Sb Nanoparticles Embedded in the N-Doped Carbon Fibers as Binder-Free Anode for Flexible Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
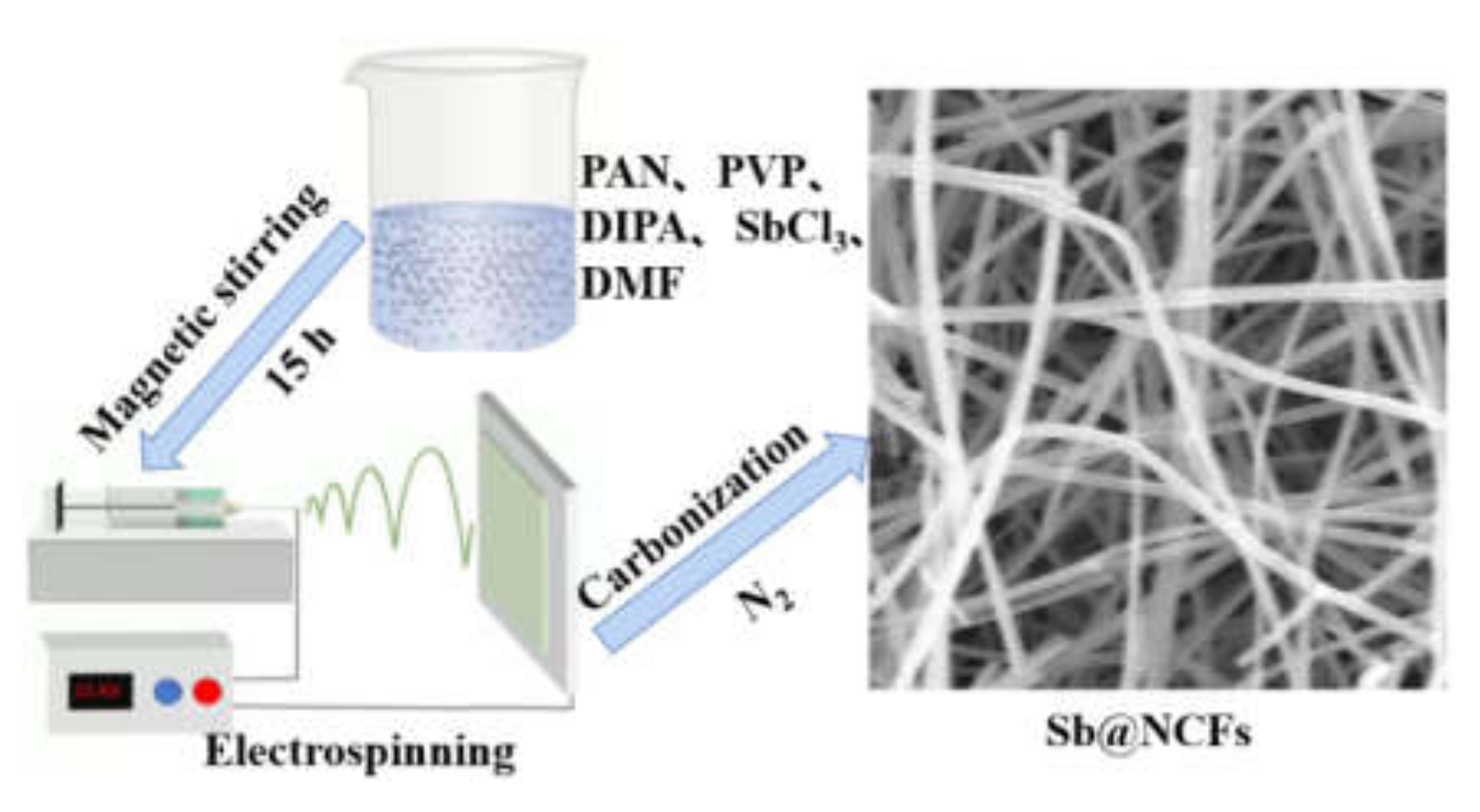
2.2. Synthesis of Sb@NCFs
2.3. Material Characterizations
2.4. Electrochemical Characterizations
3. Results and Discussion
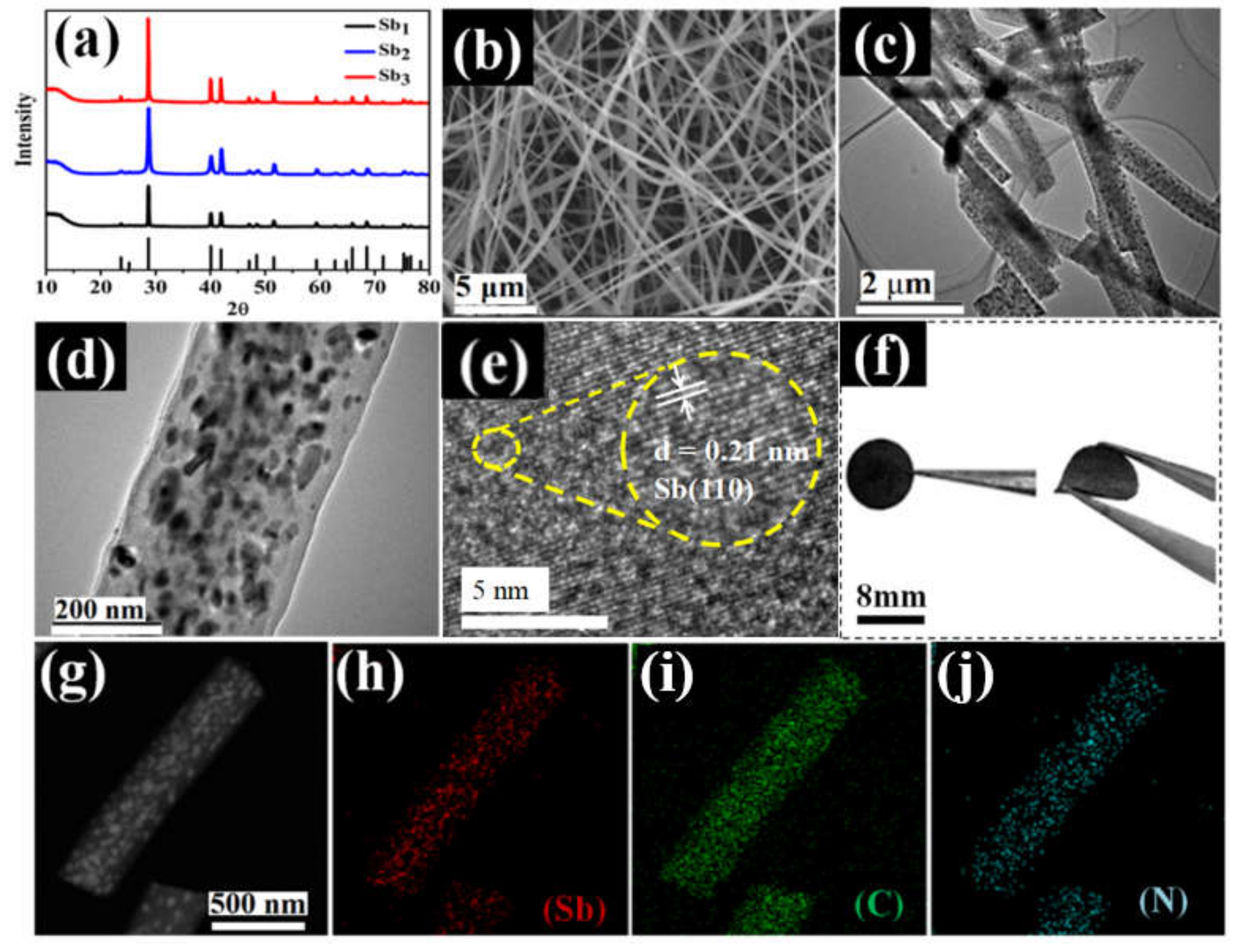
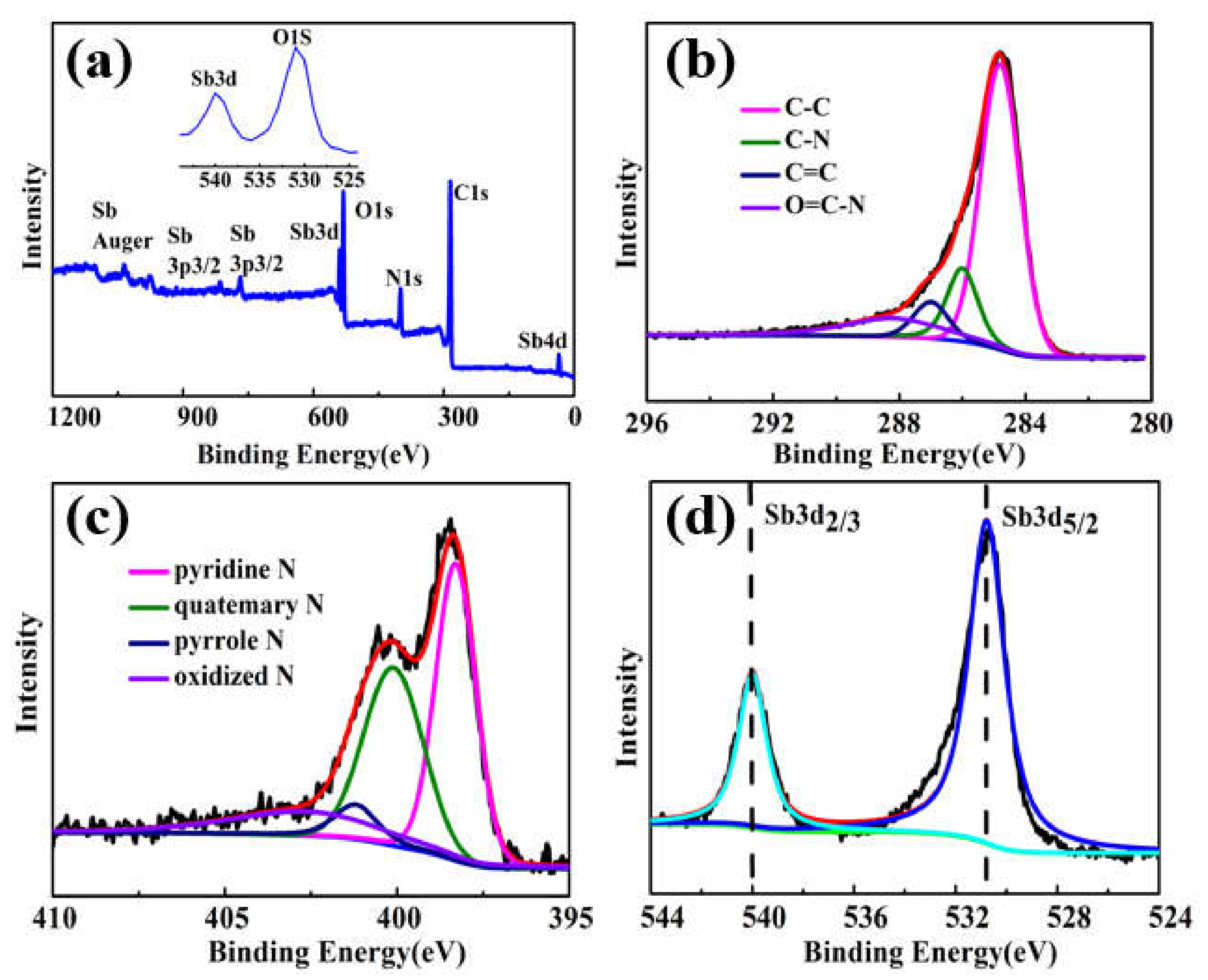
3.1. Characterizations of Sb@NCFs Composite
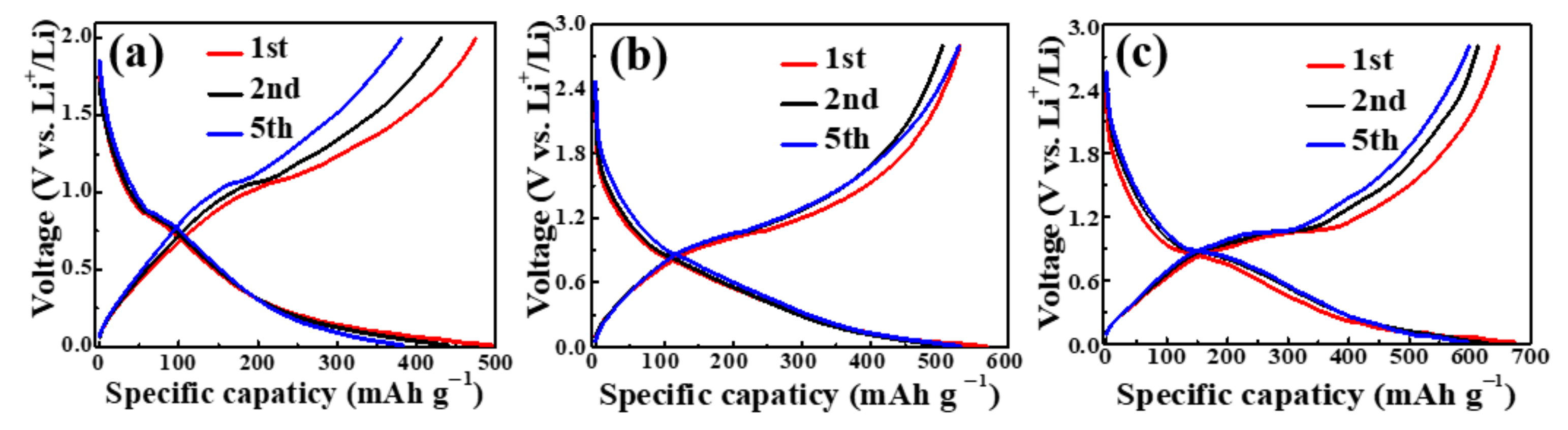
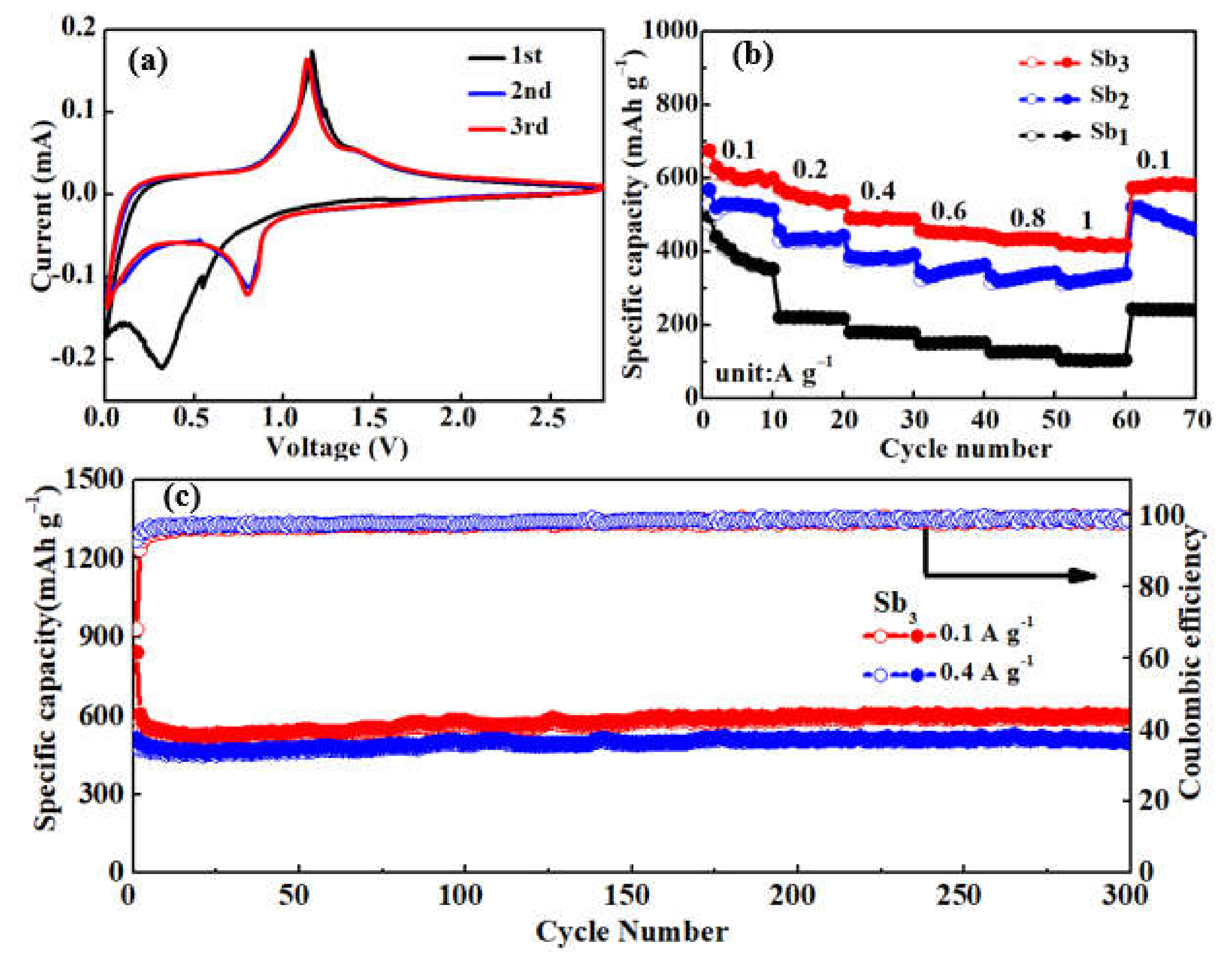
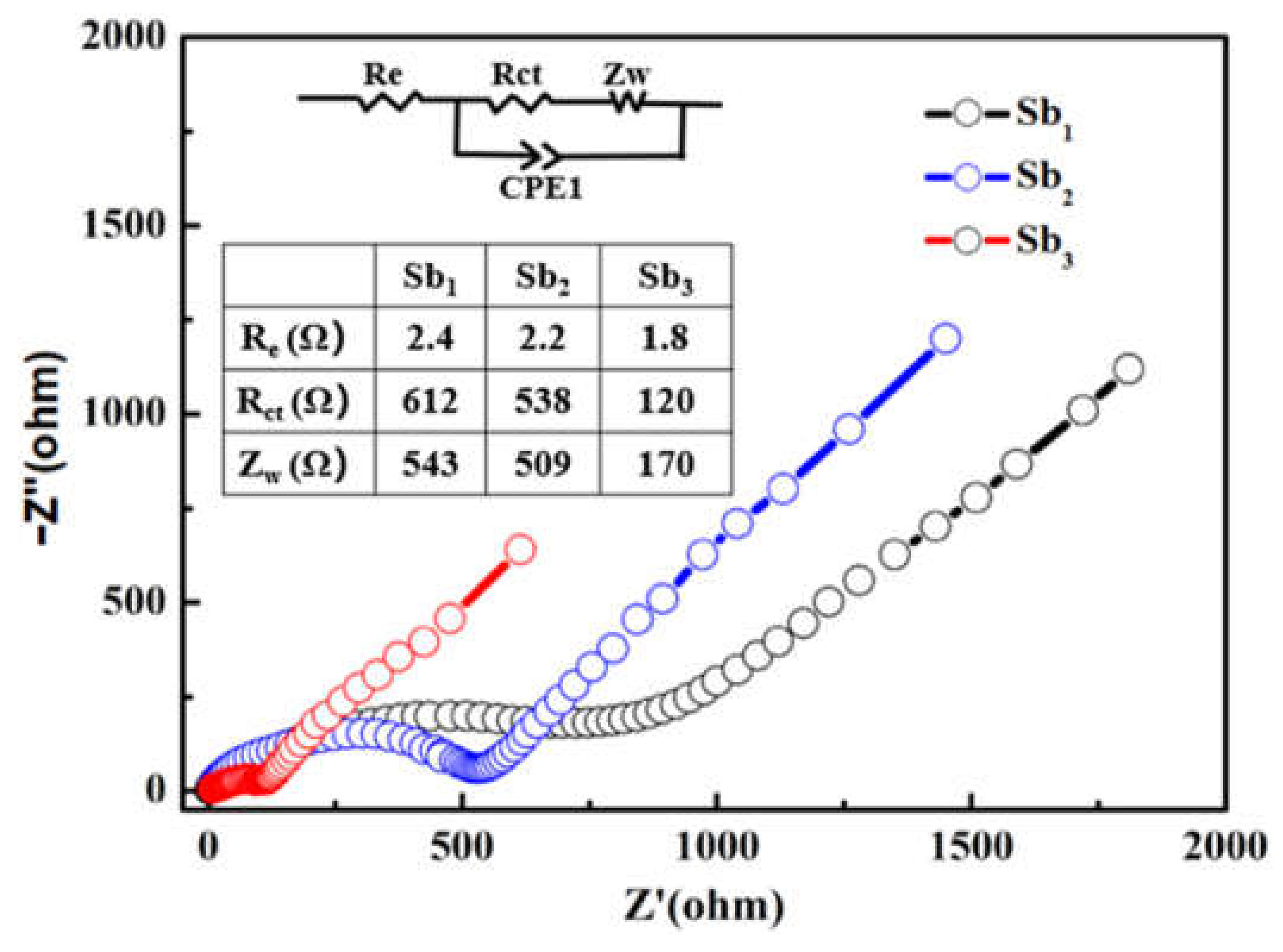
3.2. Electrochemical Performance of Sb@NCFs Composite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qin, W.; Zhou, N.; Wu, C.; Xie, M.; Sun, H.; Guo, Y.; Pan, L. Mini-review on the redox additives in aqueous electrolyte for high performance supercapacitors. ACS Omega 2020, 5, 3801–3808. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Hamilton, D.; Manjunatha, K.N.; Minakshi, M. Design, development and thermal analysis of reusable Li- ion battery module for future mobile and stationary applications. Energies 2020, 13, 1477. [Google Scholar] [CrossRef]
- Liu, J.; Wang, M.; Wang, Q.; Zhao, X.; Song, Y.; Zhao, T.; Sun, J. Sea urchin-like Si@MnO2@rGO as anodes for high-performance lithium-ion batteries. Nanomaterials 2022, 12, 285. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Whittingham, M.S. Challenges and development of tin-based anode with high volumetric capacity for Li-ion batteries Electrochem. Energy R. 2020, 3, 643–655. [Google Scholar]
- Gulzar, U.; Li, T.; Bai, X.; Goriparti, S.; Brescia, R.; Capiglia, C.; Zaccaria, R.P. Nitrogen-doped single walled carbon nanohorns enabling effective utilization of Ge nanocrystals for next generation lithium ion batteries. Electrochim. Acta 2019, 298, 89–96. [Google Scholar] [CrossRef]
- Yi, Z.; Han, Q.G.; Zan, P.; Wu, Y.M.; Cheng, Y.; Wang, L.M. Sb nanoparticles encapsulated into porous carbon matrixes for high-performance lithium-ion battery anodes. J. Power Sources 2016, 331, 16–21. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, T.; Bian, Z.; Yang, J.; Zheng, S. Tuning particle and phase formation of Sn/carbon nanofibers composite towards stable lithium-ion storage. J. Power Sources 2020, 453, 227467. [Google Scholar] [CrossRef]
