Natural Cotton Cellulose-Supported TiO2 Quantum Dots for the Highly Efficient Photocatalytic Degradation of Dyes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of TQDs/CC Photocatalyst
2.3. Characterization
2.4. Photocatalytic Measurements
3. Results and Discussion
3.1. Preparation of TQDs/CC Photocatalyst
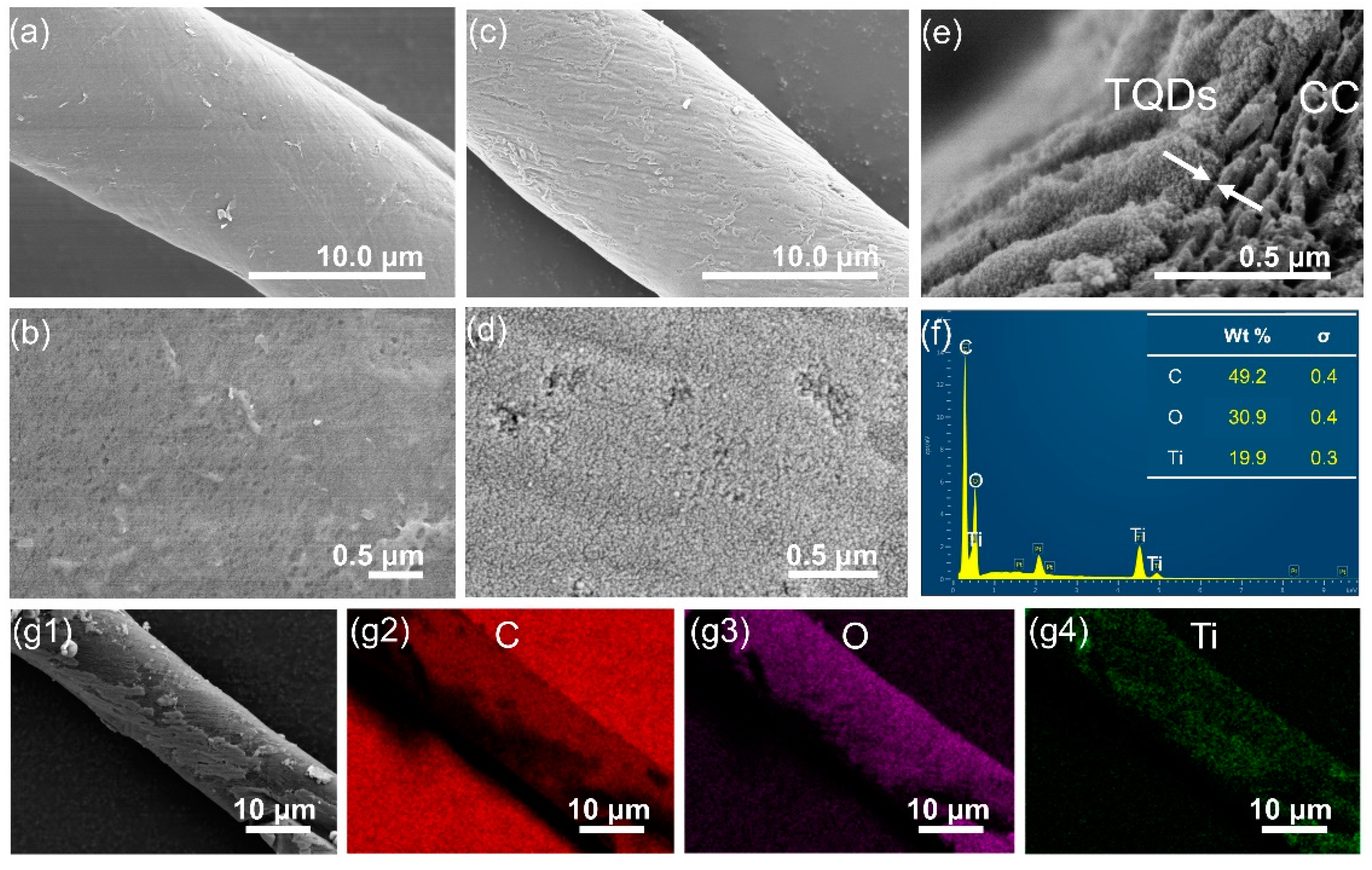
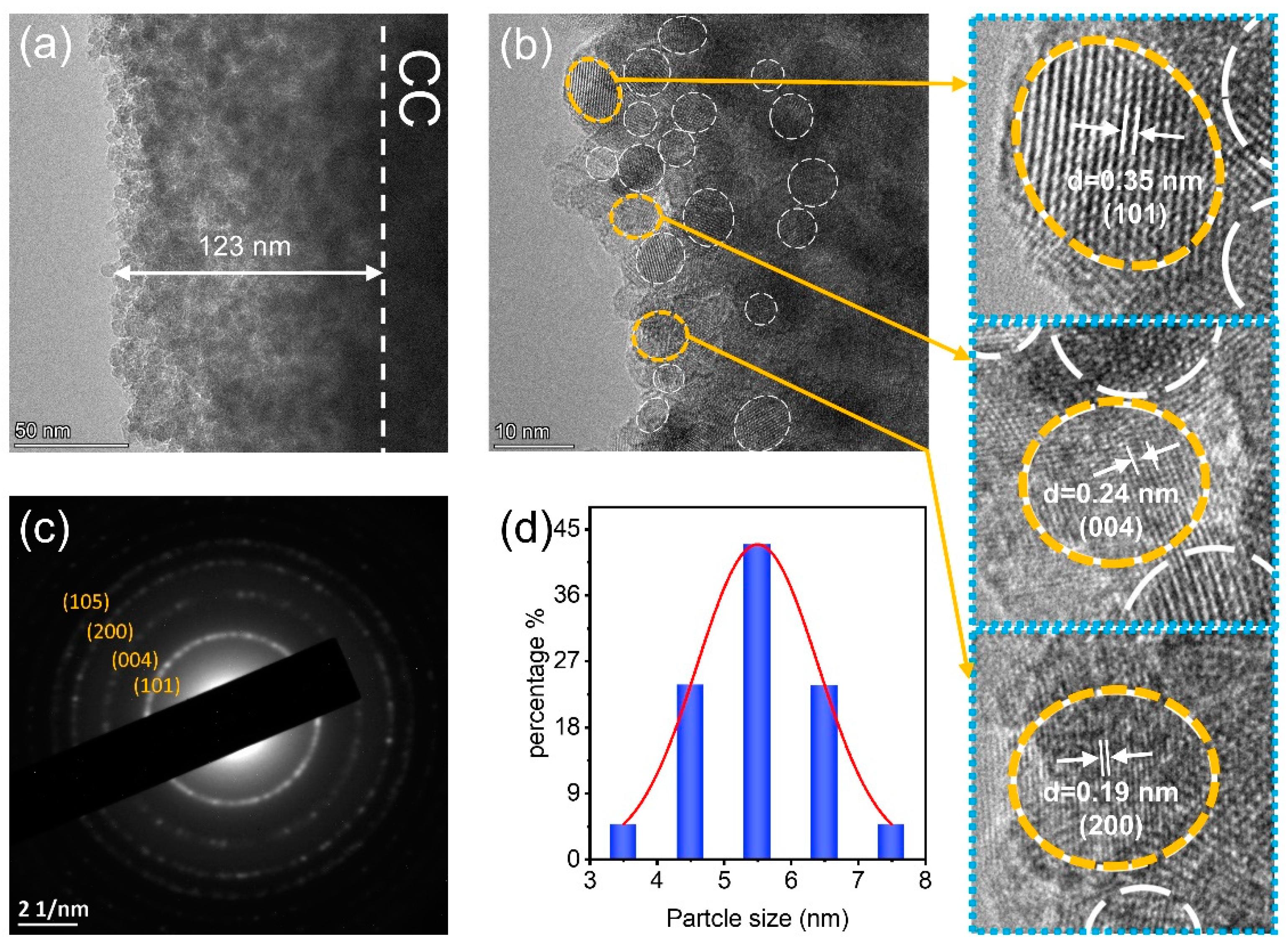
3.2. Characterizations of TQDs/CC Photocatalyst
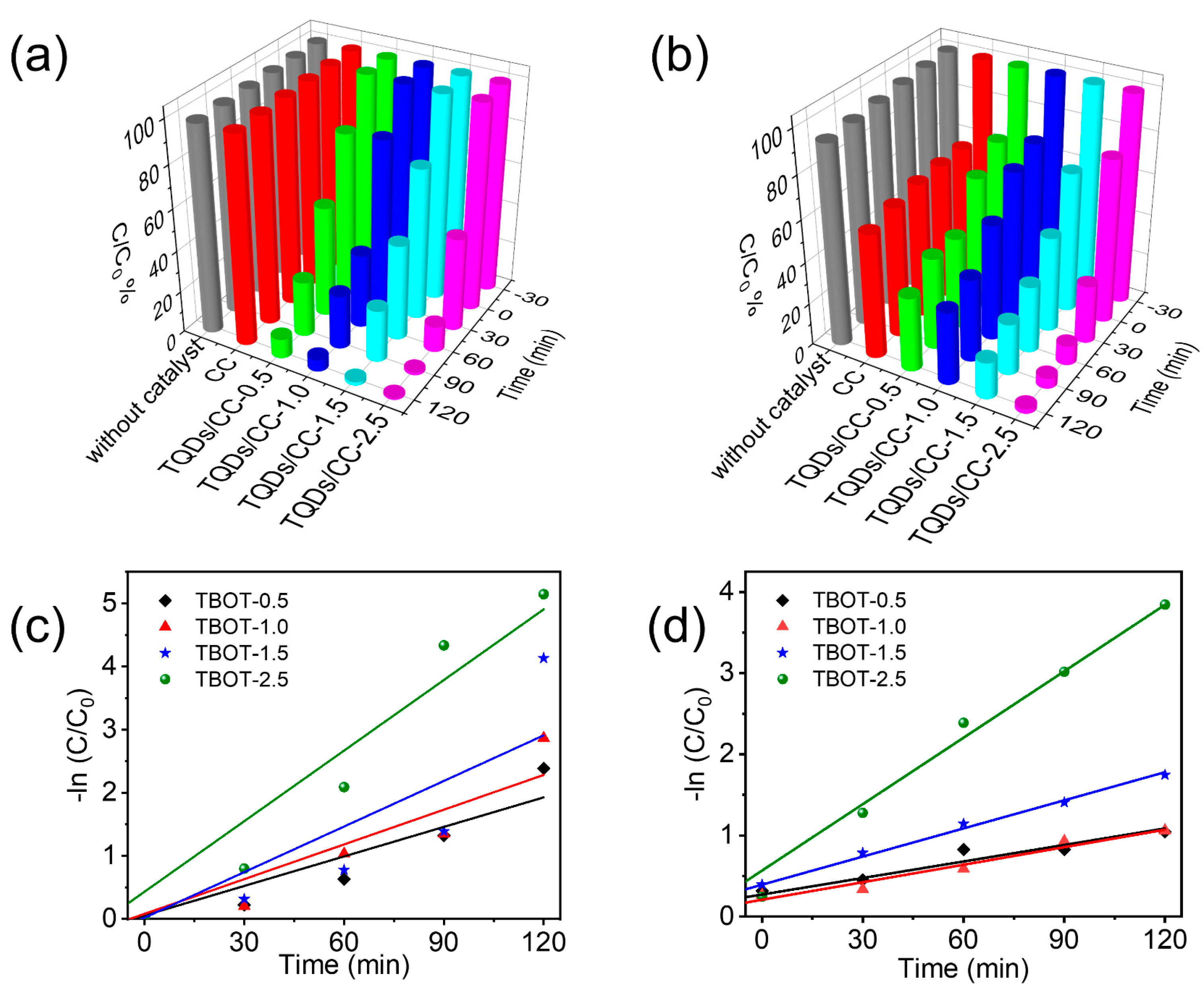
3.3. Photocatalytic Property of TQDs/CC Photocatalyst
3.4. Reusability and Stability of TQDs/CC Photocatalyst
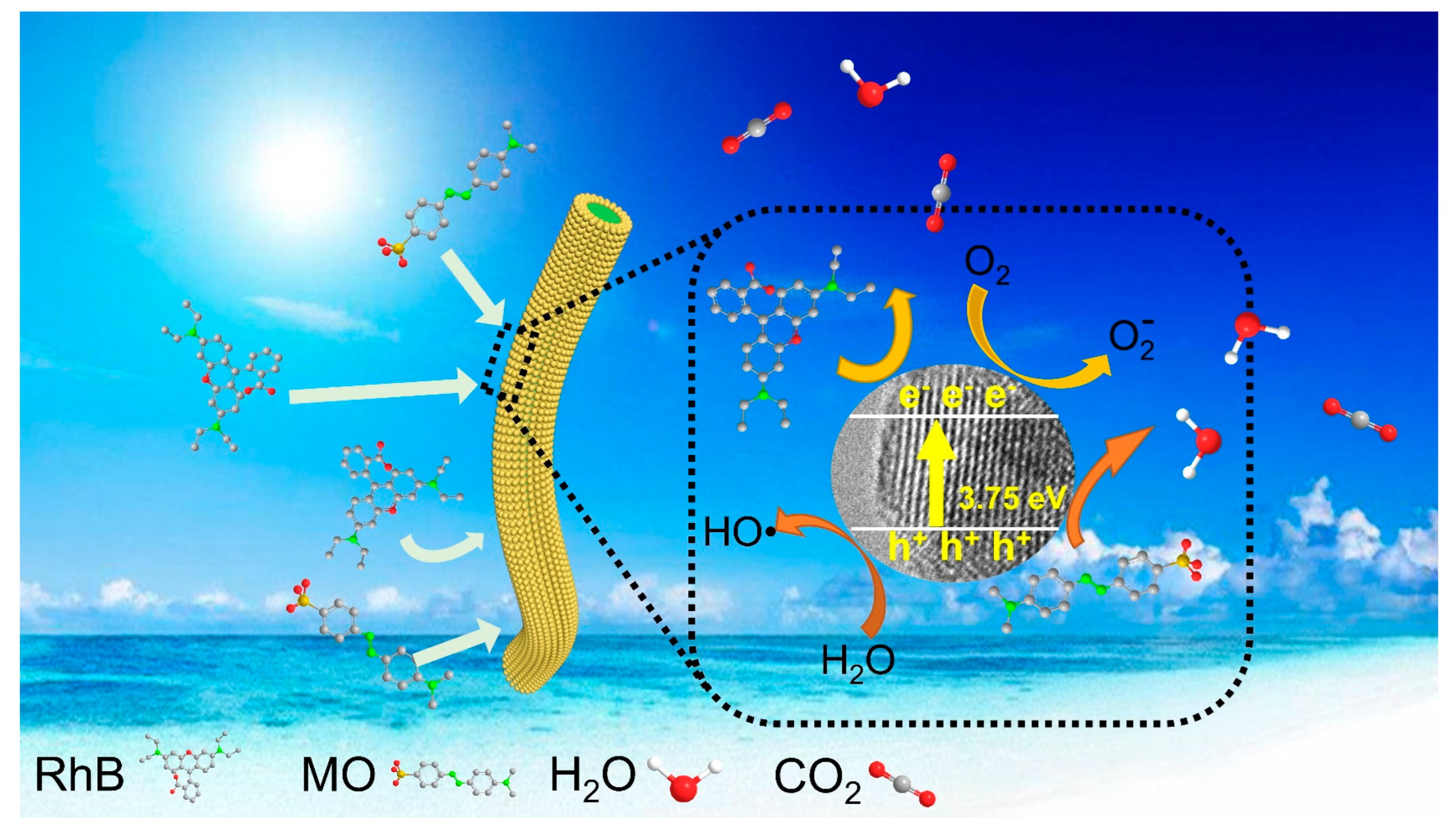
3.5. Hypothesis of Photodegradation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Escher, B.I.; Stapleton, H.M.; Schymanski, E.L. Tracking complex mixtures of chemicals in our changing environment. Science 2020, 367, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, W.; El-Gawad, H.A.; Handal, H.; Galal, H.; Mousa, H.; El-Sayed, B.; Mekkey, S.; Ibrahem, I.; Labib, A. Remarkable recycling process of ZnO quantum dots for photodegradation of reactive yellow dye and solar photocatalytic treatment process of industrial wastewater. Nanomaterials 2022, 12, 2642. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, D.; Zeng, G.; Chi, H.; Li, L.; He, Y.; Ke, F.; Xiao, J.-D.; Ye, S. Dysprosium doped CoFe2O4 with enhanced magnetic property and photodegradation activity of methyl orange. Mater. Lett. 2021, 284, 128966. [Google Scholar] [CrossRef]
- Khatri, A.; Peerzada, M.H.; Mohsin, M.; White, M. A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. J. Clean. Prod. 2015, 87, 50–57. [Google Scholar] [CrossRef]
- Mora, S.L.; Cadavid, Y.; Ch, E.M.C.; Vélez, J.M.; Buitrago-Sierra, R.; Santa, J.F. Plantain fibers obtained from pseudostems residues for efficient color degradation of indigo carmine dye. Ind. Crops Prod. 2018, 126, 302–308. [Google Scholar] [CrossRef]
