Biocompatible and Antimicrobial Cellulose Acetate-Collagen Films Containing MWCNTs Decorated with TiO2 Nanoparticles for Potential Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. MWCNT_TiO2 Synthesis
2.2. Biocomposite Materials Synthesis
2.3. Structural and Morphological Analyses
2.3.1. Fourier Transformed Infrared Spectroscopy (ATR–FTIR)
2.3.2. Raman Spectroscopy Analysis
2.3.3. X-ray Diffraction Analysis (XRD)
2.3.4. Transmission Electron Microscopy (TEM)
2.3.5. Scanning Electron Microscopy (SEM)
2.4. Absorption Kinetics
2.5. Hardness Measurements
2.6. Antimicrobial Analysis
2.6.1. Microbial Strains and Growth Conditions
2.6.2. Qualitative Antimicrobial Assay—Growth Inhibition
2.6.3. Monospecific Biofilm Development
2.6.4. Evaluation of the Planktonic Development of Microorganisms
2.7. Statistical Analysis
2.8. Cellular Viability Assays
2.8.1. MTT Assay
2.8.2. Fluorescence Microscopy
3. Results and Discussions
3.1. Characterisation of MWCNT_TiO2
3.1.1. FTIR Spectroscopy
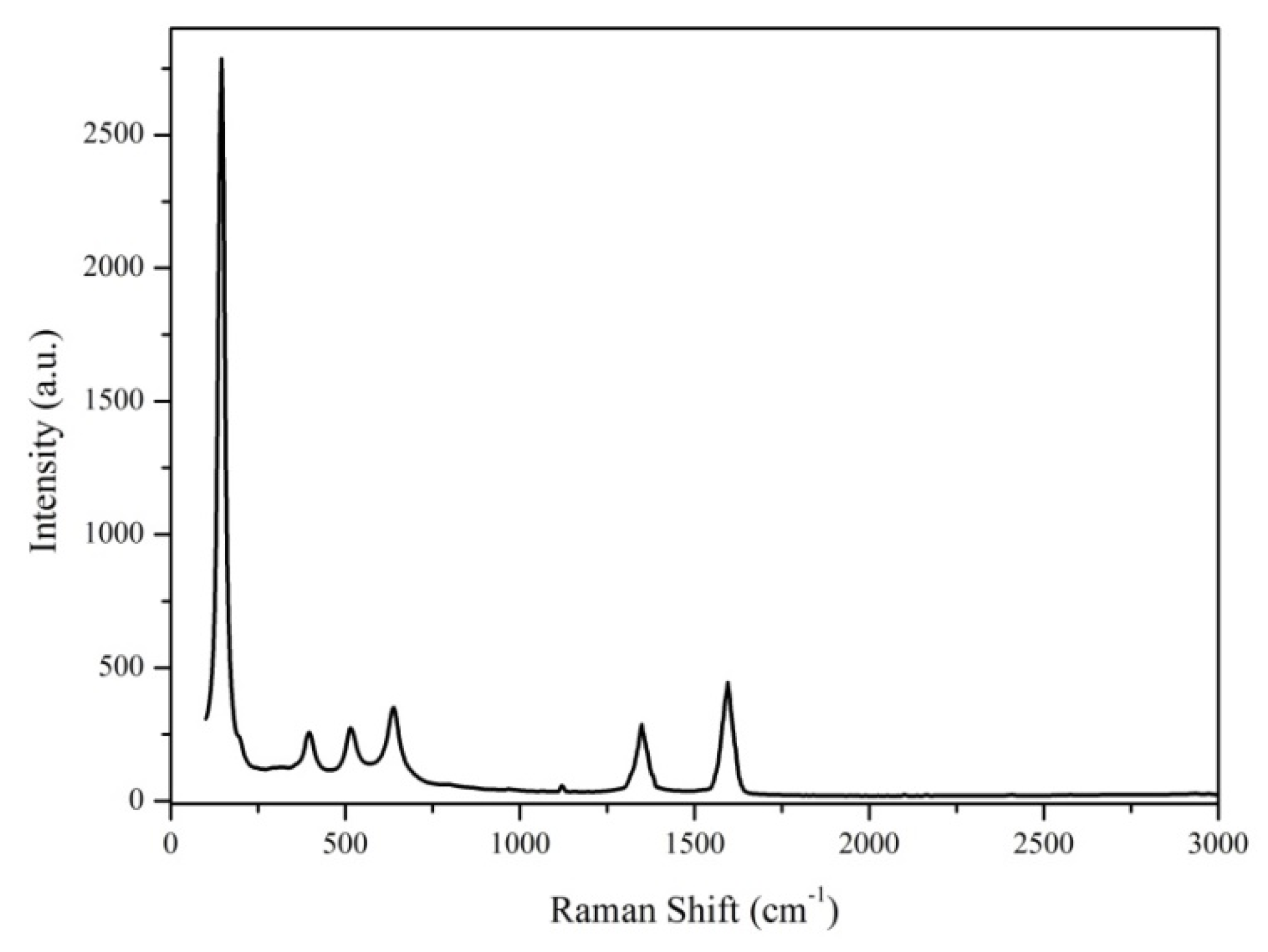
3.1.2. Raman Analysis
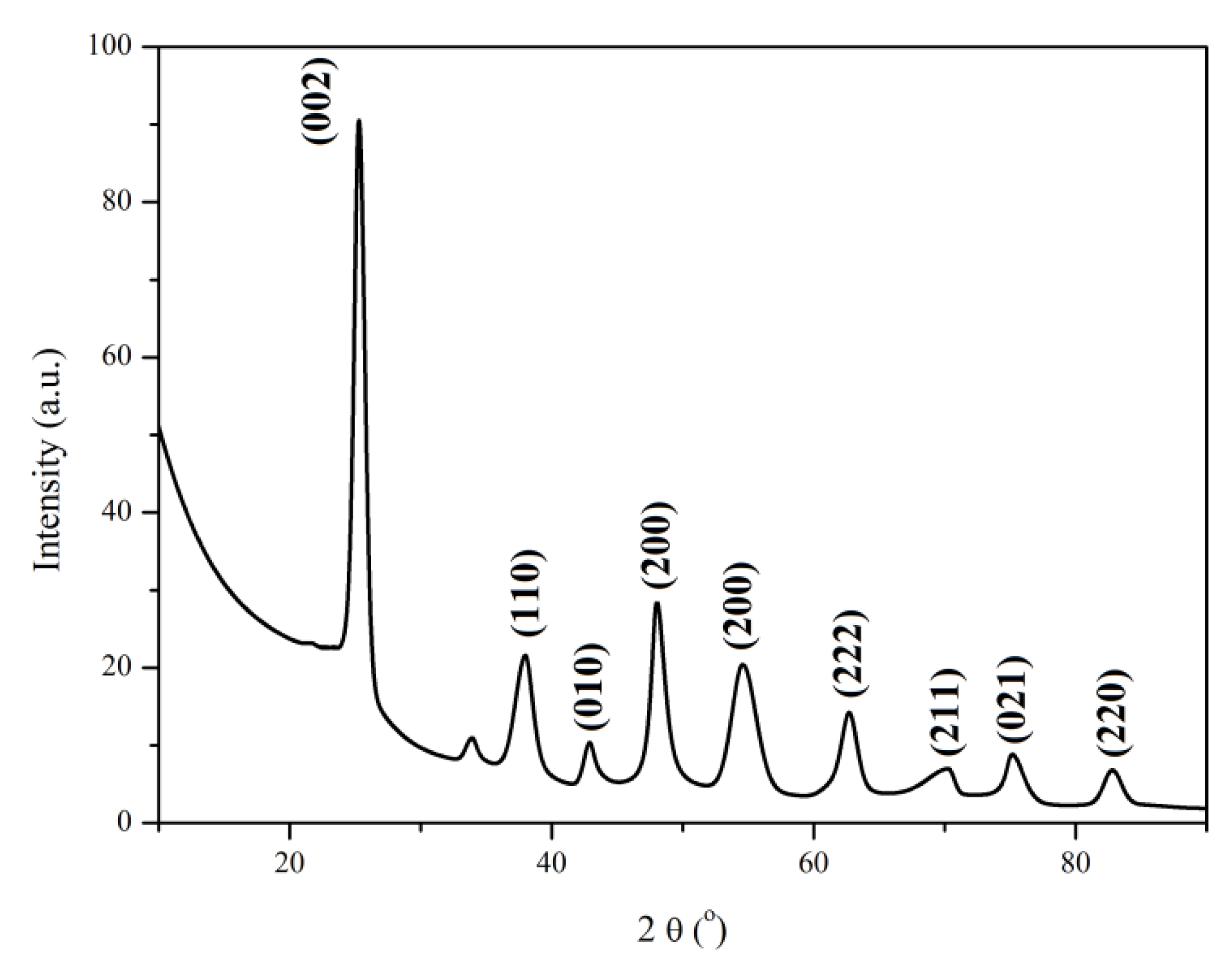
3.1.3. XRD Analysis
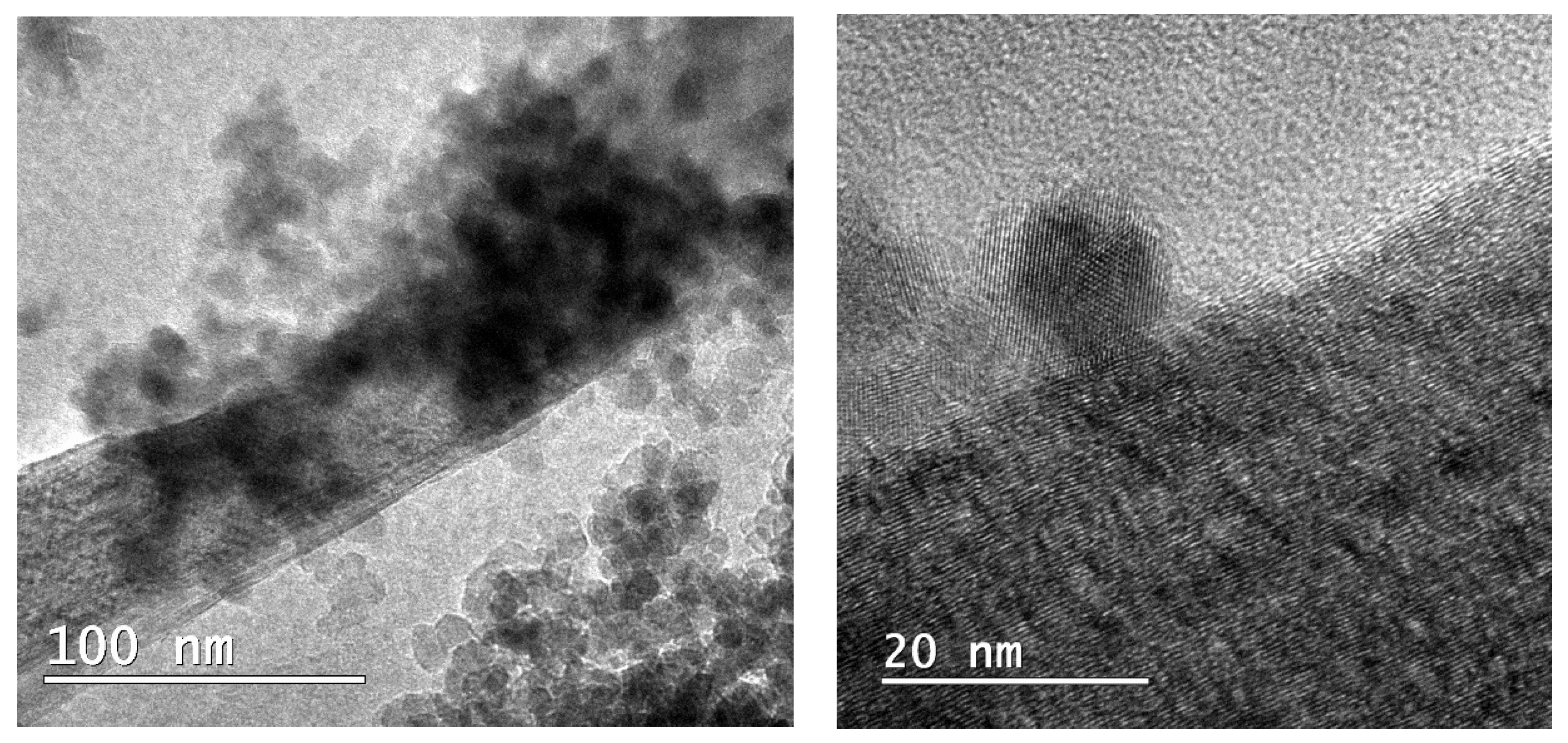
3.1.4. TEM Analysis
3.2. Characterisation and Investigation of MWCNT_TiO2@CC Films
3.2.1. FTIR Spectroscopy
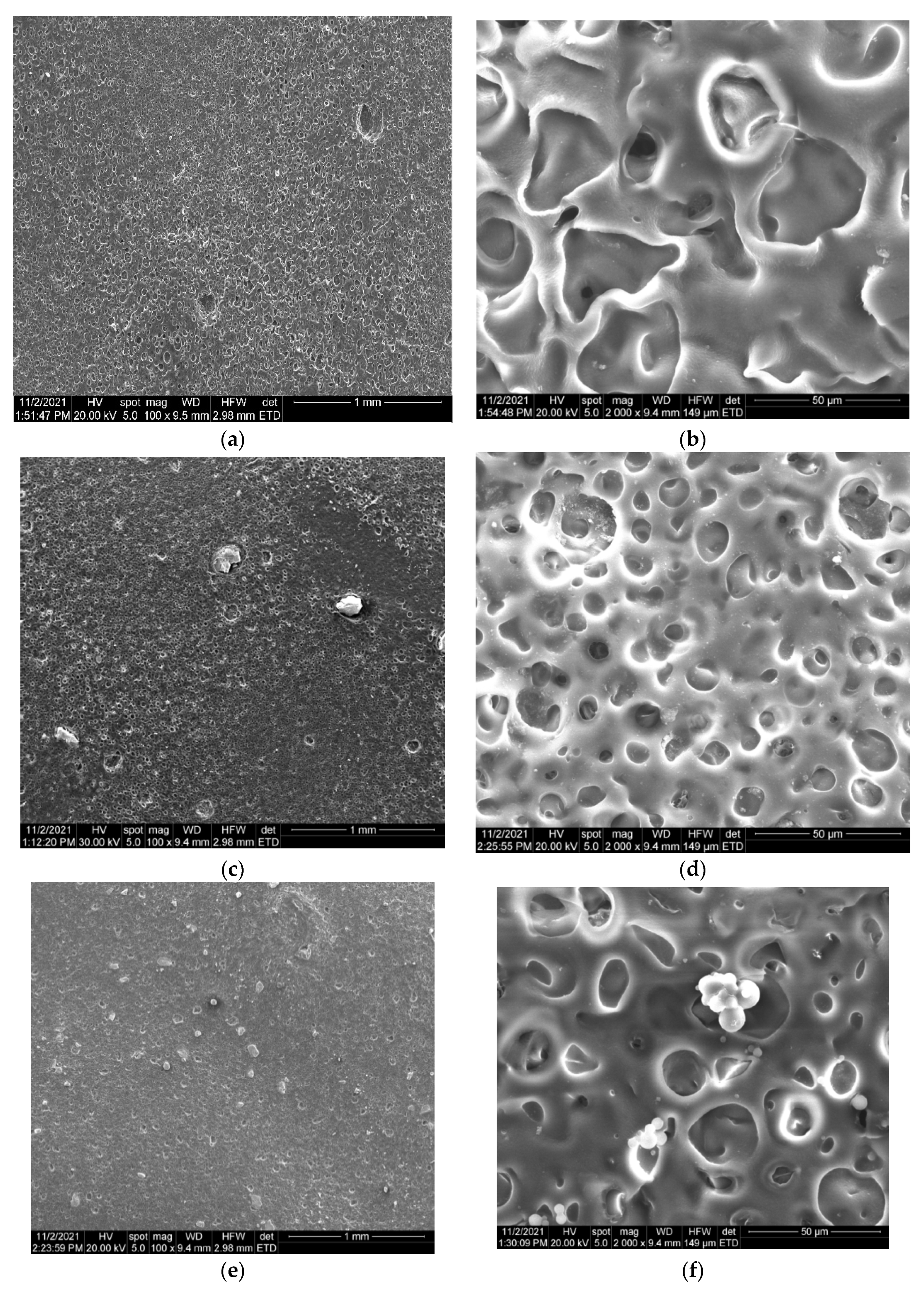
3.2.2. SEM Analysis
3.2.3. Absorption Kinetics
3.2.4. Hardness
3.2.5. Antimicrobial Analysis
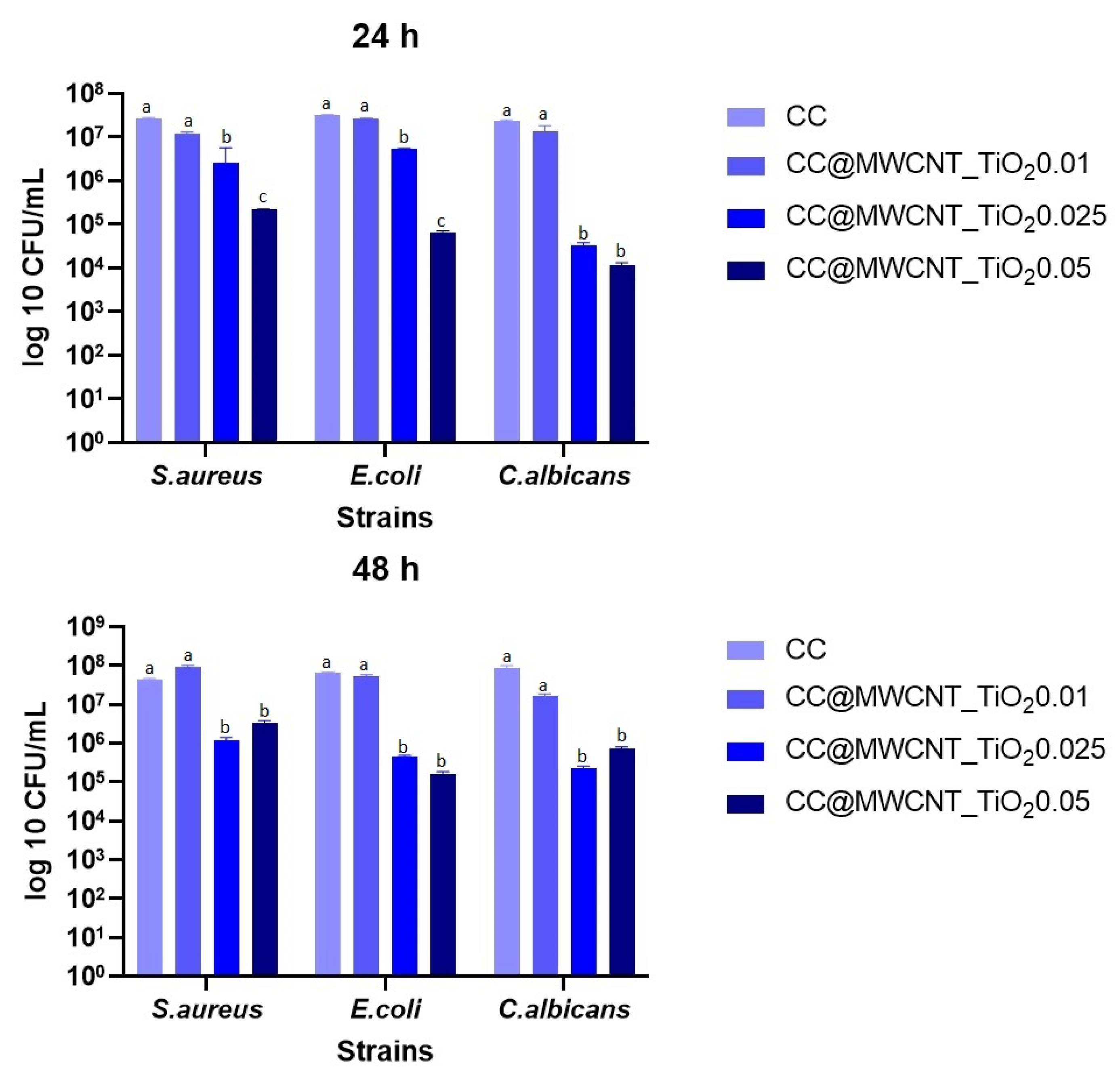
Qualitative Antimicrobial Assay—Growth Inhibition
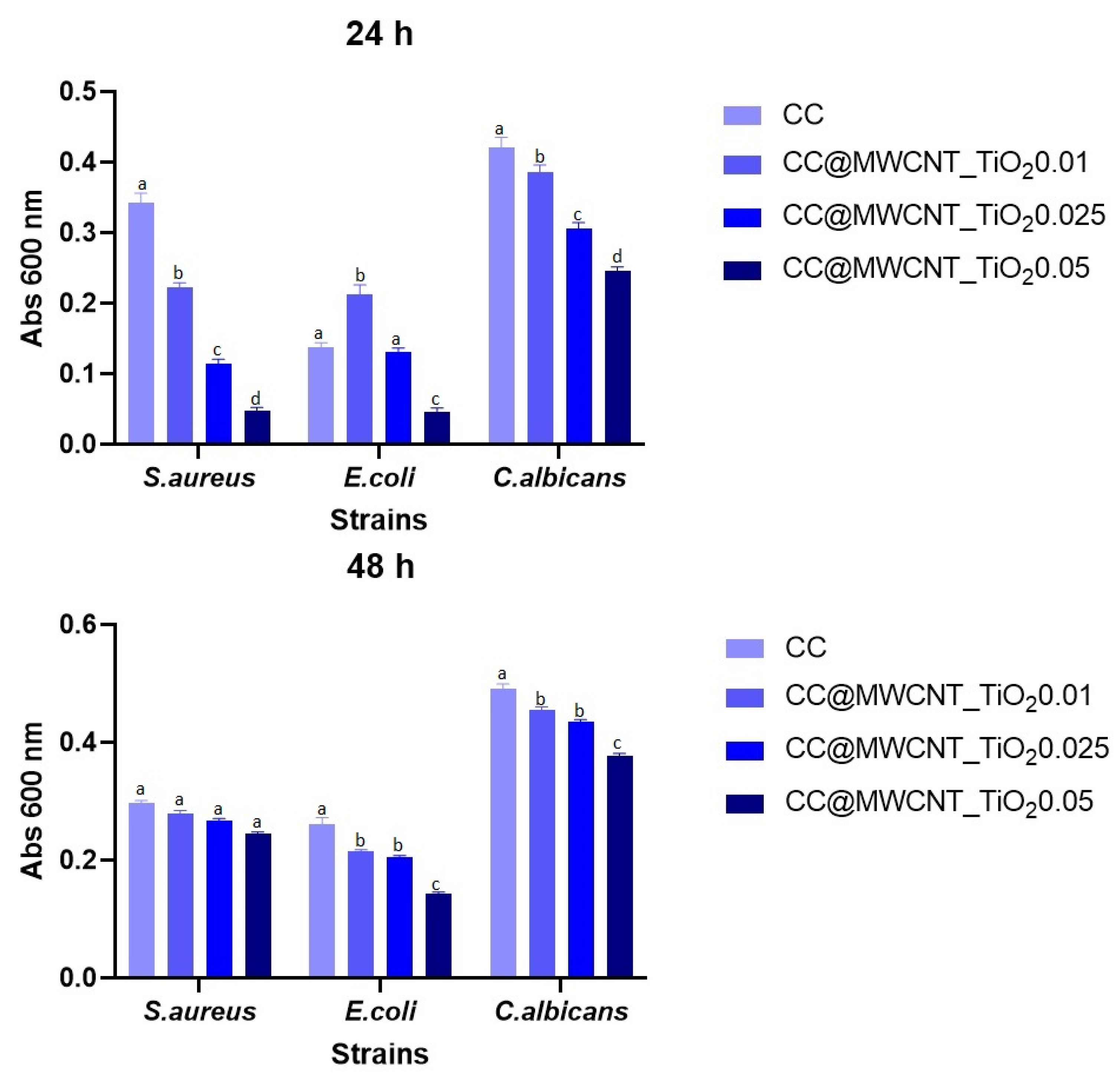
Monospecific Biofilm Development
Evaluation of the Planktonic Development of Microorganisms
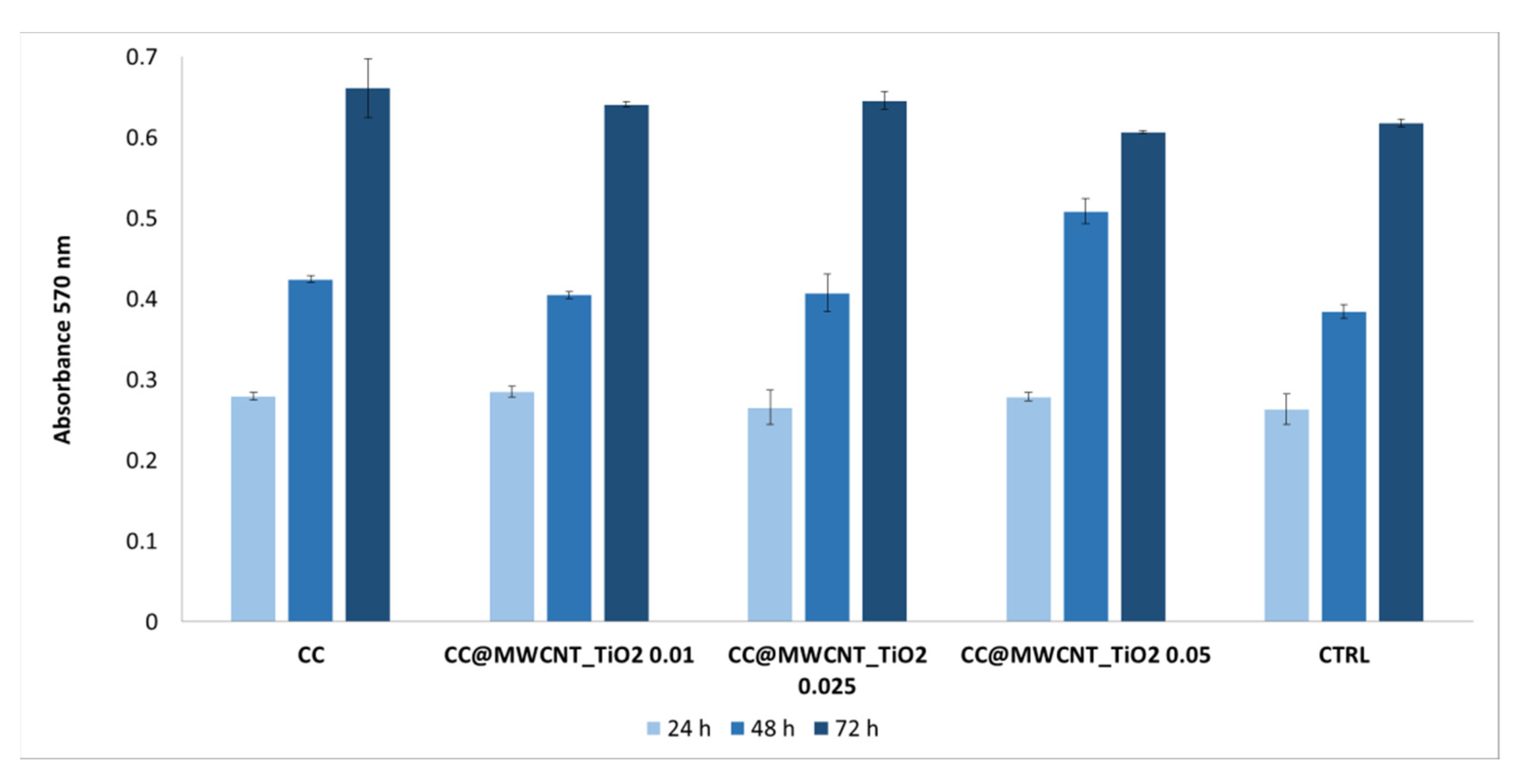
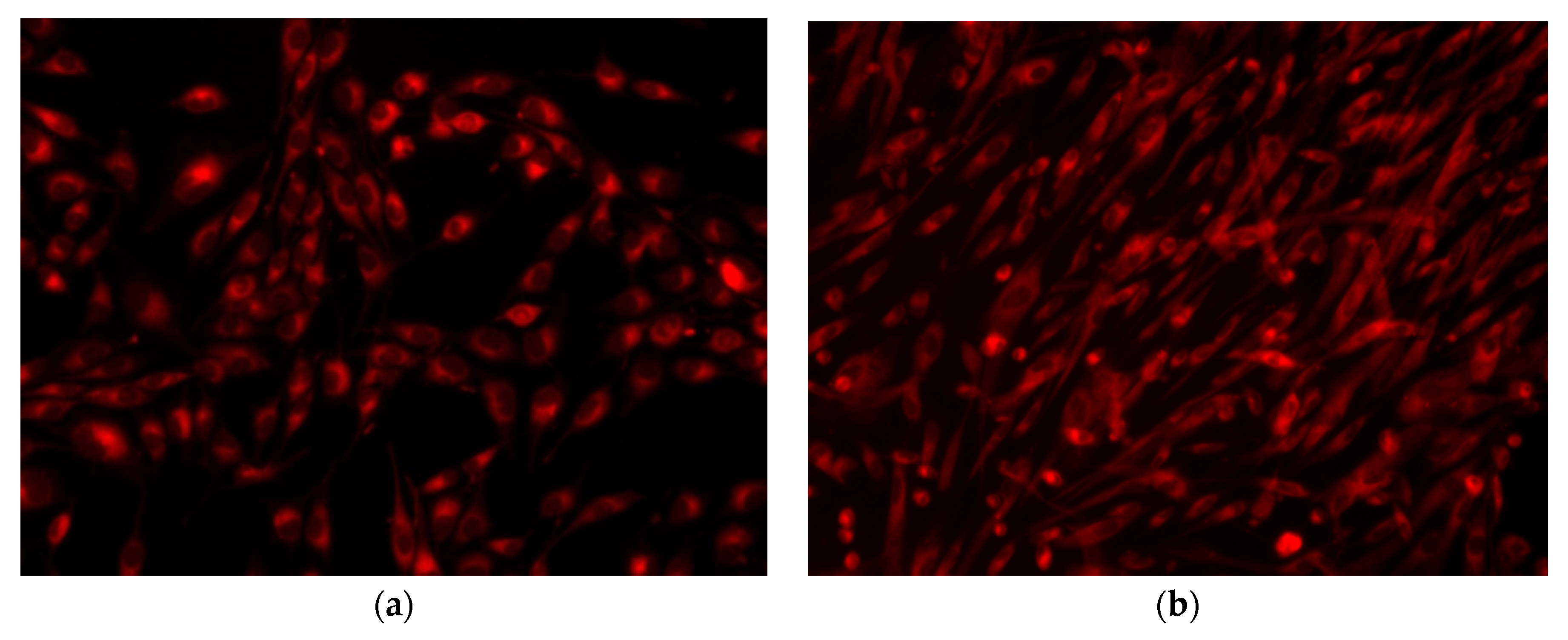
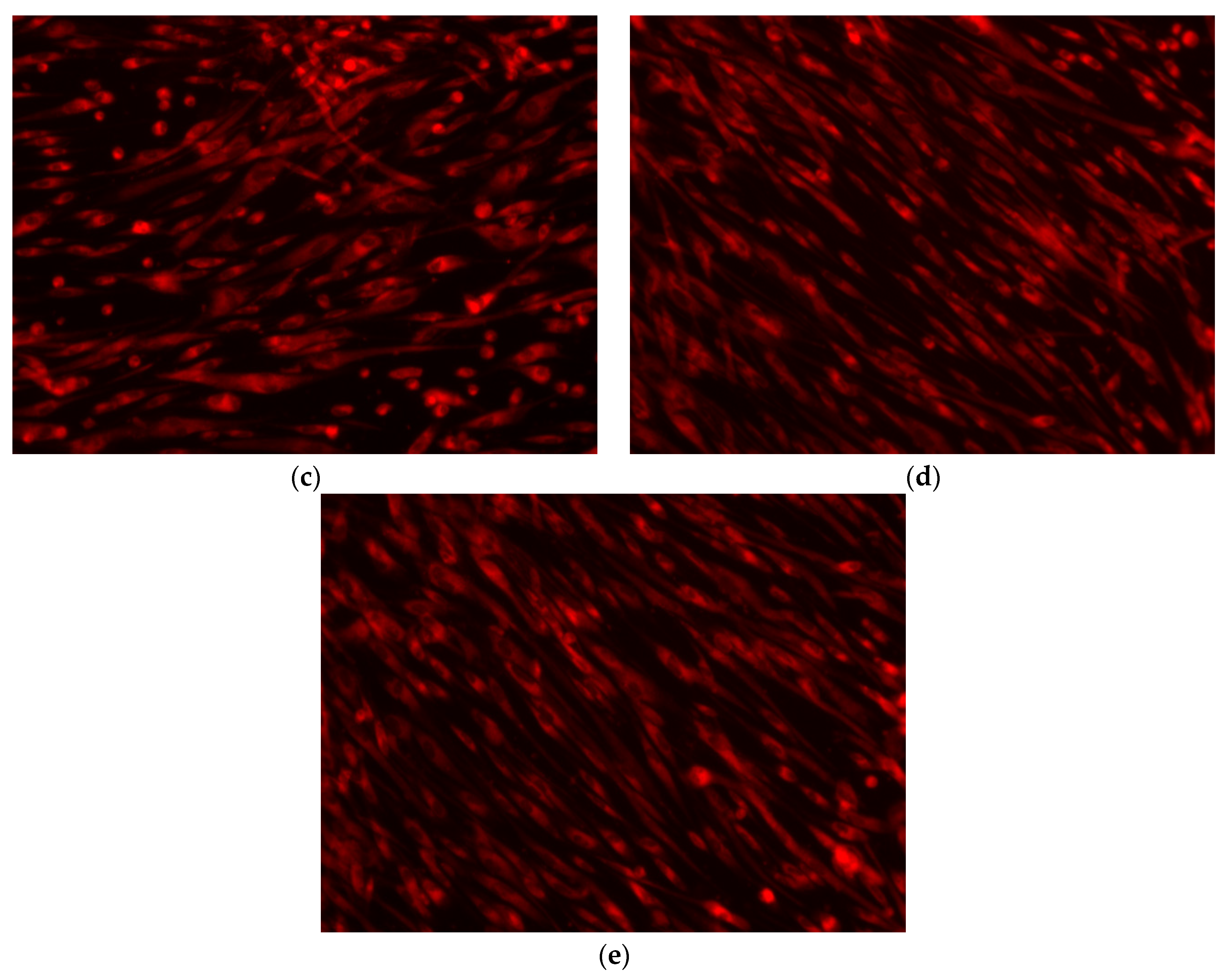
