Nanocomposite-Enhanced Efficient Evaporation System for Solar-Driven Seawater Desalination—An Optimized Design for Clean Water Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2 Nanoparticles
2.3. Synthesis of TiO2/AC Nanocomposites
2.4. Fabrication of TiO2/AC Solar Evaporator
2.5. Solar Evaporation Setup
2.6. Photothermal Conversion Efficiency
3. Results
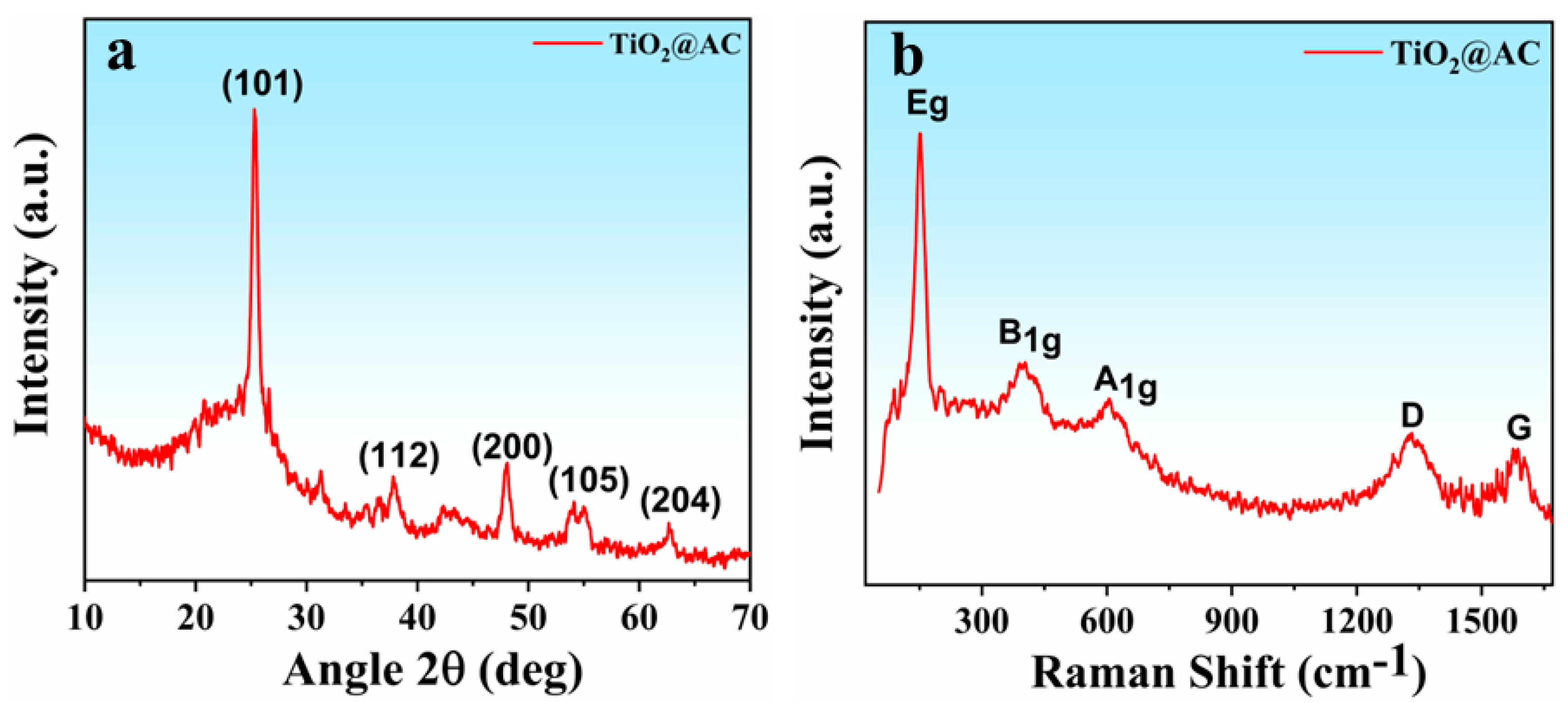
3.1. Structural Analysis
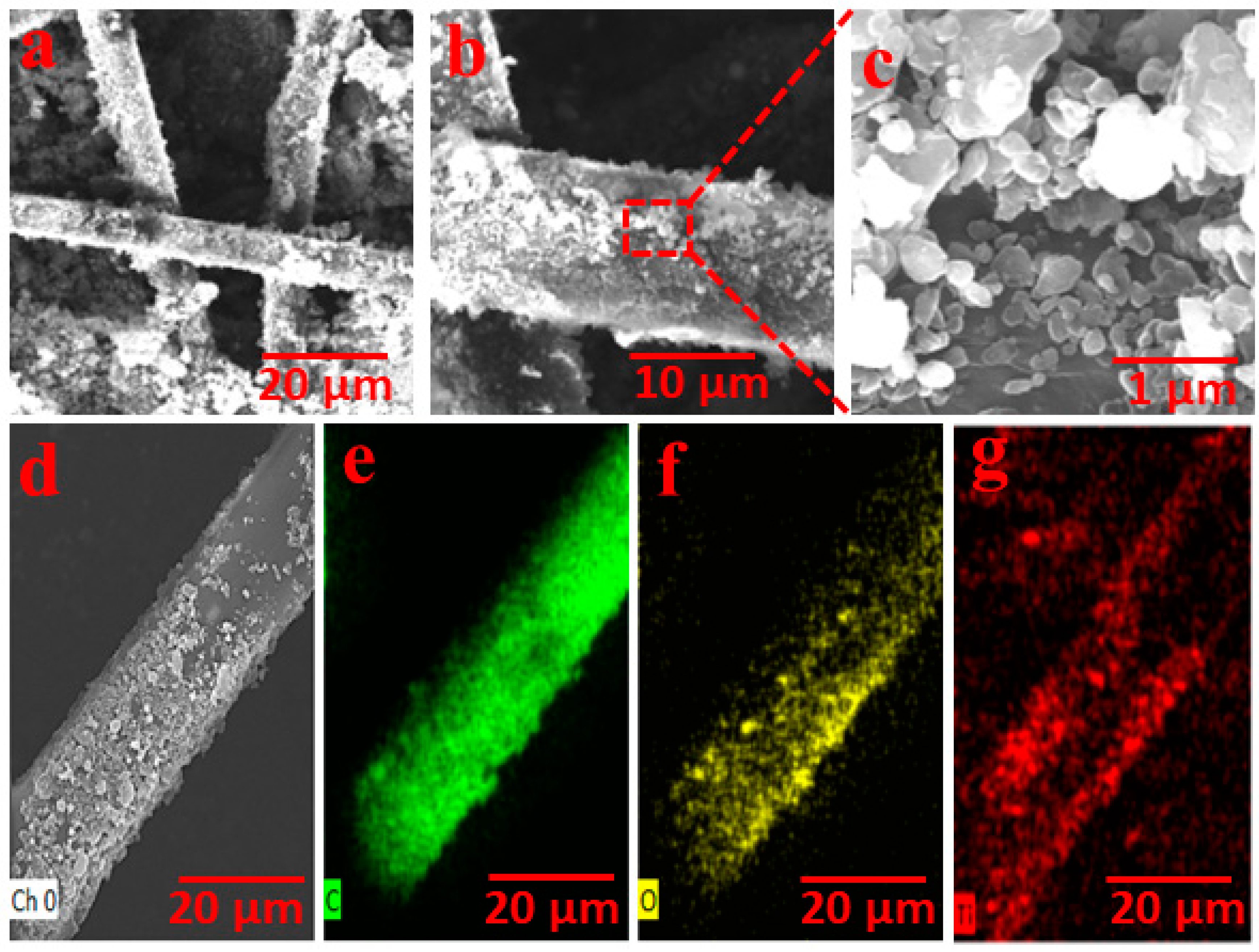
3.2. Morphological Analysis
3.3. Solar Absorption and Interfacial Surface Temperature
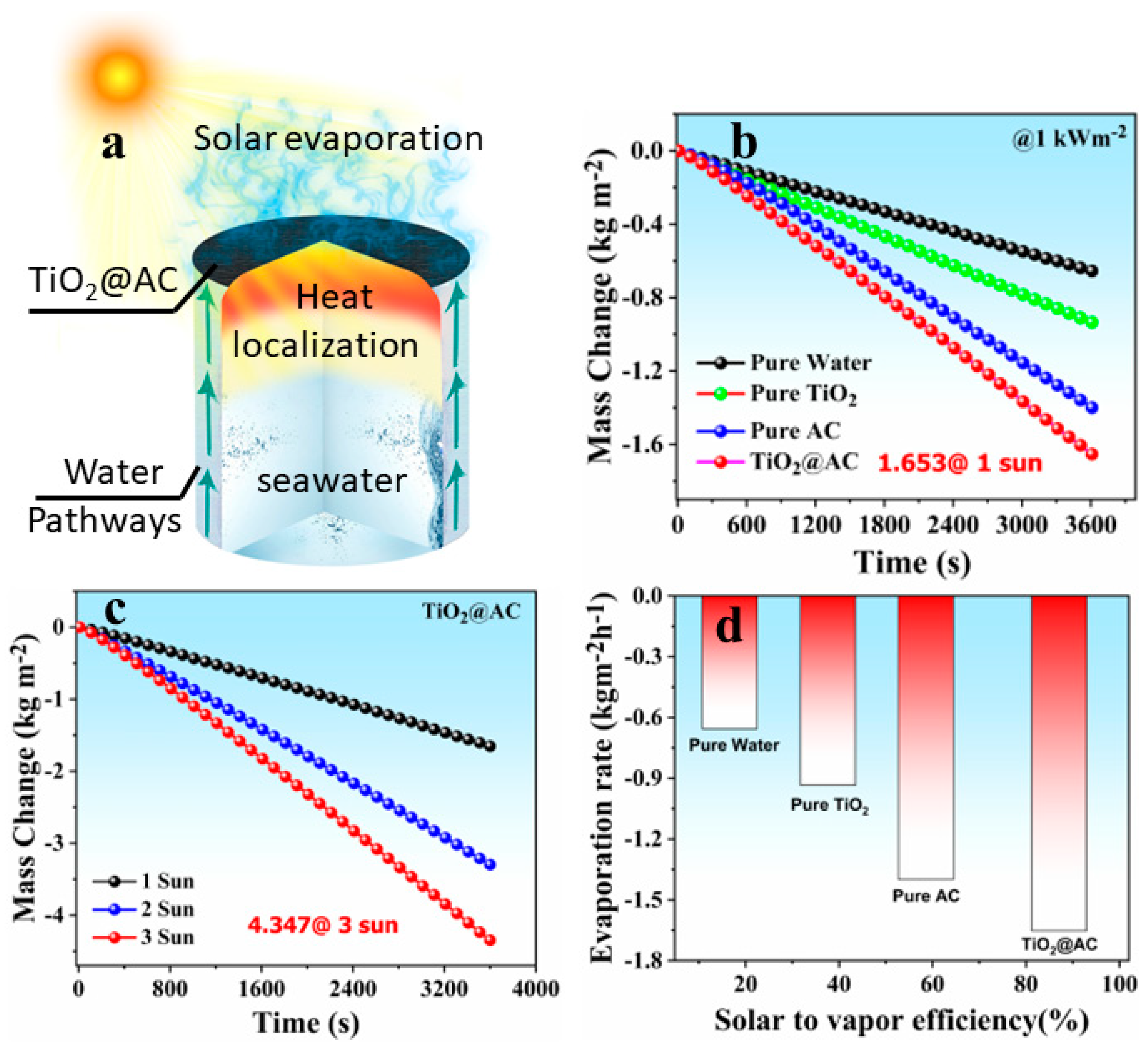
3.4. Solar Evaporation Performance
3.5. Purity and Reliability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irshad, M.S.; Wang, X.; Abbas, A.; Yu, F.; Li, J.; Wang, J.; Mei, T.; Qian, J.; Wu, S.; Javed, M.Q. Salt-resistant carbon dots modified solar steam system enhanced by chemical advection. Carbon 2021, 176, 313–326. [Google Scholar] [CrossRef]
- Arshad, N.; Ahmed, I.; Irshad, M.S.; Li, H.R.; Wang, X.; Ahmad, S.; Sharaf, M.; Firdausi, M.; Zaindin, M.; Atif, M. Super Hydrophilic Activated Carbon Decorated Nanopolymer Foam for Scalable, Energy Efficient Photothermal Steam Generation, as an Effective Desalination System. Nanomaterials 2020, 10, 2510. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Guo, Z.; Xu, Y.; Chen, Z.; Irshad, M.S.; Qian, J.; Mei, T.; Wang, X. Biomass-Derived Bilayer Solar Evaporator with Enhanced Energy Utilization for High-Efficiency Water Generation. ACS Appl. Mater. Interfaces 2020, 12, 57155–57164. [Google Scholar] [CrossRef]