- Xue, Z.H.; Luan, D.; Zhang, H.; Lou, X.W. Single-atom catalysts for photocatalytic energy conversion. Joule 2022, 6, 92–133. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Tan, Y.H.; Khalid, M.; Karri, R.R.; Walvekar, R.; Abdullah, E.C.; Nizamuddin, S.; Mazari, S.A. A comprehensive review on magnetic carbon nanotubes and carbon nanotube-based buckypaper for removal of heavy metals and dyes. J. Hazard. Mater. 2021, 413, 125375. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Liu, Y.; Zhang, J.; Wang, C.; Zang, S.; Li, Y.; Zhang, P.; Li, X. In situ construction of a C3N5 nanosheet/Bi2WO6 nanodot S-scheme heterojunction with enhanced structural defects for the efficient photocatalytic removal of tetracycline and Cr(VI). Inorg. Chem. Front. 2022, 9, 2479–2497. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M.N. Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef]
- Nie, J.; Sun, Y.; Zhou, Y.; Kumar, M.; Usman, M.; Li, J.; Shao, J.; Wang, L.; Tsang, D.C.W. Bioremediation of water containing pesticides by microalgae: Mechanisms, methods, and prospects for future research. Sci. Total Environ. 2020, 707, 136080. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Zhao, Y.; Bai, H.; Wen, T.; Kang, S.; Song, G.; Song, S.; Komarneni, S. Removal of heavy metals and dyes by clay-based adsorbents: From natural clays to 1D and 2D nano-composites. Chem. Eng. J. 2021, 420, 127574. [Google Scholar] [CrossRef]
- Dey, P.; Mahapatra, B.S.; Juyal, V.K.; Pramanick, B.; Negi, M.S.; Paul, J.; Singh, S.P. Flax processing waste–A low-cost, potential biosorbent for treatment of heavy metal, dye and organic matter contaminated industrial wastewater. Ind. Crops Prod. 2021, 174, 114195. [Google Scholar] [CrossRef]
- Dekkouche, S.; Morales-Torres, S.; Ribeiro, A.R.; Faria, J.L.; Fontàs, C.; Kebiche-Senhadji, O.; Silva, A.M.T. In situ growth and crystallization of TiO2 on polymeric membranes for the photocatalytic degradation of diclofenac and 17α-ethinylestradiol. Chem. Eng. J. 2022, 427, 131476. [Google Scholar] [CrossRef]