3.2.6. Cellular Viability/Proliferation Assay (MTT Assay) and Fluorescence Microscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coates, A.; Hu, Y.; Bax, R.; Page, C. The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug Discov. 2002, 1, 895–910. [Google Scholar] [CrossRef]
- Grigore, M.E.; Holban, A.M.; Grumezescu, A.M. Nanotherapeutics in the management of infections and cancer. In Nanobiomaterials Science, Development and Evaluation; Elsevier: Amsterdam, The Netherlands, 2017; pp. 163–189. [Google Scholar]
- Amábile-Cuevas, C.F. Global Perspectives of Antibiotic Resistance. In Antimicrobial Resistance in Developing Countries; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–13. [Google Scholar]
- Leseva, M.; Arguirova, M.; Nashev, D.; Zamfirova, E.; Hadzhyiski, O. Nosocomial infections in burn patients: Etiology, antimicrobial resistance, means to control. Ann. Burns Fire Disasters 2013, 26, 5. [Google Scholar] [PubMed]
- Corcione, S.; Lupia, T.; De Rosa, F.G. Host and Microbiota Interaction Study Group. M.I.S. Microbiome in the setting of burn patients: Implications for infections and clinical outcomes. Burns Trauma 2020, 8, 1664–3224. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.V. Nosocomial infections due to Pseudomonas. J. Infect. Dis. 1974, 130, S4–S7. [Google Scholar] [CrossRef]
- Al-Dahmoshi, H.; Al-Obaidi, R.D.; Al-Khafaji, N. Pseudomonas aeruginosa: Diseases, Biofilm and Antibiotic Resistance. In Pseudomonas Aeruginosa-Biofilm Formation, Infections and Treatments; Intech Open: London, UK, 2020. [Google Scholar]
- Neacsu, I.A.; Leau, S.-A.; Marin, S.; Holban, A.M.; Vasile, B.-S.; Nicoara, A.-I.; Ene, V.L.; Bleotu, C.; Albu Kaya, M.G.; Ficai, A. Collagen-Carboxymethylcellulose Biocomposite Wound-Dressings with Antimicrobial Activity. Materials 2021, 14, 1153. [Google Scholar] [CrossRef] [PubMed]
- Weller, C.D.; Team, V.; Sussman, G. First-line interactive wound dressing update: A comprehensive review of the evidence. Front. Pharmacol. 2020, 11, 155. [Google Scholar] [CrossRef] [Green Version]
