Enhancement of the Electrochemical Performances of Composite Solid-State Electrolytes by Doping with Graphene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Graphene
2.2. Preparation of LATP Powder
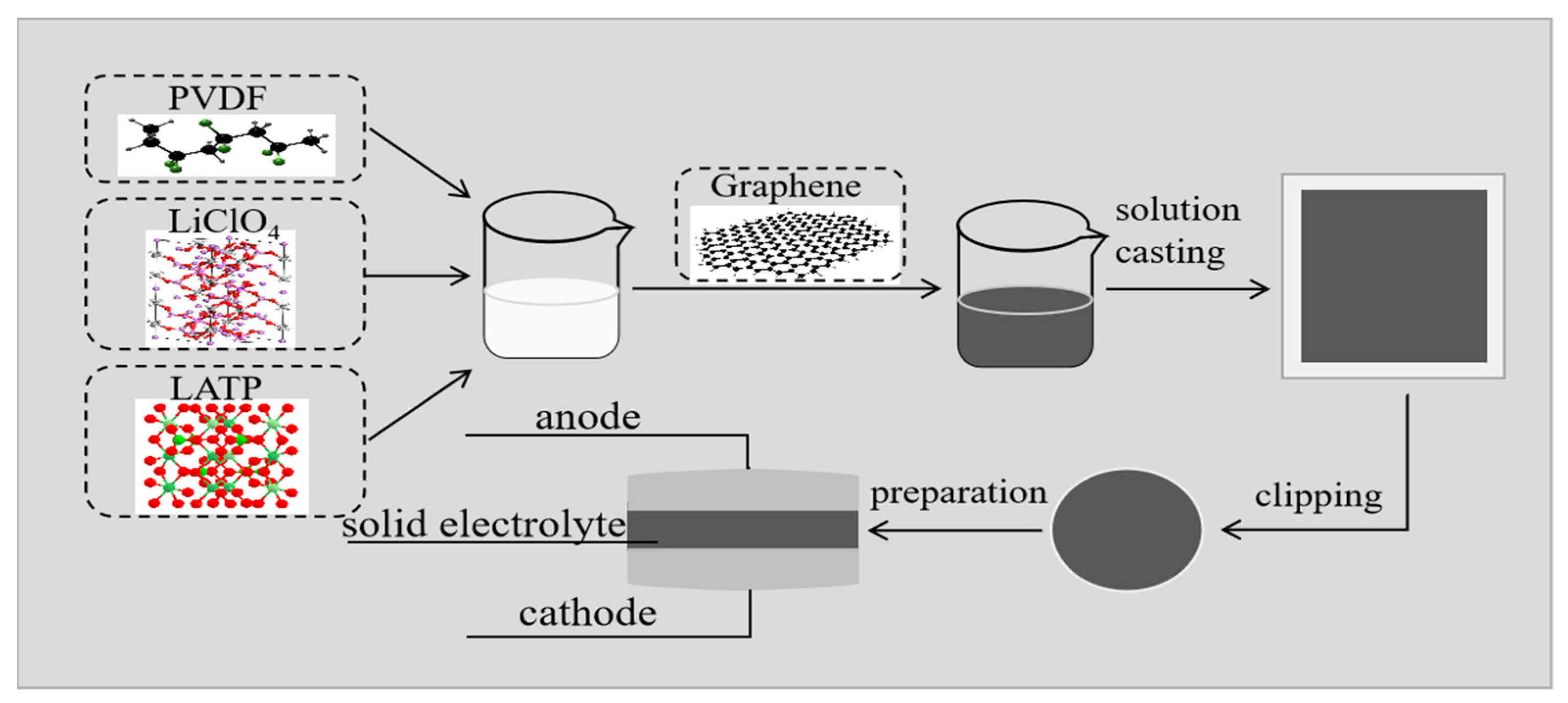
2.3. Preparation of PLLG Composite Solid Electrolyte Membrane
2.4. Battery Assembly
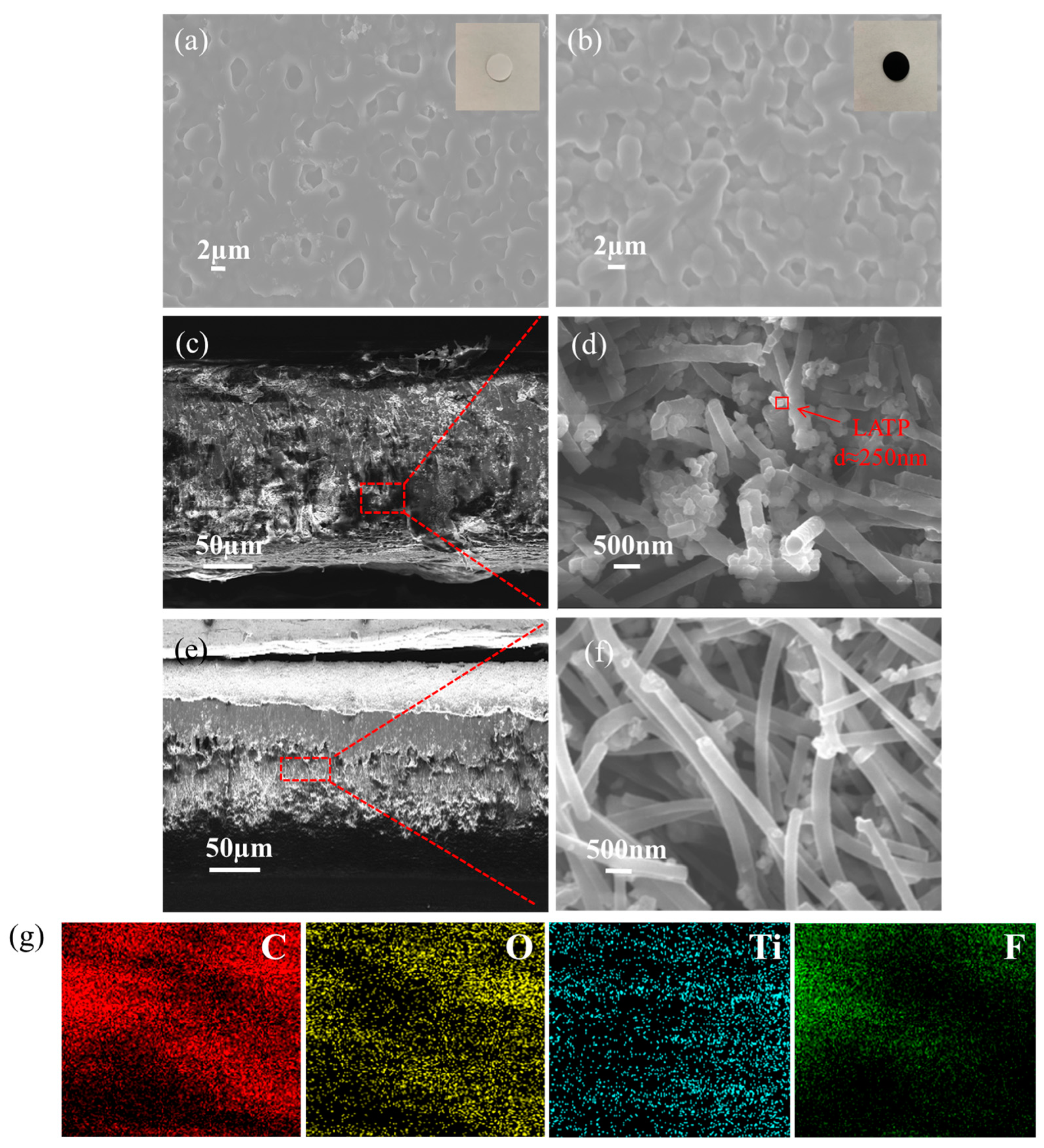
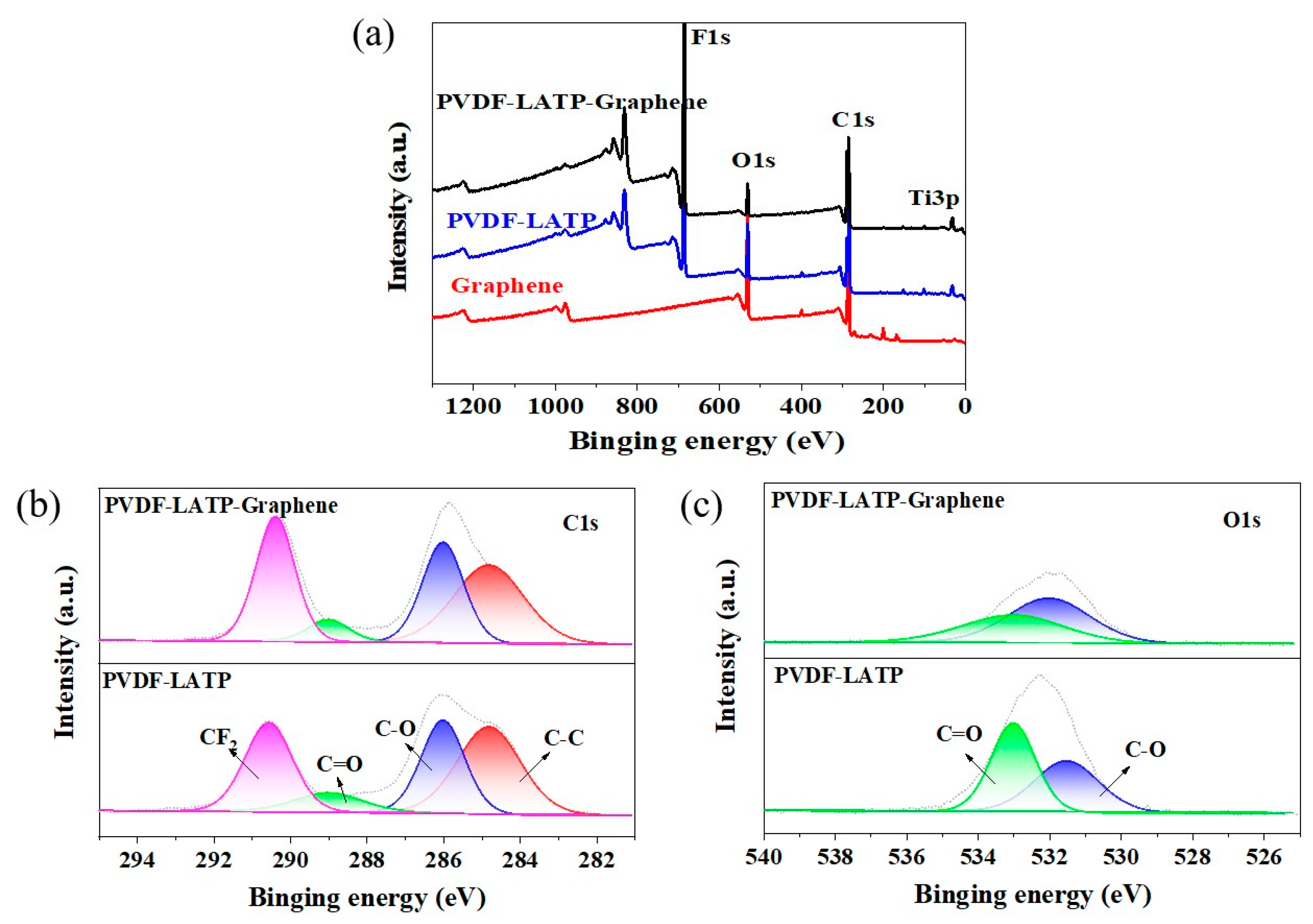
2.5. Physical Characterizations
2.6. Electrochemical Measurements
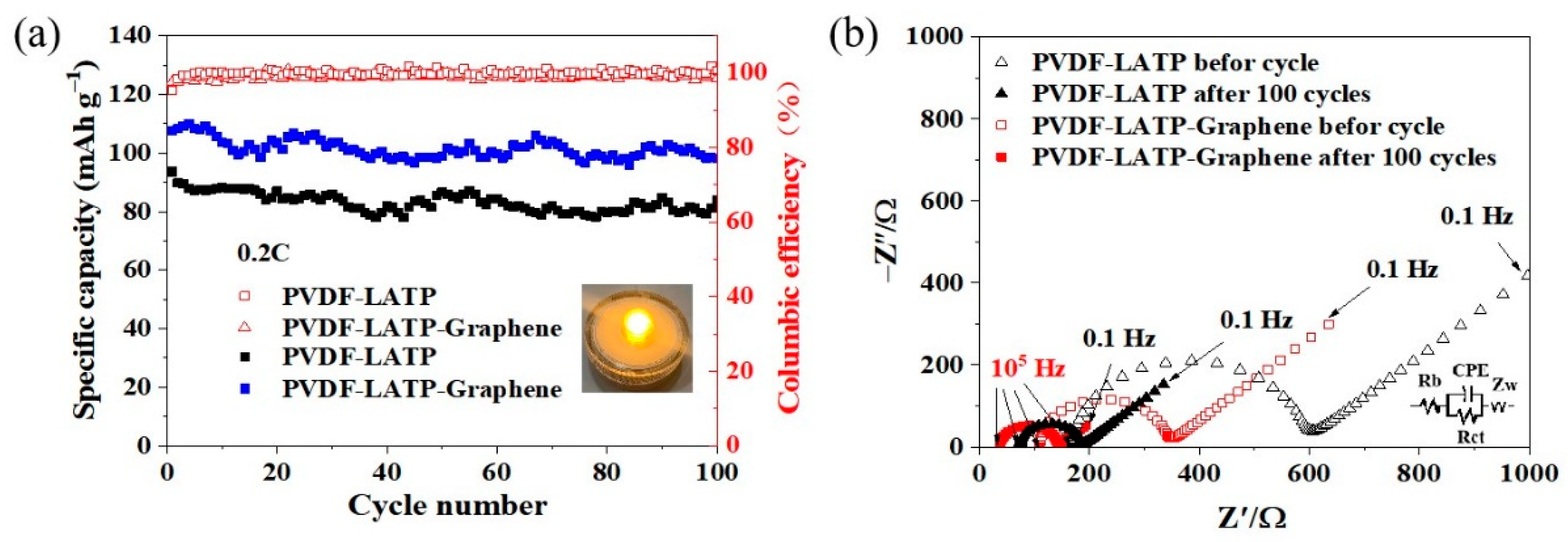
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jia, W.S.; Li, Z.L.; Wu, Z.R.; Wang, L.P.; Wu, B.; Wang, Y.H.; Cao, Y.; Li, J.Z. Graphene oxide as a filler to improve the performance of PAN-LiClO4 flexible solid polymer electrolyte. Solid State Ion. 2018, 315, 7–13. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhao, C.Z.; Yao, Y.X.; Liu, H.; Zhang, Q. Recent advances in energy chemistry between solid-state electrolyte and safe lithium-metal anodes. Chem 2019, 5, 74–96. [Google Scholar] [CrossRef]
- Wang, J.L.; Yan, X.F.; Zhang, Z.; Guo, R.N.; Ying, H.J.; Han, G.R.; Han, W.Q. Rational design of an electron/ion dual-conductive cathode framework for high-performance all-solid-state lithium batteries. ACS Appl. Mater. Interfaces 2020, 12, 41323–41332. [Google Scholar] [CrossRef] [PubMed]
