Recent Advances of Transition Metal Chalcogenides as Cathode Materials for Aqueous Zinc-Ion Batteries
Abstract
:1. Introduction
2. The Electrochemical Performance of TMCs Cathodes
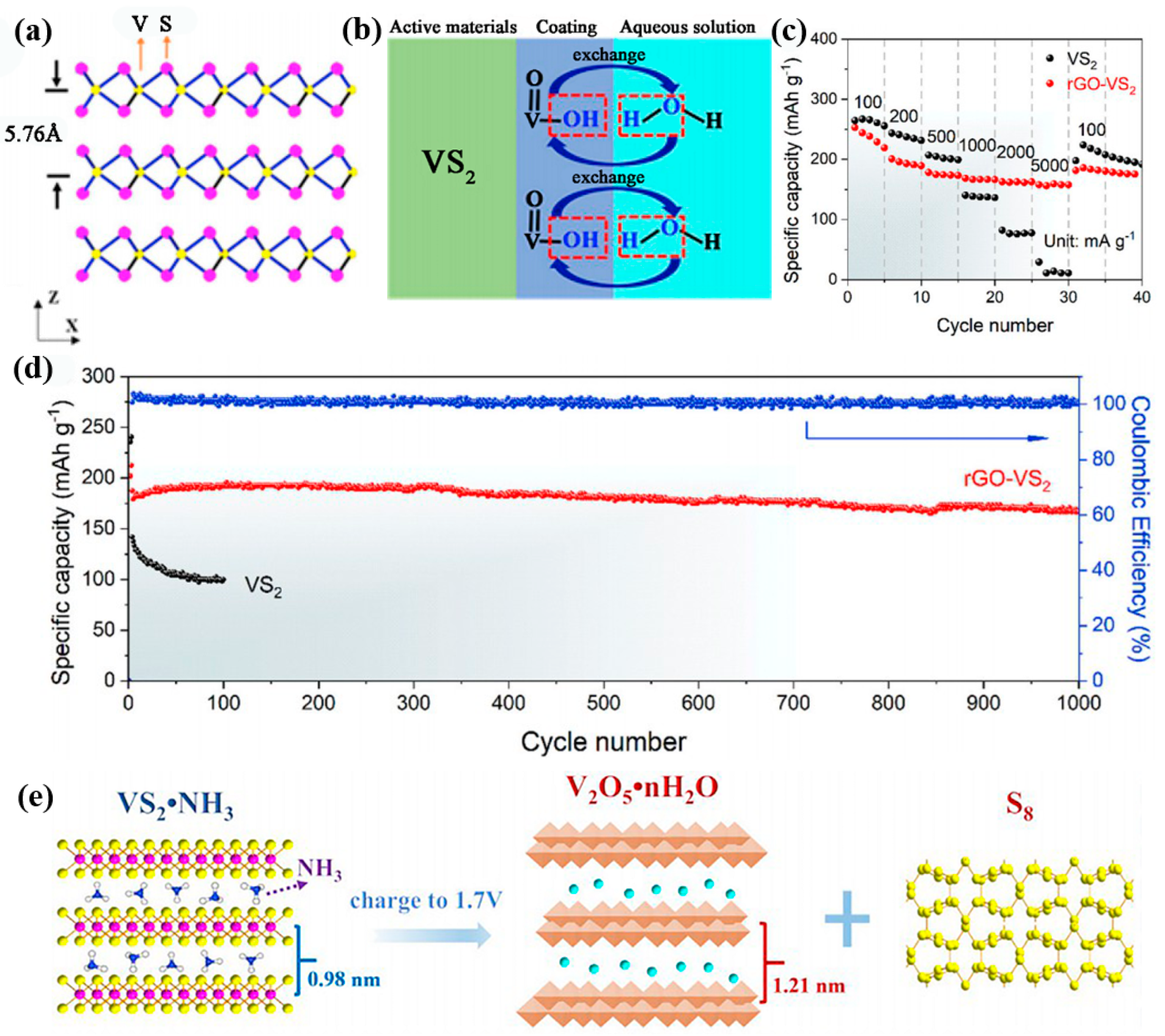
2.1. VS2 and VS4
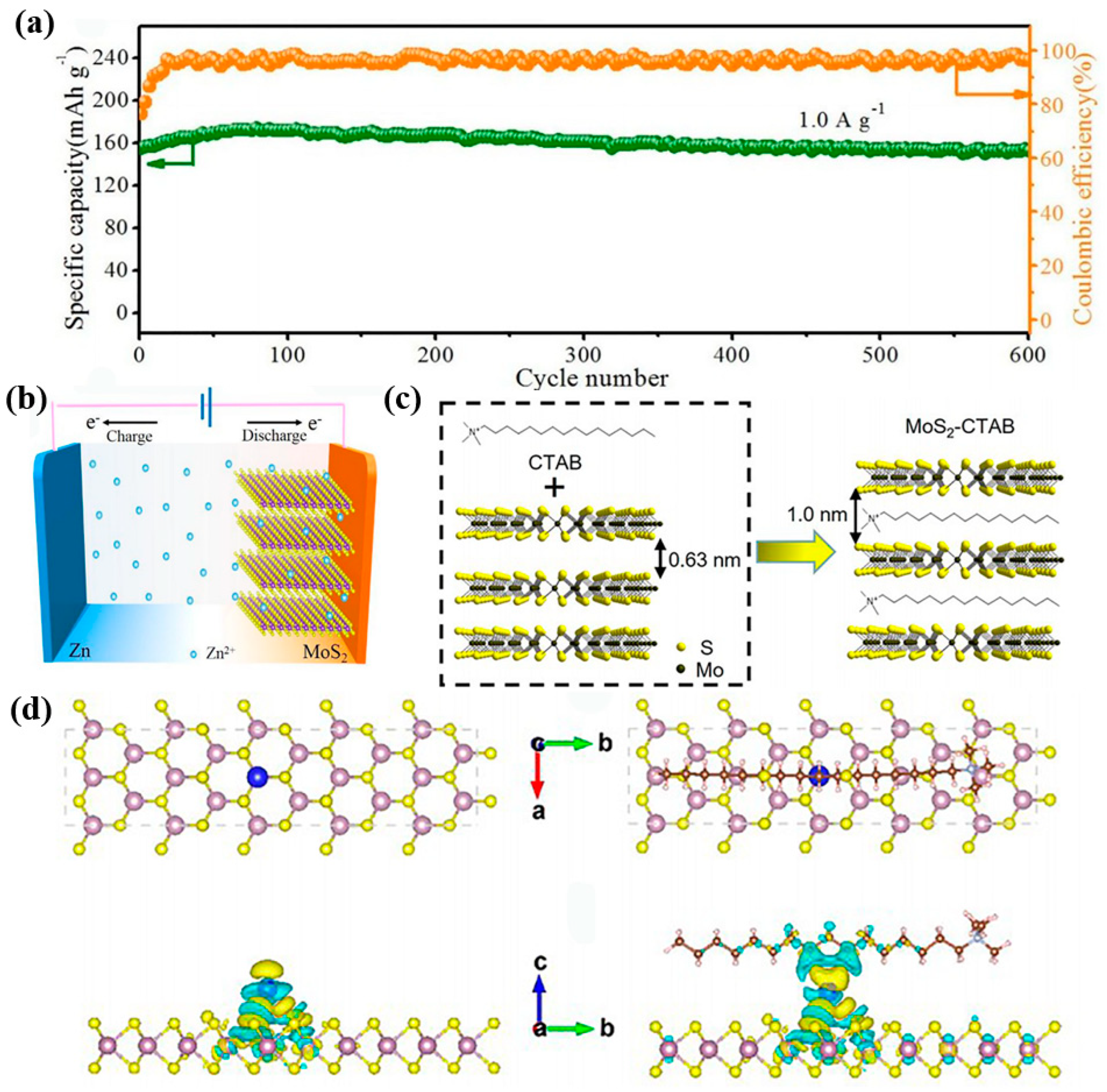
2.2. MoS2
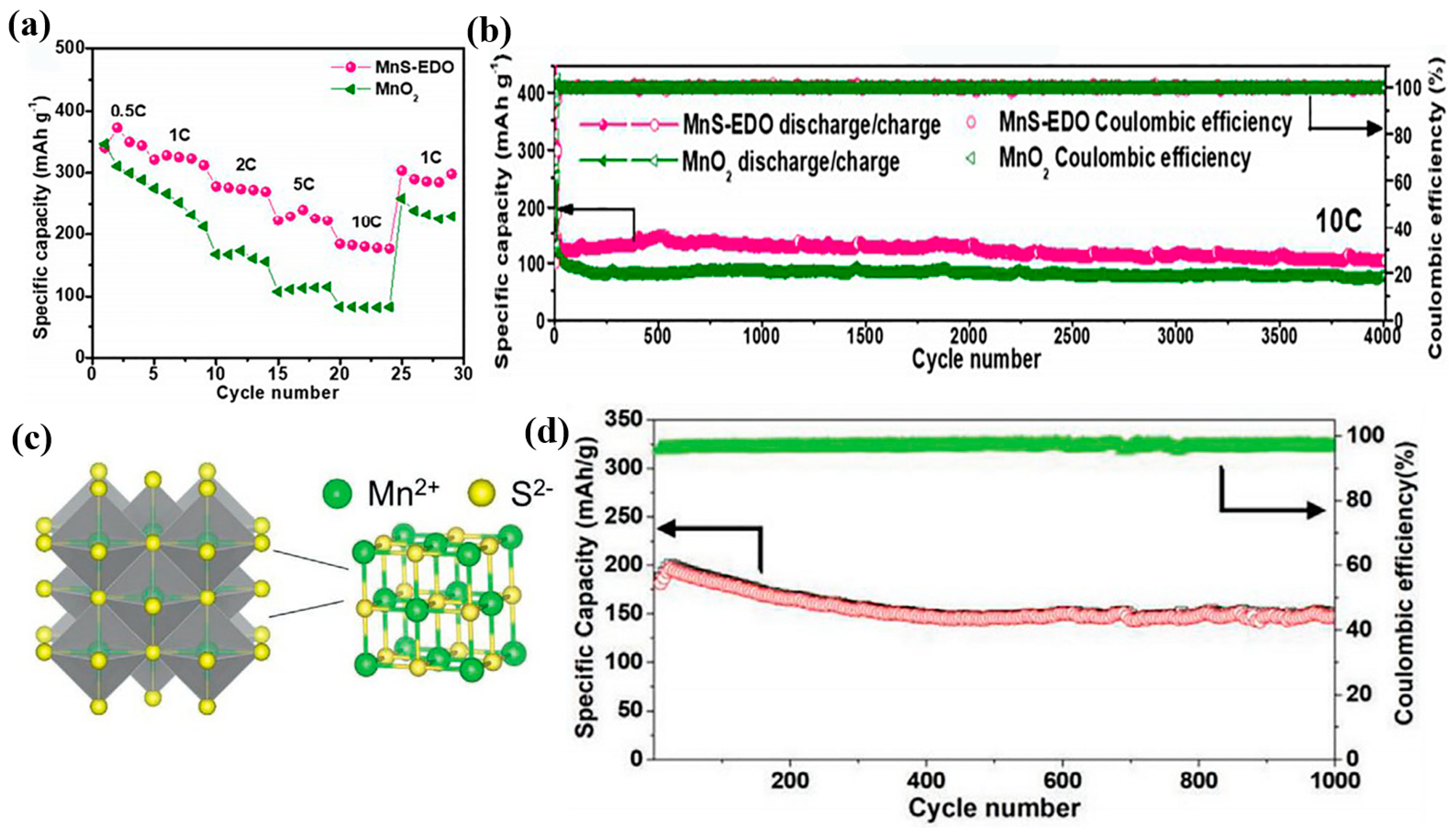
2.3. MnS
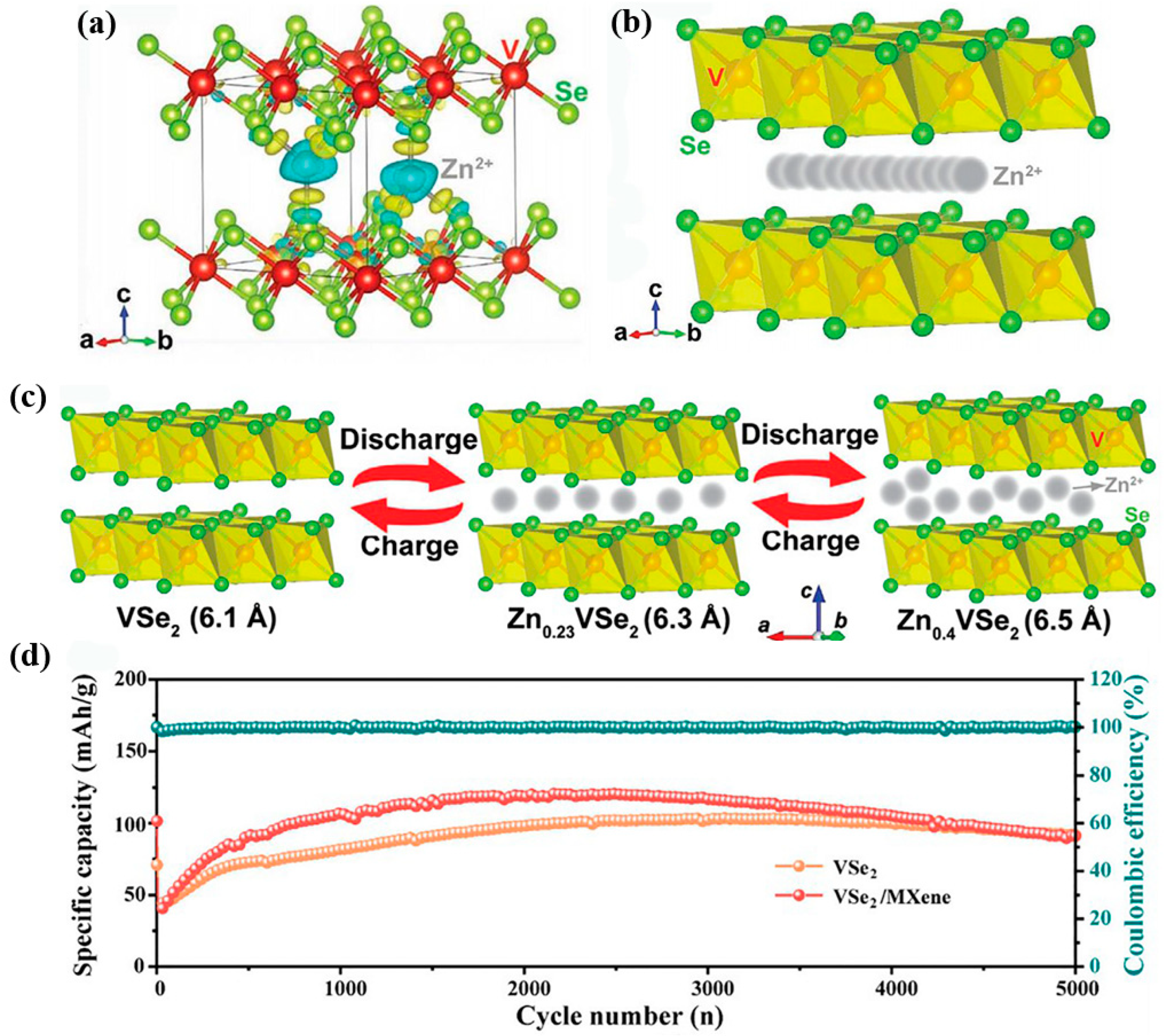
2.4. VSe2
2.5. Ni3S2
3. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Goodenough, J.B. Energy storage materials: A perspective. Energy Storage Mater. 2015, 1, 158–161. [Google Scholar] [CrossRef]
- Zhao, D.P.; Dai, M.Z.; Zhao, Y.; Liu, H.Q.; Liu, Y.; Wu, X. Improving electrocatalytic activities of FeCo2O4@FeCo2S4@PPy electrodes by surface/interface regulation. Nano Energy 2020, 72, 104715. [Google Scholar] [CrossRef]
- Ruan, P.; Liang, S.; Lu, B.; Fan, H.J.; Zhou, J. Design strategies for high-energy-density aqueous zinc batteries. Angew. Chem. Int. Ed. 2022, 61, e202200598. [Google Scholar] [CrossRef]
- Liu, C.; Wu, X.; Wang, B. Performance modulation of energy storage devices: A case of Ni-Co-S electrode materials. Chem. Eng. J. 2020, 392, 123651. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, Y.; Geng, H.; Liu, C.; Lu, B.; Rui, X.; Yan, Q. Pathways towards high energy aqueous rechargeable batteries. Coord. Chem. Rev. 2020, 424, 213521. [Google Scholar] [CrossRef]
- Ju, Z.; Zhao, Q.; Chao, D.; Hou, Y.; Pan, H.; Sun, W.; Yuan, Z.; Li, H.; Ma, T.; Su, D.; et al. Energetic Aqueous Batteries. Adv. Energy Mater. 2022, 12, 2201074. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wu, X. Toward long-life aqueous zinc ion batteries by constructing stable zinc anodes. Chem. Rec. 2022, e202200088. [Google Scholar] [CrossRef]
- Li, C.; Xie, X.; Liang, S.; Zhou, J. Issues and future perspective on zinc metal anode for rechargeable aqueous zinc-ion batteries. Energy Environ. Mater. 2020, 3, 146–159. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X. Hydrogen and sodium ions co-intercalated vanadium dioxide electrode materials with enhanced zinc ion storage capacity. Nano Energy 2021, 86, 106124. [Google Scholar] [CrossRef]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent advances in Zn-ion batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Yang, J.; Yin, B.; Sun, Y.; Pan, H.; Sun, W.; Jia, B.; Zhang, S.; Ma, T. Zinc Anode for Mild Aqueous Zinc-Ion Batteries: Challenges, Strategies, and Perspectives. Nano-Micro Lett. 2022, 14, 42. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wu, X.; Cho, Y.R. Enhanced electrochemical performance of Zn/VOx batteries by a carbon-encapsulation strategy. ACS Appl. Mater. Interfaces 2022, 14, 11654–11662. [Google Scholar] [CrossRef]
- Li, H.F.; Ma, L.T.; Han, C.P.; Wang, Z.F.; Liu, Z.X.; Tang, Z.J.; Zhi, C.Y. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X. Review of vanadium-based electrode materials for rechargeable aqueous zinc ion batteries. J. Energy Chem. 2021, 56, 223–237. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Jin, X.; Han, Y.; Lin, Y.; Lei, Z.; Wang, S.; Qin, L.; Jiao, S.; Cao, R. A hollow-structured manganese oxide cathode for stable Zn-MnO2 batteries. Nanomaterials 2018, 8, 301. [Google Scholar] [CrossRef]
- Liu, B. Transition metal dichalcogenides for high-performance aqueous zinc ion batteries. Batteries 2022, 8, 62. [Google Scholar] [CrossRef]
- Trócoli, R.; La Mantia, F. An aqueous zinc-ion battery based on copper hexacyanoferrate. ChemSusChem 2015, 8, 481–485. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, P.F.; Liu, H.Q.; Wu, X.; Zhi, C.Y. Tetragonal VO2 hollow nanospheres as robust cathode material for aqueous zinc ion batteries. Mater. Today Energy 2020, 17, 100431. [Google Scholar] [CrossRef]
- Yang, Q.; Mo, F.; Liu, Z.; Ma, L.; Li, X.; Fang, D.; Chen, S.; Zhang, S.; Zhi, C. Activating C-coordinated iron of iron hexacyanoferrate for Zn hybrid-ion batteries with 10000-cycle lifespan and superior rate capability. Adv. Mater. 2019, 31, 1901521. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Yamauchi, Y.; Alothman, Z.A.; Kaneti, Y.V.; Wu, X. Enhanced zinc ion storage capability of V2O5 electrode materials with hollow interior cavities. Batt. Supercap. 2021, 4, 1867–1873. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, X.; Veder, J.P.; Shao, Z. Self-recovery chemistry and cobalt-catalyzed electrochemical deposition of cathode for boosting performance of aqueous zinc-ion batteries. iScience 2020, 23, 100943. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Wu, X.; Cho, Y.R. High performance aqueous zinc battery enabled by potassium ion stabilization. J. Colloid Interface Sci. 2022, 628, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Cao, X.; Wu, X.J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.H.; et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Naveed, A.; Yang, H.; Shao, Y.; Yang, J.; Yanna, N.; Liu, J.; Shi, S.; Zhang, L.; Ye, A.; He, B.; et al. A Highly reversible Zn anode with intrinsically safe organic electrolyte for long-cycle-life batteries. Adv. Mater. 2019, 31, 1900668. [Google Scholar] [CrossRef]
- Liu, W.; Hao, J.; Xu, C.; Mou, J.; Dong, L.; Jiang, F.; Kang, Z.; Wu, J.; Jiang, B.; Kang, F. Investigation of zinc ion storage of transition metal oxides, sulfides, and borides in zinc ion battery systems. Chem. Commun. 2017, 53, 6872–6874. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, W.; Zhao, R.; Yang, Z.; Li, W.; Chao, D.; Qiao, S.Z.; Zhao, D. Sulfur-based aqueous batteries: Electrochemistry and strategies. J. Am. Chem. Soc. 2021, 143, 15475–15489. [Google Scholar] [CrossRef]
- Gao, M.R.; Xu, Y.F.; Jiang, J.; Yu, S.H. Nanostructured metal chalcogenides: Synthesis, modification, and applications in energy conversion and storage devices. Chem. Soc. Rev. 2013, 42, 2986–3017. [Google Scholar] [CrossRef]
- Gong, J.; Li, H.; Zhang, K.; Zhang, Z.; Cao, J.; Shao, Z.; Tang, C.; Fu, S.; Wang, Q.; Wu, X. Zinc-ion storage mechanism of polyaniline for rechargeable aqueous zinc-ion batteries. Nanomaterials 2022, 12, 1438. [Google Scholar] [CrossRef]
- He, P.; Yan, M.; Zhang, G.; Sun, R.; Chen, L.; An, Q.; Mai, L. Layered VS2 nanosheet-based aqueous Zn ion battery cathode. Adv. Energy Mater. 2017, 7, 1601920. [Google Scholar] [CrossRef]
- Pu, X.; Song, T.; Tang, L.; Tao, Y.; Cao, T.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y. Rose-like vanadium disulfide coated by hydrophilic hydroxyvanadium oxide with improved electrochemical performance as cathode material for aqueous zinc-ion batteries. J. Power Source 2019, 437, 226917. [Google Scholar] [CrossRef]
- Chen, T.; Zhu, X.; Chen, X.; Zhang, Q.; Li, Y.; Peng, W.; Zhang, F.; Fan, X. VS2 nanosheets vertically grown on graphene as high-performance cathodes for aqueous zinc-ion batteries. J. Power Source 2020, 477, 228652. [Google Scholar] [CrossRef]