- Abazari, M.F.; Gholizadeh, S.; Karizi, S.Z.; Birgani, N.H.; Abazari, D.; Paknia, S.; Derakhshankhah, H.; Allahyari, Z.; Amini, S.M.; Hamidi, M. Recent Advances in Cellulose-Based Structures as the Wound-Healing Biomaterials: A Clinically Oriented Review. Appl. Sci. 2021, 11, 7769. [Google Scholar] [CrossRef]
- Grigore, M.E.; Grumezescu, A.M.; Holban, A.M.; Mogoşanu, G.D.; Andronescu, E. Collagen-nanoparticles composites for wound healing and infection control. Metals 2017, 7, 516. [Google Scholar] [CrossRef] [Green Version]
- Nurwito, B.S.; Maulida, H.N. Electrospun fibers as a wound dressing material using combination of cellulose acetate/collagen seeding stem cell. Asian J. Microbiol. Biotechnol. Environ. Sci. 2018, 20, s43–s47. [Google Scholar]
- Ramanathan, G.; Seleenmary Sobhanadhas, L.S.; Sekar Jeyakumar, G.F.; Devi, V.; Sivagnanam, U.T.; Fardim, P. Fabrication of biohybrid cellulose acetate-collagen bilayer matrices as nanofibrous spongy dressing material for wound-healing application. Biomacromolecules 2020, 21, 2512–2524. [Google Scholar] [CrossRef]
- Samadian, H.; Salehi, M.; Farzamfar, S.; Vaez, A.; Ehterami, A.; Sahrapeyma, H.; Goodarzi, A.; Ghorbani, S. In vitro and in vivo evaluation of electrospun cellulose acetate/gelatin/hydroxyapatite nanocomposite mats for wound dressing applications. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 964–974. [Google Scholar] [CrossRef] [Green Version]
- Naomi, R.; Fauzi, M.B. Cellulose/collagen dressings for diabetic foot ulcer: A review. Pharmaceutics 2020, 12, 881. [Google Scholar] [CrossRef] [PubMed]