- Li, N.; Lu, X.; He, M.; Duan, X.; Yan, B.; Chen, G.; Wang, S. Catalytic membrane-based oxidation-filtration systems for organic wastewater purification: A review. J. Hazard. Mater. 2021, 414, 125478. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, M.; Wang, L.; Fei, Y.; Wang, S.; Núñez-Delgado, A.; Bokhari, A.; Race, M.; Khataee, A.; Klemeš, J.J.; et al. Photocatalytic degradation of xanthate in flotation plant tailings by TiO2/graphene nanocomposites. Chem. Eng. J. 2022, 431, 134104. [Google Scholar] [CrossRef]
- Xiong, L.; Tang, J. Strategies and challenges on selectivity of photocatalytic oxidation of organic substances. Adv. Energy Mater. 2021, 11, 2003216. [Google Scholar] [CrossRef]
- Karpuraranjith, M.; Chen, Y.; Rajaboopathi, S.; Ramadoss, M.; Srinivas, K.; Yang, D.; Wang, B. Three-dimensional porous MoS2 nanobox embedded g-C3N4@TiO2 architecture for highly efficient photocatalytic degradation of organic pollutant. J. Colloid Interface Sci. 2022, 605, 613–623. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, X.; Wang, L.; Wu, F.; Liu, S.; Chang, C.; Luo, X. A simple strategy to design 3-layered Au-TiO2 dual nanoparticles immobilized cellulose membranes with enhanced photocatalytic activity. Carbohydr. Polym. 2020, 231, 115694. [Google Scholar] [CrossRef]
- Singh, J.; Juneja, S.; Soni, R.K.; Bhattacharya, J. Sunlight mediated enhanced photocatalytic activity of TiO2 nanoparticles functionalized CuO-Cu2O nanorods for removal of methylene blue and oxytetracycline hydrochloride. J. Colloid Interface Sci. 2021, 590, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, S.; Zhang, D.; Li, G.; Leung, M.K.H. Controlling charge transfer in quantum-size titania for photocatalytic applications. Appl. Catal. B Environ. 2017, 215, 85–92. [Google Scholar] [CrossRef]
- Wang, X.; Xia, R.; Muhire, E.; Jiang, S.; Huo, X.; Gao, M. Highly enhanced photocatalytic performance of TiO2 nanosheets through constructing TiO2/TiO2 quantum dots homojunction. Appl. Surf. Sci. 2018, 459, 9–15. [Google Scholar] [CrossRef]
- Wu, Z.G.; Ren, Z.M.; Li, L.; Lv, L.; Chen, Z. Hydrothermal synthesis of TiO2 quantum dots with mixed titanium precursors. Sep. Purif. Technol. 2020, 251, 117328. [Google Scholar] [CrossRef]