- Shi, Q.; Zhou, J.; Ullah, S.; Yang, X.; Tokarska, K.; Trzebicka, B.; Ta, H.Q.; Rümmeli, M.H. A review of recent developments in Si/C composite materials for Li-ion batteries. Energy Storage Mater. 2021, 34, 735–754. [Google Scholar] [CrossRef]
- Jayaraman, S.; Aravindan, V.; Ulaganathan, M.; Ling, W.C.; Ramakrishna, S.; Madhavi, S. Ultralong durability of porous α-Fe2O3 nanofibers in practical Li-ion configuration with LiMn2O4 cathode. Adv. Sci. 2015, 2, 1500050. [Google Scholar] [CrossRef] [PubMed]
- Kitta, M.; Kohyama, M. Nanoscale controlled Li-insertion reaction induced by scanning electron-beam irradiation in a Li4Ti5O12 electrode material for lithium-ion batteries. Phys. Chem. Chem. Phys. 2017, 19, 11581–11587. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zeng, G.; Chen, J.; Lu, C.; Wen, Z. 3D graphene network encapsulating SnO2 hollow spheres as a high-performance anode material for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 4535–4542. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Minakshi, M.; Bahri, P.A.; Paul, S.; Kumari, P.; Divakaran, A.M.; Manjunatha, K.N. Rational design on materials for developing next generation lithium-ion secondary battery. Prog. Solid State Chem. 2021, 62, 100298. [Google Scholar] [CrossRef]
- Solmaz, R.; Karahan, B.D. Effect of vinylene carbonate as electrolyte additive for Mn2O3/NiMnO3 anodes of lithium-ion batteries. Ionics 2021, 27, 2813–2824. [Google Scholar] [CrossRef]
- Miao, Y.E.; Huang, Y.P.; Zhang, L.; Fan, W.; Lai, F.L.; Liu, T.X. Electrospun porous carbon nanofiber@MoS2 core/sheath fiber membranes as highly flexible and binder-free anodes for lithium-ion batteries. Nanoscale 2015, 7, 11093–11101. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Lian, F.; Guan, L.; Zhang, Y.; Ding, F. Adapting FeS2 micron particles as an electrode material for lithium-ion batteries via simultaneous construction of CNT internal networks and external cages. J. Mater. Chem. A 2019, 7, 991–997. [Google Scholar] [CrossRef]