- ur Rehman, S.R.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Tariq, M.; Hasan, A. Reduced graphene oxide incorporated GelMA hydrogel promotes angiogenesis for wound healing applications. Int. J. Nanomed. 2019, 14, 9603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barroso, A.; Mestre, H.; Ascenso, A.; Simões, S.; Reis, C. Nanomaterials in wound healing: From material sciences to wound healing applications. Nano Select 2020, 1, 443–460. [Google Scholar] [CrossRef]
- David, M.E.; Ion, R.-M.; Grigorescu, R.M.; Iancu, L.; Holban, A.M.; Nicoara, A.I.; Alexandrescu, E.; Somoghi, R.; Ganciarov, M.; Vasilievici, G. Hybrid Materials Based on Multi-Walled Carbon Nanotubes and Nanoparticles with Antimicrobial Properties. Nanomaterials 2021, 11, 1415. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Khouy, R.A.; Nosrati, A.; Khodaei, M.; Banitalebi-Dehkordi, M.; Ashrafi-Dehkordi, K.; Sanami, S.; Alizadeh, Z. Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis. J. Nanobiotechnol. 2021, 19, 1–21. [Google Scholar] [CrossRef]
- Ravanbakhsh, H.; Bao, G.; Mongeau, L. Carbon nanotubes promote cell migration in hydrogels. Sci. Rep. 2020, 10, 1–10. [Google Scholar]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 2020, 102250. [Google Scholar] [CrossRef]
- Laganà, P.; Visalli, G.; Facciolà, A.; Ciarello, M.P.; Laganà, A.; Iannazzo, D.; Di Pietro, A. Is the Antibacterial Activity of Multi-Walled Carbon Nanotubes (MWCNTs) Related to Antibiotic Resistance? An Assessment in Clinical Isolates. Int. J. Environ. Res. 2021, 18, 9310. [Google Scholar] [CrossRef] [PubMed]
- Vagos, M.R.; Moreira, J.M.; Soares, O.S.; Pereira, M.F.; Mergulhão, F.J. Incorporation of carbon nanotubes in polydimethylsiloxane to control Escherichia coli adhesion. Polym. Compos. 2019, 40 (Suppl. 2), E1697–E1704. [Google Scholar] [CrossRef]