- Wei, Z.; Arshad, N.; Hui, C.; Irshad, M.S.; Mushtaq, N.; Hussain, S.; Shah, M.; Naqvi, S.Z.H.; Rizwan, M.; Shahzad, N.; et al. Interfacial Photothermal Heat Accumulation for Simultaneous Salt Rejection and Freshwater Generation; an Efficient Solar Energy Harvester. Nanomaterials 2022, 12, 3206. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Z.; Guo, Z.; Irshad, M.S.; Yu, L.; Qian, J.; Mei, T.; Wang, X. Molybdenum Carbide/Carbon-Based Chitosan Hydrogel as an Effective Solar Water Evaporation Accelerator. ACS Sustain. Chem. Eng. 2020, 8, 7139–7149. [Google Scholar] [CrossRef]
- Irshad, M.S.; Arshad, N.; Wang, X. Nanoenabled Photothermal Materials for Clean Water Production. Glob. Chall. 2020, 5, 2000055. [Google Scholar] [CrossRef]
- Lu, Y.; Arshad, N.; Irshad, M.S.; Ahmed, I.; Ahmad, S.; Alshahrani, L.A.; Yousaf, M.; Sayed, A.E.; Nauman, M. Fe2O3 Nanoparticles Deposited over Self-Floating Facial Sponge for Facile Interfacial Seawater Solar Desalination. Crystals 2021, 11, 1509. [Google Scholar] [CrossRef]
- Wei, Z.; Arshad, N.; Irshad, M.S.; Idrees, M.; Ahmed, I.; Li, H.; Qazi, H.H.; Yousaf, M.; Alshahrani, L.A.; Lu, Y. A Scalable Prototype by In Situ Polymerization of Biodegradables, Cross-Linked Molecular Mode of Vapor Transport, and Metal Ion Rejection for Solar-Driven Seawater Desalination. Crystals 2021, 11, 1489. [Google Scholar] [CrossRef]
- Blanco, J.; Malato, S.; Fernández-Ibañez, P.; Alarcón, D.; Gernjak, W.; Maldonado, M.I. Review of feasible solar energy applications to water processes. Renew. Sustain. Energy Rev. 2009, 13, 1437–1445. [Google Scholar] [CrossRef]