- Xiao, X.; Ni, L.; Chen, G.; Ai, G.; Li, J.; Qiu, T.; Liu, X. Two-dimensional NiSe2 nanosheets on carbon fiber cloth for high-performance lithium-ion batteries. J. Alloy. Compd. 2020, 821, 153218. [Google Scholar] [CrossRef]
- Ge, X.; Liu, S.; Qiao, M.; Du, Y.; Li, Y.; Bao, J.; Zhou, X. Enabling superior electrochemical properties for highly efficient po tassium storage by impregnating ultrafine Sb nanocrystals within nanochannel-containing carbon nanofibers. Angew Chem. Int. Ed. Engl. 2019, 58, 14578–14583. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Kravchyk, K.; Walter, M.; Kovalenko, M.V. Monodisperse antimony nanocrystals for high-rate Li-ion and Na-ion battery anodes: Nano versus bulk. Nano Lett. 2014, 14, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhao, M.; Qiu, J.; Lai, W.Y.; Pang, H.; Huang, W. One dimensional silver-based nanomaterials: Preparations and electrochemical applications. Small 2017, 13, 1701091. [Google Scholar] [CrossRef]
- David, M.E.; Ion, R.M.; Grigorescu, R.M.; Iancu, L.; Andrei, E.R. Nanomaterials used in conservation and restoration of cultural heritage: An up-to-date overview. Materials 2020, 13, 2064. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Li, D.; Zheng, X.; Yang, X.; Chou, S.; Zhu, Z. Effect of size and dimensionality on the band gap and conductivity of InAs, PbS, Ge, and Bi2S3 nanostructured semiconductors. Curr. Nanosci. 2016, 12, 324–329. [Google Scholar] [CrossRef]
- Ramireddy, T.; Rahman, M.M.; Xing, T.; Chen, Y.; Glushenkov, A.M. Stable anode performance of an Sb-carbon nanocomposite in lithium-ion batteries and the effect of ball milling mode in the course of its preparation. J. Mater. Chem. A 2014, 2, 4282–4291. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, J.J.; Cui, J.H.; Zhu, Y.C.; Liang, J.W.; Wang, L.L.; Qian, Y.T. Electrochemical performance of rod-like Sb-C composite as anodes for Li-ion and Na-ion batteries. J. Mater. Chem. A 2015, 3, 3276–3280. [Google Scholar] [CrossRef]