- Vagos, M.R.; Gomes, M.; Moreira, J.M.; Soares, O.S.; Pereira, M.F.; Mergulhão, F.J. Carbon nanotube/poly (Dimethylsiloxane) composite materials to reduce bacterial adhesion. Antibiotics 2020, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.J.; Berry, C.J.; Brigmon, R.L. Structural and biological properties of carbon nanotube composite films. Mater. Sci. Eng. B 2005, 123, 123–129. [Google Scholar] [CrossRef]
- Kittana, N.; Assali, M.; Abu-Rass, H.; Lutz, S.; Hindawi, R.; Ghannam, L.; Zakarneh, M.; Mousa, A. Enhancement of wound healing by single-wall/multi-wall carbon nanotubes complexed with chitosan. Int. J. Nanomed. 2018, 13, 7195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pormohammad, A.; Monych, N.K.; Ghosh, S.; Turner, D.L.; Turner, R.J. Nanomaterials in Wound Healing and Infection Control. Antibiotics 2021, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.A.; Voge, C.M.; Kariolis, M.; Stegemann, J.P. Carbon nanotubes increase the electrical conductivity of fibroblast-seeded collagen hydrogels. Acta Biomater. 2008, 4, 1583–1592. [Google Scholar] [CrossRef]
- Murugesan, B.; Pandiyan, N.; Kasinathan, K.; Rajaiah, A.; Arumuga, M.; Subramanian, P.; Sonamuthue, J.; Samayananf, S.; Ravi, V.; Marimuthub, A.; et al. Fabrication of heteroatom doped NFP-MWCNT and NFB-MWCNT nanocomposite from imidazolium ionic liquid functionalized MWCNT for antibiofilm and wound healing in Wistar rats: Synthesis, characterization, in-vitro and in-vivo studies. Mater. Sci. Eng. C 2020, 111, 110791. [Google Scholar] [CrossRef]
- Das, B.; Chattopadhyay, P.; Upadhyay, A.; Gupta, K.; Mandal, M.; Karak, N. Biophysico-chemical interfacial attributes of Fe 3 O 4 decorated MWCNT nanohybrid/bio-based hyperbranched polyurethane nanocomposite: An antibacterial wound healing material with controlled drug release potential. New J. Chem. 2014, 38, 4300–4311. [Google Scholar] [CrossRef]
