiency as strategies for economic growth and the reduction of environmental impacts. Energy Policy 2021, 149, 112031. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Zhang, Q. The relationship of renewable energy consumption to financial development and economic growth in China. Renew. Energy 2021, 170, 897–904. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, Y.; Ali, S.; Shahzad, U.; Cui, L. Exploring the role of green innovation and investment in energy for environmental quality: An empirical appraisal from provincial data of China. J. Environ. Manag. 2021, 292, 112779. [Google Scholar] [CrossRef]
- Leonard, M.D.; Michaelides, E.E.; Michaelides, D.N. Energy storage needs for the substitution of fossil fuel power plants with renewables. Renew. Energy 2020, 145, 951–962. [Google Scholar] [CrossRef]
- Yuan, K.; Shi, J.; Aftab, W.; Qin, M.; Usman, A.; Zhou, F.; Lv, Y.; Gao, S.; Zou, R. Engineering the Thermal Conductivity of Functional Phase-Change Materials for Heat Energy Conversion, Storage, and Utilization. Adv. Funct. Mater. 2019, 30, 1904228. [Google Scholar] [CrossRef]
- Sahoo, S.; Nguyen, T.T.; Shim, J.J. Mesoporous Fe–Ni–Co ternary oxide nanoflake arrays on Ni foam for high-performance supercapacitor applications. J. Ind. Eng. Chem. 2018, 63, 181–190. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Nandi, A.K. A review on the recent advances in hybrid supercapacitors. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Aziz, U. Supercapattery: Merging of battery-supercapacitor electrodes for hybrid energy storage devices. J. Energy Storage 2022, 46, 103823. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Sahoo, S.; Shim, J.J. Nanostructured 3D zinc cobaltite/nitrogen-doped reduced graphene oxide composite electrode for supercapacitor applications. J. Ind. Eng. Chem. 2017, 54, 205–217. [Google Scholar] [CrossRef]
- Maile, N.C.; Moztahida, M.; Ghani, A.A.; Hussain, M.; Tahir, K.; Kim, B.; Shinde, S.K.; Fulari, V.J.; Lee, D.S. Electrochemical synthesis of binder-free interconnected nanosheets of Mn-doped Co3O4 on Ni foam for high-performance electrochemical energy storage application. Chem. Eng. J. 2021, 421, 129767. [Google Scholar] [CrossRef]
- Shinde, S.K.; Karade, S.S.; Maile, N.C.; Yadav, H.M.; Jagadale, A.D.; Jalak, M.B.; Kim, D.-Y. Bifunctional nanoparticles decorated Ni1-XMnxCo2O4 ultrathin nanoflakes-like electrodes for supercapacitor and overall water splitting. Int. J. Energy Res. 2022, 1–23. [Google Scholar] [CrossRef]
- Li, T.; Wang, Q.; Wang, Z. Oxygen Vacancy Injection on (111) CeO2 Nanocrystal Facets for Efficient H2O2 Detection. Biosensors 2022, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Geng, G.; Chen, P.; Guan, B.; Jiang, L.; Xu, Z.; Di, D.; Tu, Z.; Hao, W.; Yi, Y.; Chen, C.; et al. Shape-controlled metal-free catalysts: Facet-sensitive catalytic activity induced by the arrangement pattern of noncovalent supramolecular chains. ACS Nano 2017, 11, 4866–4876. [Google Scholar] [CrossRef]
- Chen, W.; Xue, J.; Bao, Y.; Feng, L. Surface engineering of nano-ceria facet dependent coupling effect on Pt nanocrystals for electro-catalysis of methanol oxidation reaction. Chem. Eng. J. 2020, 381, 122752. [Google Scholar] [CrossRef]
- Zhu, J.; Mu, S. Defect engineering in carbon-based electrocatalysts: Insight into intrinsic carbon defects. Adv. Funct. Mater. 2020, 30, 2001097. [Google Scholar] [CrossRef]
- Yan, X.; Jia, Y.; Yao, X. Defective structures in metal compounds for energy-related electrocatalysis. Small Struct. 2021, 2, 2000067. [Google Scholar] [CrossRef]