- Zhang, L.H.; Qin, X.Y.; Zhao, S.Q.; Wang, A.; Luo, J.; Wang, Z.L.; Kang, F.Y.; Lin, Z.Q.; Li, B.H. Advanced matrixes for binder-free nanostructured electrodes in lithium-ion batteries. Adv. Mater. 2020, 32, 1908445. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Ni, J.; Li, L. Electrospun materials for batteries moving beyond lithium-ion technologies. Electrochem. Energy R. 2021, 5, 211–241. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Tan, Z. A review of electrospun nanofiber-based separators for rechargeable lithium-ion batteries. J. Power Sources 2019, 443, 227262. [Google Scholar] [CrossRef]
- Li, W.H.; Yang, Z.Z.; Cheng, J.X.; Zhong, X.W.; Gu, L.; Yu, Y. Germanium nanoparticles encapsulated in flexible carbon nanofibers as self-supported electrodes for high performance lithium-ion batteries. Nanoscale 2014, 6, 4532–4537. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, T.; Bian, Z.H.; Sun, H.; Pang, Y.P.; Peng, C.X.; Yang, J.H.; Zheng, S.Y. Electrospun flexible Si/C@CNF nonwoven anode for high capacity and durable lithium-ion battery. Compos. Commun. 2019, 11, 1–5. [Google Scholar] [CrossRef]
- Yang, M.; Liu, L.; Yan, H.; Zhang, W.; Su, D.; Wen, J.; Liu, W.; Yuan, Y.; Liu, J.; Wang, X. Porous nitrogen-doped Sn/C film as free-standing anodes for lithium ion batteries. Appl. Surf. Sci. 2021, 551, 149246. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, D.; Hou, Y. Free-standing and consecutive ZnSe@carbon nanofibers architectures as ultra-long lifespan anode for flexible lithium-ion batteries. Nano Energy 2022, 94, 106909. [Google Scholar] [CrossRef]
- Fei, L.; Williams, B.P.; Yoo, S.H.; Carlin, J.M.; Joo, Y.L. A general approach to fabricate free-standing metal sulfide@carbon nanofiber networks as lithium ion battery anodes. Chem. Commun. 2016, 52, 1501–1504. [Google Scholar] [CrossRef]
- Xue, X.Y.; Xing, L.L.; Chen, Y.J.; Shi, S.L.; Wang, Y.G.; Wang, T.H. Synthesis and H2S sensing properties of CuO-SnO2 core/shell PN-junction nanorods. J. Phys.Chem. C. 2008, 112, 12157–12160. [Google Scholar] [CrossRef]