- Madhlopa, A.; Johnstone, C. Numerical study of a passive solar still with separate condenser. Renew. Energy 2009, 34, 1668–1677. [Google Scholar] [CrossRef] [Green Version]
- Panchal, H.N.; Shah, P. Effect of Varying Glass cover thickness on Performance of Solar still: In a Winter Climate Conditions. Int. J. Renew. Energy Res. 2012, 1, 212–223. [Google Scholar]
- Long, Z.; Li, H.; Bu, X.; Ma, W.; Zhao, L. Solar radiation on vertical surfaces for building application in different climate zones across China. J. Renew. Sustain. Energy 2013, 5, 021418. [Google Scholar] [CrossRef]
- Liang, H.; Liao, Q.; Chen, N.; Liang, Y.; Lv, G.; Zhang, P.; Lu, B.; Qu, L. Thermal Efficiency of Solar Steam Generation Approaching 100% through Capillary Water Transport. Angew. Chem. Int. Ed. 2019, 58, 19041–19046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, M.; Chen, S.; Liang, Q.; Sheng, N.; Han, Z.; Cai, Y.; Wang, H. Scalable, self-cleaning and self-floating bi-layered bacterial cellulose biofoam for efficient solar evaporator with photocatalytic purification. Desalination 2020, 500, 114899. [Google Scholar] [CrossRef]
- Irshad, M.S.; Abbas, A.; Qazi, H.H.; Aziz, M.H.; Shah, M.; Ahmed, A.; Idrees, M. Role of point defects in hybrid phase TiO2 for resistive random-access memory (RRAM). Mater. Res. Express 2019, 6, 076311. [Google Scholar] [CrossRef]