- Pearse, A.J.; Schmitt, T.E.; Fuller, E.J.; El-Gabaly, F.; Lin, C.F.; Gerasopoulos, K.; Kozen, A.C.; Alec Talin, A.; Rubloff, G.; Gregorczyk, K.E. Nanoscale solid state batteries enabled by thermal atomic layer deposition of a lithium polyphosphazene solid state electrolyte. Chem. Mater. 2017, 29, 3740–3753. [Google Scholar] [CrossRef]
- Yi, S.; Xu, T.; Li, L.; Gao, M.; Du, K.; Zhao, H.; Bai, Y. Fast ion conductor modified double-polymer (PVDF and PEO) matrix electrolyte for solid lithium-ion batteries. Solid State Ion. 2020, 355, 115–419. [Google Scholar] [CrossRef]
- Puthirath, A.B.; Patra, S.; Pal, S.; Manoj, M.; Balan, A.P.; Jayalekshmi, S. Transparent flexible lithium ion conducting solid polymer electrolyte. J. Mater. Chem. A 2017, 5, 11152–11162. [Google Scholar] [CrossRef]
- Dirican, M.; Yan, C.; Zhu, P.; Zhang, X. Composite solid electrolytes for all-solid-state lithium batteries. Mater. Sci. Eng. R 2019, 136, 27–46. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Nie, J.H.; Li, F.; Wang, Z.L.; Sun, C.W. A durable and safe solid-state lithium battery with a hybrid electrolyte membrane. Nano Energy 2018, 45, 413–419. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.Y.-P.; Kafalas, J.A. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Liang, X.H.; Han, D.; Wang, Y.T.; Lan, L.X.; Mao, J. Preparation and performance study of a PVDF–LATP ceramic composite polymer electrolyte membrane for solid-state batteries. RSC Adv. 2018, 8, 40498–40504. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.L.; Zhang, X.X.; Liu, Y.C.; Chen, S.J.; Chen, Y.Q.; Lin, J.H.; Zhang, Y.N. Boosting the Performance of Solid-State Lithium Battery Based on Hybridizing Micron-Sized LATP in a PEO/PVDF-HFP Heterogeneous Polymer Matrix. Energy Technol. 2020, 8, 2000444. [Google Scholar] [CrossRef]
- Li, S.; Sun, G.C.; He, M.; Li, H. Organic–Inorganic Composite Electrolytes Optimized with Fluoroethylene Carbonate Additive for Quasi-Solid-State Lithium-Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 20962–20971. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lin, C.; Yan, X.H.; Wu, A.M.; Shen, S.M.; Wei, G.H.; Zhang, J.L. Mechanical property-reinforced PEO/PVDF/LiClO4/SN blend all solid polymer electrolyte for lithium ion batteries. J. Electroanal. Chem. 2020, 869, 114–156. [Google Scholar] [CrossRef]
- Xue, Z.G.; He, D.; Xie, X.L. Poly (ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Gomari, S.; Esfandeh, M.; Ghasemi, I. All-solid-state flexible nanocomposite polymer electrolytes based on poly (ethylene oxide): Lithium perchlorate using functionalized graphene. Solid State Ion. 2017, 303, 37–46. [Google Scholar] [CrossRef]