- Liu, J.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. A VS2@N-doped carbon hybrid with strong interfacial interaction for high-performance rechargeable aqueous Zn-ion batteries. J. Mater. Chem. C 2021, 9, 6308–6315. [Google Scholar] [CrossRef]
- Tan, Y.; Li, S.; Zhao, X.; Wang, Y.; Shen, Q.; Qu, X.; Liu, Y.; Jiao, L. Unexpected role of the interlayer “dead Zn2+” in strengthening the nanostructures of VS2 cathodes for high-performance aqueous Zn-ion storage. Adv. Energy Mater. 2022, 12, 2104001. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Z.; Ben, H.; Zhao, M.; Luo, J.; Chen, D.; Lu, Z.; Wang, L.; Liu, C. Boosting the zinc ion storage capacity and cycling stability of interlayer-expanded vanadium disulfide through in-situ electrochemical oxidation strategy. J. Colloid Interface Sci. 2022, 607, 68–75. [Google Scholar] [CrossRef]
- Yu, D.; Wei, Z.; Zhang, X.; Zeng, Y.; Wang, C.; Chen, G.; Shen, Z.X.; Du, F. Boosting Zn2+ and NH4+ storage in aqueous media via in-situ electrochemical induced VS2/VOx heterostructures. Adv. Funct. Mater. 2020, 31, 2008743. [Google Scholar] [CrossRef]
- Zhu, Q.; Xiao, Q.; Zhang, B.; Yan, Z.; Liu, X.; Chen, S.; Ren, Z.; Yu, Y. VS4 with a chain crystal structure used as an intercalation cathode for aqueous Zn-ion batteries. J. Mater. Chem. A 2020, 8, 10761–10766. [Google Scholar] [CrossRef]
- Ding, J.; Gao, H.; Liu, W.; Wang, S.; Wu, S.; Fang, S.; Cheng, F. Operando constructing vanadium tetrasulfide-based heterostructures enabled by extrinsic adsorbed oxygen for enhanced zinc ion storage. J. Mater. Chem. A 2021, 9, 11433–11441. [Google Scholar] [CrossRef]
- Qin, H.; Yang, Z.; Chen, L.; Chen, X.; Wang, L. A high-rate aqueous rechargeable zinc ion battery based on the VS4@rGO nanocomposite. J. Mater. Chem. A 2018, 6, 23757–23765. [Google Scholar] [CrossRef]
- Chen, K.; Li, X.; Zang, J.; Zhang, Z.; Wang, Y.; Lou, Q.; Bai, Y.; Fu, J.; Zhuang, C.; Zhang, Y.; et al. Robust VS4@rGO nanocomposite as a high-capacity and long-life cathode material for aqueous zinc-ion batteries. Nanoscale 2021, 13, 12370–12378. [Google Scholar] [CrossRef]