- Abbasi, M.S.; Irshad, M.S.; Arshad, N.; Ahmed, I.; Idrees, M.; Ahmad, S.; Wei, Z.; Sharaf, M.; Al Firdausi, M.D. Biomaterial-Induced Stable Resistive Switching Mechanism in TiO2 Thin Films: The Role of Active Interstitial Sites/Ions in Minimum Current Leakage and Superior Bioactivity. ACS Omega 2020, 5, 19050–19060. [Google Scholar] [CrossRef]
- Rao, K.G.; Ashok, C.H.; Rao, K.V.; Chakra, C.S.; Rajendar, V. Synthesis of TiO2 nanoparticles from orange fruit waste. Synthesis 2015, 1, 82–90. [Google Scholar]
- Abdullah, S.A.; Sahdan, M.Z.; Nafarizal, N.; Saim, H.; Bakri, A.S.; Rohaida, C.H.C.; Adriyanto, F.; Sari, Y. Photoluminescence study of trap-state defect on TiO2 thin films at different substrate temperature via RF magnetron sputtering. In J. Phys. Conf. Ser.; 2018; Volume 995, p. 12067. [Google Scholar]
- Pan, J.; Yu, X.; Dong, J.; Zhao, L.; Liu, L.; Liu, J.; Zhao, X.; Liu, L. Diatom-Inspired TiO2-PANi-Decorated Bilayer Photothermal Foam for Solar-Driven Clean Water Generation. ACS Appl. Mater. Interfaces 2021, 13, 58124–58133. [Google Scholar] [CrossRef]
- Rao, K.G.; Ashok, C.H.; Rao, K.V.; Chakra, C.S.; Tambur, P. Green synthesis of TiO2 nanoparticles using Aloe vera extract. Int. J. Adv. Res. Phys. Sci. 2015, 2, 28–34. [Google Scholar]
- Huang, J.; He, Y.; Wang, L.; Huang, Y.; Jiang, B. Bifunctional Au@ TiO2 core--shell nanoparticle films for clean water genera-tion by photocatalysis and solar evaporation. Energy Convers. Manag. 2017, 132, 452–459. [Google Scholar] [CrossRef]
- Amanulla, A.M.; Sundaram, R. Green synthesis of TiO2 nanoparticles using orange peel extract for antibacterial, cytotoxicity and humidity sensor applications. Mater. Today Proc. 2019, 8, 323–331. [Google Scholar] [CrossRef]