- Gahlot, S.; Kulshrestha, V. Graphene based polymer electrolyte membranes for electro-chemical energy appllications. Int. J. Hydrogen Energy 2020, 45, 17029–17056. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Wang, X.; Huang, R.Q.; Niu, S.Z.; Xu, L.; Zhang, Q.C.; Amini, A.; Cheng, C. Research progress on graphene-based materials for high-performance lithium-metal batteries. New Carbon Mater. 2021, 36, 711–728. [Google Scholar]
- Hu, Z.; Li, Z.; Xia, Z.; Jiang, T.; Wang, G.; Sun, J.Y.; Sun, P.F.; Yan, C.L.; Zhang, L. PECVD-derived graphene nanowall/lithium composite anodes towards highly stable lithium metal batteries. Energy Storage Mater. 2019, 22, 29–39. [Google Scholar] [CrossRef]
- Liu, J.Q.; Wu, X.F.; He, J.Y.; Li, J.; Lai, Y.Q. Preparation and performance of a novel gel polymer electrolyte based on poly (vinylidene fluoride)/graphene separator for lithium ion battery. Electrochim. Acta 2017, 235, 500–507. [Google Scholar] [CrossRef]
- Hou, Y.; Lv, S.; Liu, L.; Liu, X. High-quality preparation of graphene oxide via the Hummers’ method: Understanding the roles of the intercalator, oxidant, and graphite particle size. Ceram. Int. 2020, 46, 2392–2402. [Google Scholar] [CrossRef]
- Breuer, S.; Prutsch, D.; Ma, Q.; Epp, V.; Preishuber-Pflügl, F.; Tietzbc, F.; Wilkening, M. Separating bulk from grain boundary Li ion conductivity in the sol-gel prepared solid electrolyte Li1.5Al0.5Ti1.5(PO4)3. Mater. Chem. 2015, A3, 21343–21350. [Google Scholar] [CrossRef]