- Gao, S.; Ju, P.; Liu, Z.; Zhai, L.; Liu, W.; Zhang, X.; Zhou, Y.; Dong, C.; Jiang, F.; Sun, J. Electrochemically induced phase transition in a nanoflower vanadium tetrasulfide cathode for high-performance zinc-ion batteries. J. Energy Chem. 2022, 69, 356–362. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, C.; Zhang, M.; Sun, Q.; Li, S.; Banis, M.N.; Han, X.; Dong, Q.; Yang, J.; Wang, G.; et al. Enhanced sodium storage capability enabled by super wide-interlayer-spacing MoS2 integrated on carbon fibers. Nano Energy 2017, 41, 66–74. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, D.; Liu, H.; Umar, A.; Wu, X. High performance hybrid supercapacitor based on hierarchical MoS2/Ni3S2 metal chalcogenide. Chin. Chem. Lett. 2019, 30, 1105–1110. [Google Scholar] [CrossRef]
- Liang, Y.; Feng, R.; Yang, S.; Ma, H.; Liang, J.; Chen, J. Rechargeable Mg batteries with graphene-like MoS2 cathode and ultrasmall Mg nanoparticle anode. Adv. Mater. 2011, 23, 640–643. [Google Scholar] [CrossRef]
- Li, H.; Yang, Q.; Mo, F.; Liang, G.; Liu, Z.; Tang, Z.; Ma, L.; Liu, J.; Shi, Z.; Zhi, C. MoS2 nanosheets with expanded interlayer spacing for rechargeable aqueous Zn-ion batteries. Energy Storage Mater. 2019, 19, 94–101. [Google Scholar] [CrossRef]
- Jiao, Y.; Mukhopadhyay, A.; Ma, Y.; Yang, L.; Hafez, A.M.; Zhu, H. Ion transport nanotube assembled with vertically aligned metallic MoS2 for high rate lithium-ion batteries. Adv. Energy Mater. 2018, 8, 1702779. [Google Scholar] [CrossRef]
- Lei, Z.; Zhan, J.; Tang, L.; Zhang, Y.; Wang, Y. Recent development of metallic (1T) phase of molybdenum disulfide for energy conversion and storage. Adv. Energy Mater. 2018, 8, 1703482. [Google Scholar] [CrossRef]
- Huang, M.; Mai, Y.; Fan, G.; Liang, X.; Fang, Z.; Jie, X. Toward fast zinc-ion storage of MoS2 by tunable pseudocapacitance. J. Alloys Compd. 2021, 871, 159541. [Google Scholar] [CrossRef]
- Sheng, Z.; Qi, P.; Lu, Y.; Liu, G.; Chen, M.; Gan, X.; Qin, Y.; Hao, K.; Tang, Y. Nitrogen-doped metallic MoS2 derived from a metal-organic framework for aqueous rechargeable zinc-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 34495–34506. [Google Scholar] [CrossRef]