- Ng, H.K.M.; Leo, C.P. The coherence between TiO2 nanoparticles and microfibrillated cellulose in thin film for enhanced dispersal and photodegradation of dye. Prog. Org. Coat. 2019, 132, 70–75. [Google Scholar] [CrossRef]
- Wei, X.; Li, J.; Wang, S.; Zhao, Y.; Duan, H.; Ge, X. Fiber-specific overexpression of GhACO1 driven by E6 promoter improves cotton fiber quality and yield. Ind. Crops Prod. 2022, 185, 115134. [Google Scholar] [CrossRef]
- Huang, G.; Huang, J.Q.; Chen, X.Y.; Zhu, Y.X. Recent advances and future perspectives in cotton research. Annu. Rev. Plant Biol. 2021, 72, 437–462. [Google Scholar] [CrossRef]
- Ghasemzadeh, B.; Matin, A.A.; Habibi, B.; Ebadi, M. Cotton/Fe3O4@SiO2@H3PW12O40 a magnetic heterogeneous catalyst for biodiesel production: Process optimization through response surface methodology. Ind. Crops Prod. 2022, 181, 114806. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.W.; Jaafar, J.; Ismail, A.; Mutalib, M.A.; Sani, N.; Asri, S.E.; Ong, C. Physicochemical characteristic of regenerated cellulose/N-doped TiO2 nanocomposite membrane fabricated from recycled newspaper with photocatalytic activity under UV and visible light irradiation. Chem. Eng. J. 2016, 284, 202–215. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Abd Mutalib, M.; Hir, Z.A.M.; Zain, M.M.; Mohamad, A.B.; Minggu, L.J.; Awang, N.A.; Salleh, W.N. An overview on cellulose-based material in tailoring bio-hybrid nanostructured photocatalysts for water treatment and renewable energy applications. Int. J. Biol. Macromol. 2017, 103, 1232–1256. [Google Scholar] [CrossRef]
- Yu, H.Y.; Chen, G.Y.; Wang, Y.B.; Yao, J.M. A facile one-pot route for preparing cellulose nanocrystal/zinc oxide nanohybrids with high antibacterial and photocatalytic activity. Cellulose 2014, 22, 261–273. [Google Scholar] [CrossRef]
- Cao, S.; Rathi, P.; Wu, X.; Ghim, D.; Jun, Y.S.; Singamaneni, S. Cellulose nanomaterials in interfacial evaporators for desalination: A “natural” choice. Adv. Mater. 2021, 33, e2000922. [Google Scholar] [CrossRef]
- Liu, R.; Dai, L.; Si, C.L. Mussel-Inspired Cellulose-Based Nanocomposite Fibers for Adsorption and Photocatalytic Degradation. ACS Sustain. Chem. Eng. 2018, 6, 15756–15763. [Google Scholar] [CrossRef]