- Dai, X.Y.; Wang, L.P.; Xu, J.; Wang, Y.; Zhou, A.J.; Li, J.Z. Improved electrochemical performance of LiCoO2 electrodes with ZnO coating by radio frequency magnetron sputtering. ACS Appl. Mater. Interfaces 2014, 6, 15853–15859. [Google Scholar] [CrossRef] [PubMed]
- Sheng, O.W.; Jin, C.B.; Luo, J.M.; Yuan, H.D.; Huang, H.; Gan, Y.P.; Zhang, J.; Xia, Y.; Liang, C.; Zhang, W.K.; et al. Mg2B2O5 nanowire enabled multifunctional solid-state electrolytes with high ionic conductivity, excellent mechanical properties, and flame-retardant performance. Nano Lett. 2018, 18, 3104–3112. [Google Scholar] [CrossRef]
- Cech, O.; Klvac, O.; Benesova, P.; Maca, J.; Cudek, P.; Vanýsek, P. Synthesizing a LiFePO4/graphene composite with electrochemically prepared few-layer graphene. J. Energy Storage 2019, 22, 373–377. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, R.; Zhao, Q.N.; Liu, W.; Lu, G.J.; Hu, X.L.; Sun, J.; Wang, R.H.; Jiang, X.P.; Hu, N.; et al. Hydroxyapatite nanowire-reinforced poly (ethylene oxide)-based polymer solid electrolyte for application in high-temperature lithium batteries. ACS Appl. Mater. Interfaces 2020, 12, 54637–54643. [Google Scholar] [CrossRef]
- Wen, J.; Zhao, Q.N.; Jiang, X.P.; Ji, G.P.; Wang, R.H.; Lu, G.J.; Long, J.F.; Hu, N.; Xu, C.H. Graphene oxide enabled flexible PEO-based solid polymer electrolyte for all-solid-state lithium metal battery. ACS Appl. Energy Mater. 2021, 4, 3660–3669. [Google Scholar] [CrossRef]