- Chen, S.-X.; Chang, S.-P.; Chang, S.-J.; Hsieh, W.-K.; Lin, C.-H. Highly Stable Ultrathin TiO2Based Resistive Random Access Memory with Low Operation Voltage. ECS J. Solid State Sci. Technol. 2018, 7, Q3183–Q3188. [Google Scholar] [CrossRef]
- Zimbone, M.; Buccheri, M.; Cacciato, G.; Sanz, R.; Rappazzo, G.; Boninelli, S.; Reitano, R.; Romano, L.; Privitera, V.; Grimaldi, M. Photocatalytical and antibacterial activity of TiO2 nanoparticles obtained by laser ablation in water. Appl. Catal. B Environ. 2014, 165, 487–494. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Hu, Y.; Wang, X. Commercially Available Activated Carbon Fiber Felt Enables Efficient Solar Steam Generation. ACS Appl. Mater. Interfaces 2018, 10, 9362–9368. [Google Scholar] [CrossRef]
- Kalagatur, N.K.; Karthick, K.; Allen, J.A.; Ghosh, O.S.N.; Chandranayaka, S.; Gupta, V.K.; Krishna, K.; Mudili, V. Application of Activated Carbon Derived from Seed Shells of Jatropha curcas for Decontamination of Zearalenone Mycotoxin. Front. Pharmacol. 2017, 8, 760. [Google Scholar] [CrossRef]
- Irshad, M.S.; Wang, X.; Arshad, N.; Javed, M.Q.; Shamim, T.; Guo, Z.; Li, H.R.; Wang, J.; Mei, T. Bifunctional in-situ pol-ymerized nanocomposites for convective solar desalination and enhanced photo-thermoelectric power generation. Environ. Sci. Nano 2022, 9, 1685–1698. [Google Scholar]
- Srinivasu, P.; Singh, S.P.; Islam, A.; Han, L. Novel Approach for the Synthesis of Nanocrystalline Anatase Titania and Their Photovoltaic Application. Adv. Optoelectron. 2011, 2011, 539382. [Google Scholar] [CrossRef]