- David, M.E.; Grigorescu, R.M.; Iancu, L.; Andrei, E.R.; Somoghi, R.; Frone, A.N.; Atkinson, I.; Predoana, L.; Nicoara, A.-I.; Ion, R.-M. Synthesis and characterization of multi-walled carbon nanotubes decorated with hydroxyapatite. Fuller. Nanotub. 2021, 29, 423–430. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Naddafan, M.; Shaker Alattar, A.; Dehghani, Z.; Malekfar, R. Detecting compressive strain by evaluation of Raman spectroscopy of the multiwall Carbon nanotubes/TiO2 nanocomposites. Int. J. Nano Dimens. 2020, 11, 168–176. [Google Scholar]
- Xie, Y.; Qian, H.; Zhong, Y.; Guo, H.; Hu, Y. Facile low-temperature synthesis of carbon nanotube/nanohybrids with enhanced visible-light-driven photocatalytic activity. Int. J. Photoenergy 2012, 2012, 682138. [Google Scholar] [CrossRef] [Green Version]
- Wongaree, M.; Chiarakorn, S.; Chuangchote, S. Photocatalytic improvement under visible light in nanoparticles by carbon nanotube incorporation. J. Nanomater. 2015, 2015, 689306. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.F.; Zaid, M.H.M.; Liza, S.; Matori, K.A.; Mamat, M.S.; Hazan, M.A.; Yaakob, Y. Effect of CNT on microstructural properties of Zn2SiO4/CNT composite via dry powder processing. Mater. Res. Express 2020, 7, 105601. [Google Scholar] [CrossRef]
- Gopi, S.; Pius, A.; Kargl, R.; Kleinschek, K.S.; Thomas, S. Fabrication of cellulose acetate/chitosan blend films as efficient adsorbent for anionic water pollutants. Polym. Bull. 2019, 76, 1557–1571. [Google Scholar] [CrossRef]
- Atieh, M.A.; Bakather, O.Y.; Al-Tawbini, B.; Bukhari, A.A.; Abuilaiwi, F.A.; Fettouhi, M.B. Effect of carboxylic functional group functionalized on carbon nanotubes surface on the removal of lead from water. Bioinorg. Chem. Appl. 2010, 2010, 603978. [Google Scholar] [CrossRef]