- Bao, W.; Hu, Z.Y.; Wang, Y.Y.; Jiang, J.H.; Huo, S.K.; Fan, W.Z.; Chen, W.J.; Jing, X.; Long, X.Y.; Zhang, Y. Poly (ionic liquid)-functionalized graphene oxide towards ambient temperature operation of all-solid-state PEO-based polymer electrolyte lithium metal batteries. Chem. Eng. J. 2022, 437, 135420. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Feng, X.; Zhang, Z.; Gao, H.; Huang, J. Silica-assisted cross-linked polymer electrolyte membrane with high electrochemical stability for lithium-ion batteries. J. Colloid Interface Sci. 2021, 594, 1–8. [Google Scholar] [CrossRef]
- Naveen Kumar, K.; Saijyothi, K.; Kang, M.; Ratnakaram, Y.C.; Hari Krishna, K.; Jin, D.; Lee, Y.M. Improved electrical properties of Fe nanofiller impregnated PEO + PVP: Li+ blended polymer electrolytes for lithium battery applications. Appl. Phys. A-Mater. 2016, 122, 698. [Google Scholar] [CrossRef]
- Li, B.Y.; Su, Q.M.; Yu, L.T.; Dong, S.J.; Zhang, M.; Ding, S.K.; Du, G.H.; Xu, B.S. Ultrathin, flexible, and sandwiched structure composite polymer electrolyte membrane for solid-state lithium batteries. J. Membr. Sci. 2021, 618, 118734. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly (vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Farivar, F.; Yap, P.L.; Hassan, K.; Tung, T.T.; Tran, D.N.H.; Pollard, A.J.; Losic, D. Unlocking thermogravimetric analysis (TGA) in the fight against “Fake graphene” materials. Carbon 2021, 179, 505–513. [Google Scholar] [CrossRef]