- Liu, L.; Dong, P.; Liu, R.; Zhou, Q.; Wang, X.; Yi, G.; Cheng, B. Preparation and self-assembly of uniform TiO2/SiO2 composite submicrospheres. J. Colloid Interface Sci. 2005, 288, 1–5. [Google Scholar] [CrossRef]
- Yu, J.C.C.; Nguyen, V.H.; Lasek, J.; Wu, J.C.S. Titania nanosheet photocatalysts with dominantly exposed (001) reactive facets for photocatalytic NOx abatement. Appl. Catal. B Environ. 2017, 219, 391–400. [Google Scholar] [CrossRef]
- Walid, A.; Daoud, S.K.L.; Tung, W.S.; Xin, J.H.; Cheuk, K.; Qi, K. Self-cleaning keratins. Chem. Mater. 2008, 20, 1242–1244. [Google Scholar]
- Zhang, G.; Wang, D.; Yan, J.; Xiao, Y.; Gu, W.; Zang, C. Study on the photocatalytic and antibacterial properties of TiO2 nanoparticles-coated cotton fabrics. Materials 2019, 12, 2010. [Google Scholar] [CrossRef]
- Abulizi, A.; Okitsu, K.; Zhu, J.J. Ultrasound assisted reduction of graphene oxide to graphene in L-ascorbic acid aqueous solutions: Kinetics and effects of various factors on the rate of graphene formation. Ultrason. Sonochem. 2014, 21, 1174–1181. [Google Scholar] [CrossRef]
- Chu, S.; Miao, Y.; Qian, Y.; Ke, F.; Chen, P.; Jiang, C.; Chen, X. Synthesis of uniform layer of TiO2 nanoparticles coated on natural cellulose micrometer-sized fibers through a facile one-step solvothermal method. Cellulose 2019, 26, 4757–4765. [Google Scholar] [CrossRef]
- Joost, U.; Šutka, A.; Oja, M.; Smits, K.; Döbelin, N.; Loot, A.; Järvekülg, M.; Hirsimäki, M.; Valden, M.; Nõmmiste, E. Reversible photodoping of TiO2 nanoparticles for photochromic applications. Chem. Mater. 2018, 30, 8968–8974. [Google Scholar] [CrossRef]
- Liang, Y.C.; Li, T.H. Sputtering-assisted synthesis of copper oxide–titanium oxide nanorods and their photoactive performances. Nanomaterials 2022, 12, 2634. [Google Scholar] [CrossRef]