- Kochkina, N.E.; Butikova, O.A. Effect of fibrous TiO2 filler on the structural, mechanical, barrier and optical characteristics of biodegradable maize starch/PVA composite films. Int. J. Biol. 2019, 139, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Arakha, M.; Saleem, M.; Mallick, B.C.; Jha, S. The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khashan, K.S.; Sulaiman, G.M.; Abdulameer, F.A.; Albukhaty, S.; Ibrahem, M.A.; Al-Muhimeed, T.; AlObaid, A.A. Antibacterial Activity of TiO2 Nanoparticles Prepared by One-Step Laser Ablation in Liquid. Appl. Sci. 2021, 11, 4623. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Guo, X.; Li, W.; Chen, J.; Liu, Q.; Xu, Q.; Wang, Q.; Yang, H.; Shui, Y. Effects of different TiO2 nanoparticles concentrations on the physical and antibacterial activities of chitosan-based coating film. Nanomaterials 2020, 10, 1365. [Google Scholar] [CrossRef]
- Zare-Zardini, H.; Shanbedi, M.; Soltaninejad, H.; Mohammadzadeh, M.; Amiri, A.; Hamidieh, A.A.; Ferdowsian, F.; Alemi, A.; Hoseinkhani, S.; Fesahat, F. The Effect of Temperature and Acidity on Antimicrobial Activities of Pristine MWCNTs and MWCNTs-Arg. J. Nanosci. Nanotechnol. 2020, 16, 127–136. [Google Scholar]
- Chłopek, J.; Czajkowska, B.; Szaraniec, B.; Frackowiak, E.; Szostak, K.; Beguin, F. In vitro studies of carbon nanotubes biocompatibility. Carbon 2006, 44, 1106–1111. [Google Scholar] [CrossRef]
- Singh, S.; Shi, T.; Duffin, R.; Albrecht, C.; van Berlo, D.; Höhr, D.; Fubini, B.; Martra, G.; Fenoglio, I.; Borm, P.J. Endocytosis, oxidative stress and IL-8 expression in human lung epithelial cells upon treatment with fine and ultrafine TiO2: Role of the specific surface area and of surface methylation of the particles. Toxicol. Appl. Pharmacol. 2007, 222, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, M.; Giardina, C.; Hubbard, A. Silica-induced caspase activation in mouse alveolar macrophages is dependent upon mitochondrial integrity and aspartic proteolysis. Toxicol. Sci. 2003, 76, 91–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Sample No. | Sample Coding | Ratio of Cellulose Acetate/Collagen (g/g) | Observation |