- Liu, J.; Xu, P.; Liang, J.; Liu, H.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Boosting aqueous zinc-ion storage in MoS2 via controllable phase. Chem. Eng. J. 2020, 389, 124405. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, W.; Ren, X.; Yin, Y.; Zhao, Y.; Ren, Z.; Sun, Y.; Lei, Q.; Wang, J.; Wang, L.; et al. A volume self-regulation MoS2 superstructure cathode for stable and high mass-loaded Zn-ion storage. ACS Nano 2022, 16, 12095–12106. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, X. Strategies for constructing manganese-based oxide electrode materials for aqueous rechargeable zinc-ion batteries. Chin. Chem. Lett. 2022, 33, 1236–1244. [Google Scholar] [CrossRef]
- Minakshi, M.; Singh, P.; Issa, T.B.; Thurgate, S.; Marco, R.D. Lithium insertion into manganese dioxide electrode in MnO2/Zn aqueous battery. J. Power Source 2004, 138, 319–322. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry With H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Zhou, W.; Ye, C.; Zhang, Q.; Chen, Y.; Gu, L.; Davey, K.; Qiao, S.Z. An electrolytic Zn-MnO2 battery for high-voltage and scalable energy storage. Angew. Chem. Int. Ed. 2019, 58, 7823–7828. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, W.; Xu, Y.; Zeng, Z.; Tian, H.; Velayutham, M.; Shi, W.; Li, W.; Wang, C.; Reed, D.; et al. Charging activation and desulfurization of MnS unlock the active sites and electrochemical reactivity for Zn-ion batteries. Nano Energy 2020, 75, 104869. [Google Scholar] [CrossRef]
- Ma, S.C.; Sun, M.; Sun, B.Y.; Li, D.; Liu, W.L.; Ren, M.M.; Kong, F.G.; Wang, S.J.; Guo, Z.X. In situ preparation of manganese sulfide on reduced graphene oxide sheets as cathode for rechargeable aqueous zinc-ion battery. J. Solid State Chem. 2021, 299, 122166. [Google Scholar] [CrossRef]
- Jiang, K.; Zhou, Z.; Wen, X.; Weng, Q. Fabrications of high-performance planar zinc-ion microbatteries by engraved soft templates. Small 2021, 17, 2007389. [Google Scholar] [CrossRef]
- Lv, R.; Robinson, J.A.; Schaak, R.E.; Sun, D.; Sun, Y.; Mallouk, T.E.; Terrones, M. Transition metal dichalcogenides and beyond: Synthesis, properties, and applications of single- and few-layer nanosheets. Acc. Chem. Res. 2015, 48, 56–64. [Google Scholar] [CrossRef]
- Xu, K.; Chen, P.; Li, X.; Wu, C.; Guo, Y.; Zhao, J.; Wu, X.; Xie, Y. Ultrathin nanosheets of vanadium diselenide: A metallic two-dimensional material with ferromagnetic charge-density-wave behavior. Angew. Chem. Int. Ed. 2013, 52, 10477. [Google Scholar] [CrossRef]