- Park, D.W.; Natalia, A.; Cañas, N.A.; Wagner, N.; Friedrich, K.A. Novel solvent-free direct coating process for battery electrodes and their electrochemical performance. J. Power Sources 2016, 306, 758–763. [Google Scholar] [CrossRef] [Green Version]
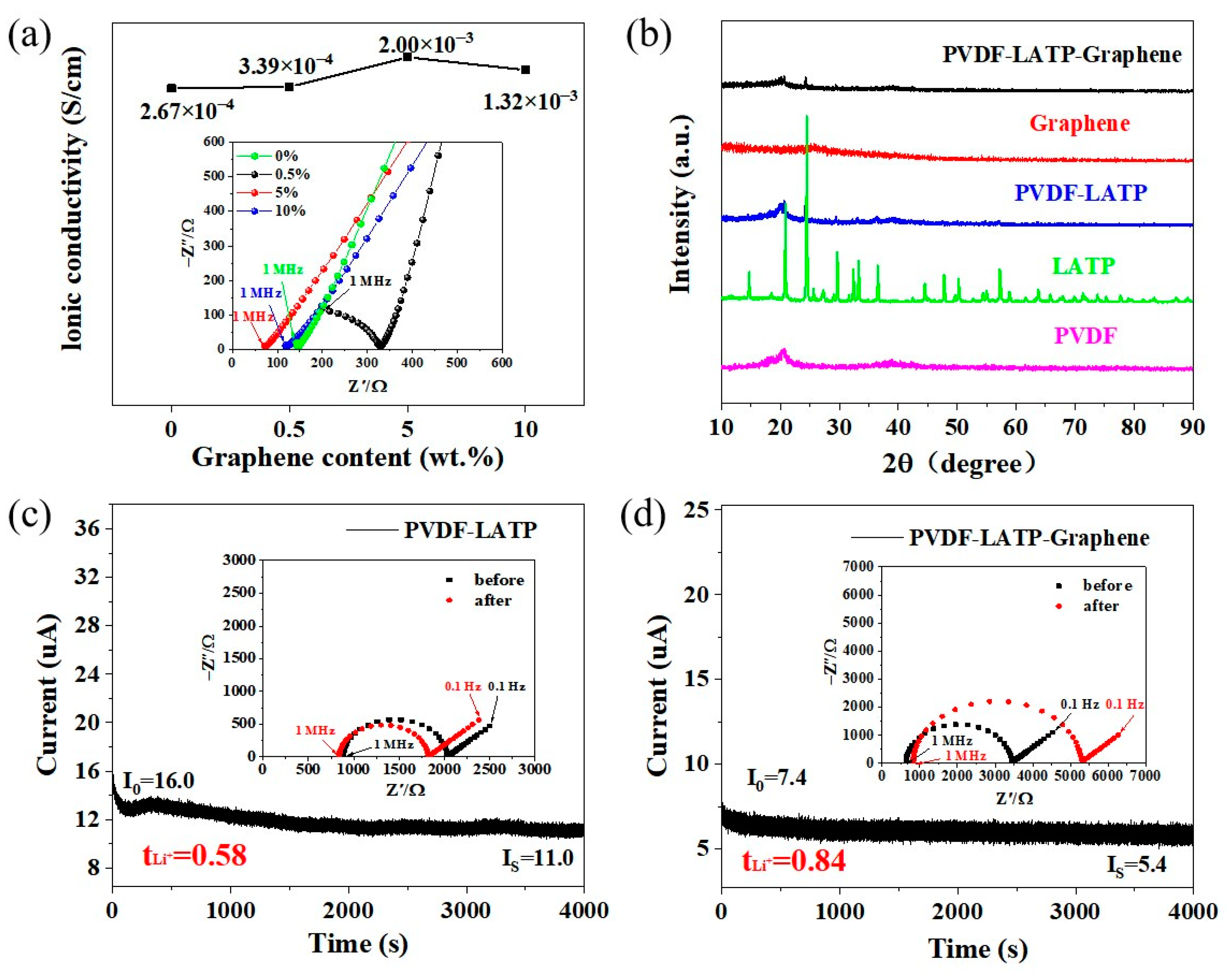


| Electrolytes | ||
|---|---|---|
| PLL | PLLG | |
| I0/µA | 16 | 7.4 |
| Is/µA | 11 | 5.4 |
| R0/Ω | 2040 | 3450 |
| Rs/Ω | 1820 | 5300 |
| V/mV | 50 | 50 |
| tLi+ | 0.58 | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Huang, D.; Lan, L.; Yang, G.; Huang, J. Enhancement of the Electrochemical Performances of Composite Solid-State Electrolytes by Doping with Graphene. Nanomaterials 2022, 12, 3216. https://doi.org/10.3390/nano12183216
Liang X, Huang D, Lan L, Yang G, Huang J. Enhancement of the Electrochemical Performances of Composite Solid-State Electrolytes by Doping with Graphene. Nanomaterials. 2022; 12(18):3216. https://doi.org/10.3390/nano12183216
Chicago/Turabian StyleLiang, Xinghua, Dongxue Huang, Linxiao Lan, Guanhua Yang, and Jianling Huang. 2022. "Enhancement of the Electrochemical Performances of Composite Solid-State Electrolytes by Doping with Graphene" Nanomaterials 12, no. 18: 3216. https://doi.org/10.3390/nano12183216