- Fu, J.Z.; Liu, H.; Liao, L.B.; Fan, P.; Wang, Z.; Wu, Y.Y.; Zhang, Z.W.; Hai, Y.; Lv, G.C.; Mei, L.F.; et al. Ultrathin Si/CNTspaper-like composite for flexible Li-ion battery anode with high volumetric capacity. Front. Chem. 2018, 6, 624. [Google Scholar] [CrossRef]
- Köse, H.; Aydin, A.O.; Dombaycioğlu, Ş. Graphene-based architectures of tin and zinc oxide nanocomposites for free-standing binder-free Li-ion anodes. Int. J. Energy Res. 2018, 42, 4710–4718. [Google Scholar] [CrossRef]
- Liao, H.-S.; Lin, J.; Liu, Y.; Huang, P.; Jin, A.; Chen, X.Y. Self-assembly mechanisms of nanofibers from peptide amphiphiles in solution and on substrate surfaces. Nanoscale 2016, 8, 14814–14820. [Google Scholar] [CrossRef]
- Rolandi, M.; Rolandi, R. Self-assembled chitin nanofibers and applications. Adv. Colloid Interface 2014, 207, 216–222. [Google Scholar] [CrossRef]
- Jessl, S.; Copic, D.; Engelke, S.; Ahmad, S.; De Volder, M. Hydrothermal coating of patterned carbon nanotube forest for structured lithium-ion battery electrodes. Small 2019, 15, e1901201. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; He, X.; Li, J.; Gao, X.; Jia, J. Porous CuO/SnO2 composite nanofibers fabricated by electrospinning and their H2S sensing properties. Sens. Actuators B-Chem. 2012, 165, 82–87. [Google Scholar] [CrossRef]
- Lu, X.F.; Wang, C.; Favier, F.; Pinna, N. Electrospun nanomaterials for supercapacitor electrodes: Designed architectures and electrochemical performance. Adv. Energy Mater. 2017, 7, 1601301. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, W.; Wu, C.; Fan, Q.G.; Zhang, W.X.; Hu, X.L.; Huang, Y.H. Self-wrapped Sb/C nanocomposite as anode material for high-performance sodium-ion batteries. Nano Energy 2015, 16, 479–487. [Google Scholar] [CrossRef]
- Zhong, L.F.; Tang, A.D.; Wen, X.; Yan, P.; Wang, J.J.; Tan, L.; Chen, J. New finding on Sb (2–3 nm) nanoparticles and carbon simultaneous anchored on the porous palygorskite with enhanced catalytic activity. J. Alloys Compd. 2018, 743, 394–402. [Google Scholar] [CrossRef]