|---|---|---|---|
| 1 | CC | 1:1 | - |
| 2 | CC@MWCNT_TiO2 0.01 | 1:1 | 0.01 g of MWCNT_TiO2 was added over the solution |
| 3 | CC@MWCNT_TiO2 0.025 | 1:1 | 0.025 g of MWCNT_TiO2 was added over the solution |
| 4 | CC@MWCNT_TiO2 0.05 | 1:1 | 0.05 g of MWCNT_TiO2 was added over the solution |
| Sample | 2θ (°) | 2θ (rad) | β (°) | L (Å) | L (nm) |
|---|---|---|---|---|---|
| MWCNTs [18] | 25.53 | 0.4456 | 4.08 | 20.18 | 2.018 |
| MWCNTs_TiO2 | 25.267 | 0.2204 | 0.964 | 85.39 | 8.539 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, M.E.; Ion, R.M.; Grigorescu, R.M.; Iancu, L.; Holban, A.M.; Iordache, F.; Nicoara, A.I.; Alexandrescu, E.; Somoghi, R.; Teodorescu, S.; et al. Biocompatible and Antimicrobial Cellulose Acetate-Collagen Films Containing MWCNTs Decorated with TiO2 Nanoparticles for Potential Biomedical Applications. Nanomaterials 2022, 12, 239. https://doi.org/10.3390/nano12020239
David ME, Ion RM, Grigorescu RM, Iancu L, Holban AM, Iordache F, Nicoara AI, Alexandrescu E, Somoghi R, Teodorescu S, et al. Biocompatible and Antimicrobial Cellulose Acetate-Collagen Films Containing MWCNTs Decorated with TiO2 Nanoparticles for Potential Biomedical Applications. Nanomaterials. 2022; 12(2):239. https://doi.org/10.3390/nano12020239
Chicago/Turabian StyleDavid, Madalina Elena, Rodica Mariana Ion, Ramona Marina Grigorescu, Lorena Iancu, Alina Maria Holban, Florin Iordache, Adrian Ionut Nicoara, Elvira Alexandrescu, Raluca Somoghi, Sofia Teodorescu, and et al. 2022. "Biocompatible and Antimicrobial Cellulose Acetate-Collagen Films Containing MWCNTs Decorated with TiO2 Nanoparticles for Potential Biomedical Applications" Nanomaterials 12, no. 2: 239. https://doi.org/10.3390/nano12020239



.jpg)