- Sahoo, S.; Shim, J.J. Facile synthesis of three-dimensional ternary ZnCo2O4/reduced graphene oxide/NiO composite film on nickel foam for next generation supercapacitor electrodes. ACS Sustain. Chem. Eng. 2017, 5, 241–251. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Liu, D.; Wang, Q.; Li, T.; Wang, Z. Cu2O-BiOI isotype (p-p) heterojunction: Boosted visible-light-driven photoelectrochemical activity for non-enzymatic H2O2 sensing. Appl. Surf. Sci. 2020, 521, 146434. [Google Scholar] [CrossRef]
- Shinde, S.K.; Karade, S.S.; Maile, N.C.; Yadav, H.M.; Ghodake, G.S.; Jagadale, A.D.; Kim, D.Y. Green synthesis of novel CuCo2O4 nanocomposite for stable hybrid supercapacitors by deep eutectic solvents. J. Mol. Liq. 2021, 334, 116390. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Niu, P.; Wang, S.; Shi, J.; Li, L. Porous two-Dimensional materials for photocatalytic and electrocatalytic applications. Matter 2020, 2, 1377–1413. [Google Scholar] [CrossRef]
- Chen, P.; Xu, K.; Fang, Z.; Tong, Y.; Wu, J.; Lu, X.; Peng, X.; Ding, H.; Wu, C.; Xie, Y. Metallic Co4N porous nanowire arrays activated by surface oxidation as electrocatalysts for the oxygen evolution reaction. Angew. Chem. Int. Ed. 2015, 54, 14710–14714. [Google Scholar] [CrossRef]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef]
- Filho, E.A.S.; Pieretti, E.F.; Bento, R.T.; Pillis, M.F. Effect of nitrogen-doping on the surface chemistry and corrosion stability of TiO2 films. J. Mater. Res. Technol. 2020, 9, 922–934. [Google Scholar] [CrossRef]
- Yun, M.; Ma, Y.; Cai, Z.; Ji, H.; Han, J.; Wang, M.; Tong, Z.; Xiao, L.; Jia, S.; Chen, X. Holey graphene interpenetrating networks for boosting high-capacitive Co3O4 electrodes via an electrophoretic deposition process. Ceram. Int. 2021, 47, 27210–27216. [Google Scholar] [CrossRef]
- Ma, Y.; Ji, B.; Lv, X.; Xiong, D.; Zhao, X.; Xie, H.; Zhang, Z. Confined heterogeneous catalysis by boron nitride-Co3O4 nanosheet cluster for peroxymonosulfate oxidation toward ranitidine removal. Chem. Eng. J. 2022, 435, 135126. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, X.; Willis, W.S.; Song, D.; Huang, Y.; Zhao, J.; Li, B.; Lei, Y. Nitrogen-doped hollow Co3O4 nanofibers for both solid-state pH sensing and improved non-enzymatic glucose sensing. Electroanalysis 2019, 31, 678–687. [Google Scholar] [CrossRef]
- Yin, C.; Liu, Y.; Xia, Q.; Kang, S.; Li, X.; Wang, Y.; Cui, L. Oxygen vacancy-rich nitrogen-doped Co3O4 nanosheets as an efficient water-resistant catalyst for low temperature CO oxidation. J. Colloid Interface Sci. 2019, 553, 427–435. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, W.; Chen, X.; Peng, Y.; Song, Y.; Lv, C.; Liu, H.; Sun, J.; Yuan, D.; Li, X.; et al. Defect-rich nitrogen doped Co3O4/C porous nanocubes enable high-Efficiency bifunctional oxygen eectrocatalysis. Adv. Funct. Mater. 2019, 29, 1902875. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Wang, J.; Sui, Y.; Qi, J.; Chen, Z.; Zhang, P.; Chen, C.; Liu, W. Constructing Co(OH)F nanorods@NiCo-LDH nanocages derived from ZIF-67 for high-performance supercapacitors. Adv. Mater. Interfaces 2021, 8, 1–10. [Google Scholar] [CrossRef]
- Chhetri, K.; Kim, T.; Acharya, D.; Muthurasu, A.; Dahal, B.; Mangal, R.; Chandra, P.; Pathak, I.; Ji, S.; Hoon, T.; et al. Hollow carbon nanofibers with inside-outside decoration of bi-metallic MOF derived Ni-Fe phosphides as electrode materials for asymmetric supercapacitors. Chem. Eng. J. 2022, 450, 138363. [Google Scholar] [CrossRef]
- Bhattarai, R.M.; Chhetri, K.; Saud, S.; Teke, S.; Kim, S.J.; Mok, Y.S. Eco-friendly synthesis of cobalt molybdenum hydroxide 3d nanostructures on carbon fabric coupled with cherry flower waste-derived activated carbon for quasi-solid-state flexible asymmetric supercapacitors. ACS Appl. Nano Mater. 2022, 5, 160–175. [Google Scholar] [CrossRef]