- Lv, H.L.; Qiu, S.; Lu, G.X.; Fu, Y.; Li, X.Y.; Hu, C.X.; Liu, J.R. Nanostructured antimony/carbon composite fibers as anode material for lithium-ion battery. Electrochim. Acta 2015, 151, 214–221. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, Y.; Fan, X.L.; Ji, G.B.; Ji, X.; Wang, H.Y.; Hou, S.; Zachariah, M.R.; Wang, C.S. Extremely stable antimony-carbon composite anodes for potassium-ion batteries. Energ. Environ. Sci. 2019, 12, 615–623. [Google Scholar] [CrossRef]
- Yi, Z.; Han, Q.G.; Cheng, Y.; Wang, F.X.; Wu, Y.M.; Wang, L.M. A novel strategy to prepare Sb thin film sandwiched between the reduced graphene oxide and Ni foam as binder-free anode material for lithium-ion batteries. Electrochim. Acta 2016, 190, 804–810. [Google Scholar] [CrossRef]
- Wang, M.G.; Han, J.; Hu, Y.M.; Guo, R. Mesoporous C, N-codoped TiO2 hybrid shells with enhanced visible light photocatalytic performance. RSC Adv. 2017, 7, 15513–15520. [Google Scholar] [CrossRef]
- Yang, F.H.; Zhang, Z.A.; Du, K.; Zhao, X.X.; Chen, W.; Lai, Y.Q.; Li, J. Dopamine derived nitrogen-doped carbon sheets as anode materials for high-performance sodium ion batteries. Carbon 2015, 91, 88–95. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zhang, N.; Jiao, L.F.; Chen, J. Tin Nanodots encapsulated in porous nitrogen-doped carbon nanofibers as a free-standing anode for avanced sodium-ion batteries. Adv Mater. 2015, 27, 6702–6707. [Google Scholar] [CrossRef]
- Gurgul, J.; Rinke, M.T.; Schellenberg, I.; Pöttgen, R. The antimonide oxides REZnSbO and REMnSbO (RE = Ce, Pr)—An XPS study. Solid State Sci. 2013, 17, 122–127. [Google Scholar] [CrossRef]
- Schaff, J.E.; Roberts, J.T. Adsorbed states of acetonitrile and chloroform on amorphous and crystalline ice studied with X-ray photoelectron spectroscopy. Surf. Sci. 1999, 426, 384–394. [Google Scholar] [CrossRef]