- Yang, C.; Feng, J.; Lv, F.; Zhou, J.; Lin, C.; Wang, K.; Zhang, Y.; Yang, Y.; Wang, W.; Li, J.; et al. Metallic graphene-like VSe2 ultrathin nanosheets: Superior potassium-ion storage and their working mechanism. Adv. Mater. 2018, 30, 1800036. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, H.; Zhang, L.; Li, Q.; Xu, M.; Bao, S.J. A rough endoplasmic reticulum-like VSe2/rGO anode for superior sodium-ion capacitors. Inorg. Chem. Front. 2019, 6, 2935–2943. [Google Scholar] [CrossRef]
- Ming, F.; Liang, H.; Lei, Y.; Zhang, W.; Alshareef, H.N. Solution synthesis of VSe2 nanosheets and their alkali metal ion storage performance. Nano Energy 2018, 53, 11–16. [Google Scholar] [CrossRef]
- Wu, Z.; Lu, C.; Wang, Y.; Zhang, L.; Jiang, L.; Tian, W.; Cai, C.; Gu, Q.; Sun, Z.; Hu, L. Ultrathin VSe2 nanosheets with fast ion diffusion and robust structural stability for rechargeable zinc-ion battery cathode. Small 2020, 16, 2000698. [Google Scholar] [CrossRef]
- Cai, S.; Wu, Y.; Chen, H.; Ma, Y.; Fan, T.; Xu, M.; Bao, S.J. Why does the capacity of vanadium selenide based aqueous zinc ion batteries continue to increase during long cycles? J. Colloid Interface Sci. 2022, 615, 30–37. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, G.; Wan, H.; Chen, F.; Liu, X.; Ma, R. 2D free-standing nitrogen-doped Ni-Ni3S2 @carbon nanoplates derived from metal-organic frameworks for enhanced oxygen evolution reaction. Small 2019, 15, 1900348. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, P.; Liu, H.; Song, J.; Umar, A.; Wu, X. Toward a high performance asymmetric hybrid capacitor by electrode optimization. Inorg. Chem. Front. 2019, 6, 2824–2831. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, L.; Xie, C.; Wang, D.; Zou, Y.; Chen, R.; Wang, Y.; Jia, C.; Wang, S. Defect engineering on electrode materials for rechargeable batteries. Adv. Mater. 2020, 32, 1905923. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, H.; Qin, L.; Cao, X.; Zhou, J.; Pan, A.; Fang, G.; Liang, S. Interlayer doping in layered vanadium oxides for low-cost energy storage: Sodium-ion batteries and aqueous zinc-ion batteries. ChemNanoMat 2020, 6, 1553–1566. [Google Scholar] [CrossRef]