- Ha, Y.; Shi, L.; Chen, Z.; Wu, R. Phase-transited lysozyme-driven formation of self-supported Co3O4@C nanomeshes for overall water splitting. Adv. Sci. 2019, 6, 190027. [Google Scholar] [CrossRef]
- Gao, M.; Wang, W.; Rong, Q.; Jiang, J.; Zhang, Y.; Yu, H. Porous ZnO-coated Co3O4 nanorod as a high-energy-density supercapacitor material. ACS Appl. Mater. Interfaces 2018, 10, 23163–23173. [Google Scholar] [CrossRef]
- Li, S.; Duan, Y.; Teng, Y.; Fan, N.; Huo, Y. MOF-derived tremelliform Co3O4/NiO/Mn2O3 with excellent capacitive performance. Appl. Surf. Sci. 2019, 478, 247–254. [Google Scholar] [CrossRef]
- Cai, D.; Huang, H.; Wang, D.; Liu, B.; Wang, L.; Liu, Y.; Li, Q.; Wang, T. High-Performance Supercapacitor Electrode Based on the Unique ZnO@ Co3O4 Core/Shell Heterostructures on Nickel Foam. ACS Appl. Mater. Interfaces 2014, 6, 15905–15912. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, K.; Li, N.; Zhang, H.; Dao, W.; Shen, X.; Zhu, G.; Kong, L.; Yuan, A. Nitrogen-doped carbon dots anchored NiO/Co3O4 ultrathin nanosheets as advanced cathodes for hybrid supercapacitors. J. Colloid Interface Sci. 2020, 579, 282–289. [Google Scholar] [CrossRef]
- Wang, X.; Xia, H.; Wang, X.; Gao, J.; Shi, B.; Fang, Y. Facile synthesis ultrathin mesoporous Co3O4 nanosheets for high-energy asymmetric supercapacitor. J. Alloys Compd. 2016, 686, 969–975. [Google Scholar] [CrossRef]
- Nuamah, R.A.; Noormohammed, S.; Sarkar, D.K. Supercapacitor performance evaluation of nanostructured Ag-decorated Co-Co3O4 composite thin film electrode material. Int. J. Energy Res. 2022, 46, 13099–13110. [Google Scholar] [CrossRef]
- Kannan, V.; Choi, J.H.; Park, H.C.; Kim, H.S. Ultrahigh supercapacitance in cobalt oxide nanorod film grown by oblique angle deposition technique. Curr. Appl. Phys. 2018, 18, 1399–1402. [Google Scholar] [CrossRef]
- Barik, R.; Raulo, A.; Jha, S.; Nandan, B.; Ingole, P.P. Polymer-derived electrospun Co3O4@C porous nanofiber network for flexible, high-performance, and stable supercapacitors. ACS Appl. Energy Mater. 2020, 3, 11002–11014. [Google Scholar] [CrossRef]
- Zhang, H.; Geng, S.; Ouyang, M.; Mao, M.; Xie, F.; Riley, D.J. Using metal cation to control the microstructure of cobalt oxide in energy conversion and storage applications. Small 2022, 18, 2106391. [Google Scholar] [CrossRef]
- Yesuraj, J.; Lee, H.O.; Pandiyan, M.K.; Jayavelu, J.; Bhagavathiachari, M.; Kim, K. Bio-Engineered Hexagon-shaped Co3O4 nanoplates on deoxyribonucleic acid (DNA) scaffold: An efficient electrode material for an asymmetric supercapacitor and electrocatalysis application. J. Mol. Struct. 2022, 1256, 132499. [Google Scholar] [CrossRef]
- Kang, M.; Zhou, H.; Wen, P.; Zhao, N. Highly hierarchical porous ultrathin Co3O4 nanosheets@Ni foam for high-performance supercapacitors. ACS Appl. Energy Mater. 2021, 4, 1619–1627. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhong, T.; Wang, Z. Plasma-Engineered N-CoOx Nanowire Array as a Bifunctional Electrode for Supercapacitor and Electrocatalysis. Nanomaterials 2022, 12, 2984. https://doi.org/10.3390/nano12172984
Wang Q, Zhong T, Wang Z. Plasma-Engineered N-CoOx Nanowire Array as a Bifunctional Electrode for Supercapacitor and Electrocatalysis. Nanomaterials. 2022; 12(17):2984. https://doi.org/10.3390/nano12172984
Chicago/Turabian StyleWang, Qi, Tongtong Zhong, and Zhou Wang. 2022. "Plasma-Engineered N-CoOx Nanowire Array as a Bifunctional Electrode for Supercapacitor and Electrocatalysis" Nanomaterials 12, no. 17: 2984. https://doi.org/10.3390/nano12172984