- Wan, F.; Guo, J.Z.; Zhang, X.H.; Zhang, J.P.; Sun, H.Z.; Yan, Q.; Han, D.X.; Niu, L.; Wu, X.L. In situ binding Sb nanospheres on graphene via oxygen bonds as superior anode for ultrafast sodium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 7790–7799. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Yang, X.M.; Zhang, Y.; Hou, H.S.; Jing, M.J.; Zhu, Y.R.; Fang, L.B.; Chen, Q.Y.; Ji, X.B. Cathodically induced antimony for rechargeable Li-ion and Na-ion batteries: The influences of hexagonal and amorphous phase. J. Power Sources 2015, 282, 358–367. [Google Scholar] [CrossRef]
- He, X.M.; Pu, W.H.; Wang, L.; Ren, J.G.; Jiang, C.Y.; Wan, C.R. Synthesis of nano Sb-encapsulated pyrolytic polyacrylonitrile composite for anode material in lithium secondary batteries. Electrochim. Acta 2007, 52, 3651–3653. [Google Scholar] [CrossRef]
- Ghomi, J.S.; Akbarzadeh, Z. Ultrasonic accelerated Knoevenagel condensation by magnetically recoverable MgFe2O4 nanocatalyst: A rapid and green synthesis of coumarins under solvent-free conditions. Ultrason Sonochem 2018, 40, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cheng, J.L.; Li, X.D.; Yuan, D.M.; Ni, W.; Qu, G.X.; Guan, Q.; Zhang, Y.; Wang, B. Sulfur quantum dots wrapped by conductive polymer shell with internal void spaces for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 4049–4057. [Google Scholar] [CrossRef]
- Xu, Y.H.; Zhu, Y.J.; Liu, Y.H.; Wang, C.S. Electrochemical performance of porous carbon/tin composite anodes for sodium-ion and lithium-ion batteries. Adv. Energy Mater. 2013, 3, 128–133. [Google Scholar] [CrossRef]
- Wu, N.T.; Zhang, Y.; Guo, Y.; Liu, S.J.; Liu, H.; Wu, H. flakelike LiCoO2 with exposed {010} facets as a stable cathode material for highly reversible lithium storage. ACS Appl. Mater. Interfaces 2016, 8, 2723–2731. [Google Scholar] [CrossRef]
- Wu, L.; Pei, F.; Mao, R.J.; Wu, F.Y.; Wu, Y.; Qian, J.F.; Cao, Y.L.; Ai, X.P.; Yang, H.X. SiC-Sb-C nanocomposites as high-capacity and cycling-stable anode for sodium-ion batteries. Electrochim. Acta 2013, 87, 41–45. [Google Scholar] [CrossRef]