- Onwumere, J.; Tek, J.P.; Budnyak, T.; Chen, J.; Budnyk, S.; Karim, Z.; Thersleff, T.; Kustrowski, P.; Mathew, A.P.; Slabon, A. Celluphot: Hybrid cellulose-bismuth oxybromide membrane for pollutant removal. ACS Appl. Mater. Interfaces 2020, 12, 42891–42901. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, H.; Zhang, J.; Zelekew, O.A.; Lu, D.; Kuo, D.-H.; Lin, J. Synthesis of visible light responsive iodine-doped mesoporous TiO2 by using biological renewable lignin as template for degradation of toxic organic pollutants. Appl. Catal. B Environ. 2019, 252, 152–163. [Google Scholar] [CrossRef]
- Lyu, Y.; Asoh, T.-A.; Uyama, H. Hierarchically porous TiO2 monolith prepared using a cellulose monolith as a template. Mater. Chem. Front. 2021, 5, 3877–3885. [Google Scholar] [CrossRef]
- Wahid, F.; Zhao, X.Q.; Cui, J.X.; Wang, Y.Y.; Wang, F.P.; Jia, S.R.; Zhong, C. Fabrication of bacterial cellulose with TiO2-ZnO nanocomposites as a multifunctional membrane for water remediation. J. Colloid Interface Sci. 2022, 620, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, S.; Zhang, N.; Zhang, J. Preparation and characterization of nanocrystalline cellulose via low-intensity ultrasonic-assisted sulfuric acid hydrolysis. Cellulose 2013, 21, 335–346. [Google Scholar] [CrossRef]
- Yao, L.; Hu, S.; Wang, X.; Lin, M.; Zhang, C.; Chen, Y.; Yue, F.; Qi, H. Facile preparation of lignin-containing cellulose nanofibrils from sugarcane bagasse by mild soda-oxygen pulping. Carbohydr. Polym. 2022, 290, 119480. [Google Scholar] [CrossRef]
- Engel, J.; Bishop, S.R.; Vayssieres, L.; Tuller, H.L. In situ electrical characterization of anatase TiO2 quantumdots. Adv. Funct. Mater. 2014, 24, 4952–4958. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.X.; Qian, K.; Jia, A.; Li, D.; Shi, L.; Hu, J.; Zhu, J.; Huang, W. Structure sensitivity of Au-TiO2 strong metal-support interactions. Angew. Chem. Int. Ed. Engl. 2021, 60, 12074–12081. [Google Scholar] [CrossRef]
- You, X.; Wang, R.; Zhu, Y.; Sui, W.; Cheng, D. Comparison of adsorption properties of a cellulose-rich modified rice husk for the removal of methylene blue and aluminum (III) from their aqueous solution. Ind. Crops Prod. 2021, 170, 113687. [Google Scholar] [CrossRef]