- Tong, X.; Li, Y.; Pang, N.; Zhou, Y.; Wu, D.; Xiong, D.; Xu, S.; Wang, L.; Chu, P.K. Highly active cobalt-doped nickel sulfide porous nanocones for high-performance quasi-solid-state zinc-ion batteries. J. Energy Chem. 2022, 66, 237–249. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, T.; Chen, Y.; Wang, Q. The Ni/Ni3S2 nanocomposite derived from Ni-ZIF with superior energy storage performance as cathodes for asymmetric supercapacitor and rechargeable aqueous zinc ion battery. J. Alloys Compd. 2022, 891, 161935. [Google Scholar] [CrossRef]



| Cathodes | Anodes | Voltage (V) | Electrolyte | Capacity (mAh g−1) | Cycle Stability (mAh g−1, Cycles, A g−1) | Ref. |
|---|---|---|---|---|---|---|
| VS2@VOOH | Zinc foil | 0.4–1.0 | 3 M ZnSO4 | 165 (0.1 A g−1) | 91.4, 400, 2.5 | [31] |
| VS2 and N-doped carbon | Zinc foil | 0.2–1.8 | 3 M Zn(CF3SO3)2 | 203 (0.05 A g−1) | 144, 600, 1 | [33] |
| VS2/VOx | Zinc foil | 0.1–1.8 | 25 M ZnCl2 | 260 (0.1 A g−1) | 75%, 3000, 1 | [36] |
| VS4 | Zinc foil | 0.2–1.6 | 1 M ZnSO4 | 310 (0.1 A g−1) | 110, 500, 2.5 | [37] |
| VS4/V2O3 | Zinc foil | 0.3–1.2 | 3 M Zn(CF3SO3)2 | 163 (0.1 A g−1) | - | [38] |
| VS4@rGO | Zinc foil | 0–1.8 | 1 M Zn(CF3SO3)2 | 450 (0.5 A g−1) | 82%, 3500, 10 | [40] |
| MoS2 | Deposited zinc on carbon cloth | 0.5–1.5 | 2 M ZnSO4 | 202.6 (0.1 A g−1) | 164.5, 600, 1 | [45] |
| MoS2-CTAB | Zn anode plated on carbon paper | 0.2–1.3 | 3 M ZnSO4 | 181.8 (0.1 A g−1) | 92.8%, 2100, 10 | [51] |
| MnS | zinc powder | 0.9–1.95 | 2 M ZnSO4 and 0.1 M MnSO4 | 297 (0.1 A g−1) | 150, 1000, 1 | [58] |
| VSe2 | Zinc foil | 0.2–1.6 | 2 M ZnSO4 | 131.8 (0.1 A g−1) | 80.8%, 500, 0.1 | [64] |
| Ni/Ni3S2 | Zinc foil | 1.3–1.9 | 6 M KOH and 0.6 M ZnO | 220 (0.2 A g−1) | 93.1%, 1000, 4 | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wu, X. Recent Advances of Transition Metal Chalcogenides as Cathode Materials for Aqueous Zinc-Ion Batteries. Nanomaterials 2022, 12, 3298. https://doi.org/10.3390/nano12193298
Liu Y, Wu X. Recent Advances of Transition Metal Chalcogenides as Cathode Materials for Aqueous Zinc-Ion Batteries. Nanomaterials. 2022; 12(19):3298. https://doi.org/10.3390/nano12193298
Chicago/Turabian StyleLiu, Ying, and Xiang Wu. 2022. "Recent Advances of Transition Metal Chalcogenides as Cathode Materials for Aqueous Zinc-Ion Batteries" Nanomaterials 12, no. 19: 3298. https://doi.org/10.3390/nano12193298