- Wang, H.Q.; Pan, Q.C.; Wu, Q.; Zhang, X.H.; Huang, Y.G.; Lushington, A.; Li, Q.Y.; Sun, X.L. Ultrasmall MoS2 embedded in carbon nanosheets coated Sn/SnOx as anode material for high-rate and long life Li-ion batteries. J. Mater. Chem. A 2017, 5, 4576–4582. [Google Scholar] [CrossRef]
- Chen, W.X.; Lee, J.Y.; Liu, Z.L. The nanocomposites of carbon nanotube with Sb and SnSb0.5 as Li-ion battery anodes. Carbon 2003, 41, 959–966. [Google Scholar] [CrossRef]
- NuLi, Y.N.; Yang, J.; Jiang, M.S. Synthesis and characterization of Sb/CNT and Bi/CNT composites as anode materials for lithium-ion batteries. Mater. Lett. 2008, 62, 2092–2095. [Google Scholar] [CrossRef]
| Materials | Free-Standing (Yes or No) | Current Density (A g−1) | Cycle Numbers | Capacity (mAh g−1) | References |
|---|---|---|---|---|---|
| Sb/CNT | No | 0.05 | 30 | 287 | [59] |
| Sb@C | No | 0.3 | 20 | 408 | [52] |
| Sb/C | No | 0.23 | 250 | 550 | [22] |
| 1.15 | - | 400 | |||
| Sb/CNT | No | 0.25 | 50 | 277.4 | [60] |
| Sb/C | No | 0.1 | 100 | 315.9 | [42] |
| 0.8 | - | 246.2 | |||
| Sb@C | No | 0.1 | 100 | 478.8 | [23] |
| 0.5 | - | 369.7 | |||
| Sb/graphite | Yes | 0.1 | 50 | 424.1 | [44] |
| 1 | - | 158 | |||
| Sb/C | No | 0.2 | 100 | 565 | [6] |
| 1 | 500 | 400.5 | |||
| 5 | - | 315.4 | |||
| Sb@NCFs | Yes | 0.1 | 300 | 590 | This work |
| 0.4 | 300 | 480 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Jia, N.; Li, J.; Liu, P.; Zhao, X.; Lin, Y.; Sun, C.; Qin, W. Sb Nanoparticles Embedded in the N-Doped Carbon Fibers as Binder-Free Anode for Flexible Li-Ion Batteries. Nanomaterials 2022, 12, 3093. https://doi.org/10.3390/nano12183093
Wang X, Jia N, Li J, Liu P, Zhao X, Lin Y, Sun C, Qin W. Sb Nanoparticles Embedded in the N-Doped Carbon Fibers as Binder-Free Anode for Flexible Li-Ion Batteries. Nanomaterials. 2022; 12(18):3093. https://doi.org/10.3390/nano12183093
Chicago/Turabian StyleWang, Xin, Nanjun Jia, Jianwei Li, Pengbo Liu, Xinsheng Zhao, Yuxiao Lin, Changqing Sun, and Wei Qin. 2022. "Sb Nanoparticles Embedded in the N-Doped Carbon Fibers as Binder-Free Anode for Flexible Li-Ion Batteries" Nanomaterials 12, no. 18: 3093. https://doi.org/10.3390/nano12183093