- Challagulla, S.; Tarafder, K.; Ganesan, R.; Roy, S. Structure sensitive photocatalytic reduction of nitroarenes over TiO2. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Lota, G.; Krawczyk, P.; Lota, K.; Sierczyńska, A.; Kolanowski, Ł; Baraniak, M.; Buchwald, T. The application of activated carbon modified by ozone treatment for energy storage. J. Solid State Electrochem. 2016, 20, 2857–2864. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Irshad, M.S.; Arshad, N.; Noureen, L.; Ahmed, I.; Mushtaq, N.; Asghar, M.S.; Hayat, Q.; Ghazanfar, U.; Idrees, M.; et al. Nanocomposite-Enhanced Efficient Evaporation System for Solar-Driven Seawater Desalination—An Optimized Design for Clean Water Production. Nanomaterials 2022, 12, 3296. https://doi.org/10.3390/nano12193296
Wei Z, Irshad MS, Arshad N, Noureen L, Ahmed I, Mushtaq N, Asghar MS, Hayat Q, Ghazanfar U, Idrees M, et al. Nanocomposite-Enhanced Efficient Evaporation System for Solar-Driven Seawater Desalination—An Optimized Design for Clean Water Production. Nanomaterials. 2022; 12(19):3296. https://doi.org/10.3390/nano12193296
Chicago/Turabian StyleWei, Zhou, Muhammad Sultan Irshad, Naila Arshad, Laila Noureen, Iftikhar Ahmed, Naveed Mushtaq, Muhammad Sohail Asghar, Qaisar Hayat, Uzma Ghazanfar, Muhammad Idrees, and et al. 2022. "Nanocomposite-Enhanced Efficient Evaporation System for Solar-Driven Seawater Desalination—An Optimized Design for Clean Water Production" Nanomaterials 12, no. 19: 3296. https://doi.org/10.3390/nano12193296