- Apostolaki, M.A.; Toumazatou, A.; Antoniadou, M.; Sakellis, E.; Xenogiannopoulou, E.; Gardelis, S.; Boukos, N.; Falaras, P.; Dimoulas, A.; Likodimos, V. Graphene quantum dot-TiO2 photonic crystal films for photocatalytic applications. Nanomaterials 2020, 10, 2566. [Google Scholar] [CrossRef]
- Zheng, X.; Li, D.; Li, X.; Chen, J.; Cao, C.; Fang, J.; Wang, J.; He, Y.; Zheng, Y. Construction of ZnO/TiO2 photonic crystal heterostructures for enhanced photocatalytic properties. Appl. Catal. B Environ. 2015, 168–169, 408–415. [Google Scholar] [CrossRef]
- Wang, G.; Wang, F.; Liu, S.; Li, M.; Xie, M.; Yang, Z.; Xiang, Y.; Lv, S.; Han, W. Construction of heterojuncted photocatalyst with TiO2 quantum dots and graphene oxide nanosheets for highly efficient photocatalysis. Scr. Mater. 2021, 199, 113862. [Google Scholar] [CrossRef]
- Zhou, S.; Fu, Z.; Xia, L.; Mao, Y.; Zhao, W.; Wang, A.; Zhang, C.; Ding, C.; Xu, W. In situ synthesis of ternary hybrid nanocomposites on natural Juncus effusus fiber for adsorption and photodegradation of organic dyes. Sep. Purif. Technol. 2021, 255, 117671. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, M.; Wang, Y.; Kwok, Y.H.; Pan, W.; Szeto, W.; Huang, H.; Leung, D.Y.C. Fluorinated TiO2 coupling with α-MnO2 nanowires supported on different substrates for photocatalytic VOCs abatement under vacuum ultraviolet irradiation. Appl. Catal. B Environ. 2021, 280, 119388. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Q.; Lv, K.; Li, M. Heterojunction construction between TiO2 hollowsphere and ZnIn2S4 flower for photocatalysis application. Appl. Surf. Sci. 2017, 398, 81–88. [Google Scholar] [CrossRef]
- Zhou, S.; Xia, L.; Zhang, K.; Fu, Z.; Wang, Y.; Zhang, Q.; Zhai, L.; Mao, Y.; Xu, W. Titanium dioxide decorated natural cellulosic Juncus effusus fiber for highly efficient photodegradation towards dyes. Carbohydr. Polym. 2020, 232, 115830. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpaa, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef]
- Ni, J.; Wang, W.; Liu, D.; Zhu, Q.; Jia, J.; Tian, J.; Li, Z.; Wang, X.; Xing, Z. Oxygen vacancy-mediated sandwich-structural TiO2-x/ultrathin g-C3N4/TiO2-x direct Z-scheme heterojunction visible-light-driven photocatalyst for efficient removal of high toxic tetracycline antibiotics. J. Hazard. Mater. 2021, 408, 124432. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Wang, C.; Liu, Y.; Li, N.; Zhang, P.; Li, X. Rationally designed Ta3N5/BiOCl S-scheme heterojunction with oxygen vacancies for elimination of tetracycline antibiotic and Cr(VI): Performance, toxicity evaluation and mechanism insight. J. Mater. Sci. Technol. 2022, 123, 177–190. [Google Scholar] [CrossRef]
- Li, Y.; Cao, L.; Li, L.; Yang, C. In situ growing directional spindle TiO2 nanocrystals on cellulose fibers for enhanced Pb(2+) adsorption from water. J. Hazard. Mater. 2015, 289, 140–148. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Men, Q.; Ma, C.; Liu, Y.; Huo, P.; Yan, Y. A Z-scheme TiO2 quantum dots fragment-Bi12TiO20 composites for enhancing photocatalytic activity. Renew. Energ. 2020, 147, 856–863. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Cai, M.; Liu, Y.; Dong, K.; Zhang, J. Designing oxygen vacancy mediated bismuth molybdate (Bi2MoO6)/N-rich carbon nitride (C3N5) S-scheme heterojunctions for boosted photocatalytic removal of tetracycline antibiotic and Cr(VI): Intermediate toxicity and mechanism insight. J. Colloid. Interface Sci. 2022, 624, 219–232. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Zhang, W.; Guo, C.; Zhu, J.; Cui, J.; Xue, Z.; Chen, P. Natural Cotton Cellulose-Supported TiO2 Quantum Dots for the Highly Efficient Photocatalytic Degradation of Dyes. Nanomaterials 2022, 12, 3130. https://doi.org/10.3390/nano12183130
Shen H, Zhang W, Guo C, Zhu J, Cui J, Xue Z, Chen P. Natural Cotton Cellulose-Supported TiO2 Quantum Dots for the Highly Efficient Photocatalytic Degradation of Dyes. Nanomaterials. 2022; 12(18):3130. https://doi.org/10.3390/nano12183130
Chicago/Turabian StyleShen, Hancheng, Weiwei Zhang, Chunyun Guo, Jing Zhu, Junjie Cui, Zhonghua Xue, and Peirong Chen. 2022. "Natural Cotton Cellulose-Supported TiO2 Quantum Dots for the Highly Efficient Photocatalytic Degradation of Dyes" Nanomaterials 12, no. 18: 3130. https://doi.org/10.3390/nano12183130




