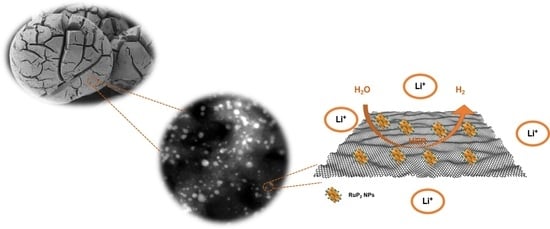
Phosphorus-Rich Ruthenium Phosphide Embedded on a 3D Porous Dual-Doped Graphitic Carbon for Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of RuP2@N-P-C
2.2. Characterization
2.3. Electrochemical Measurements
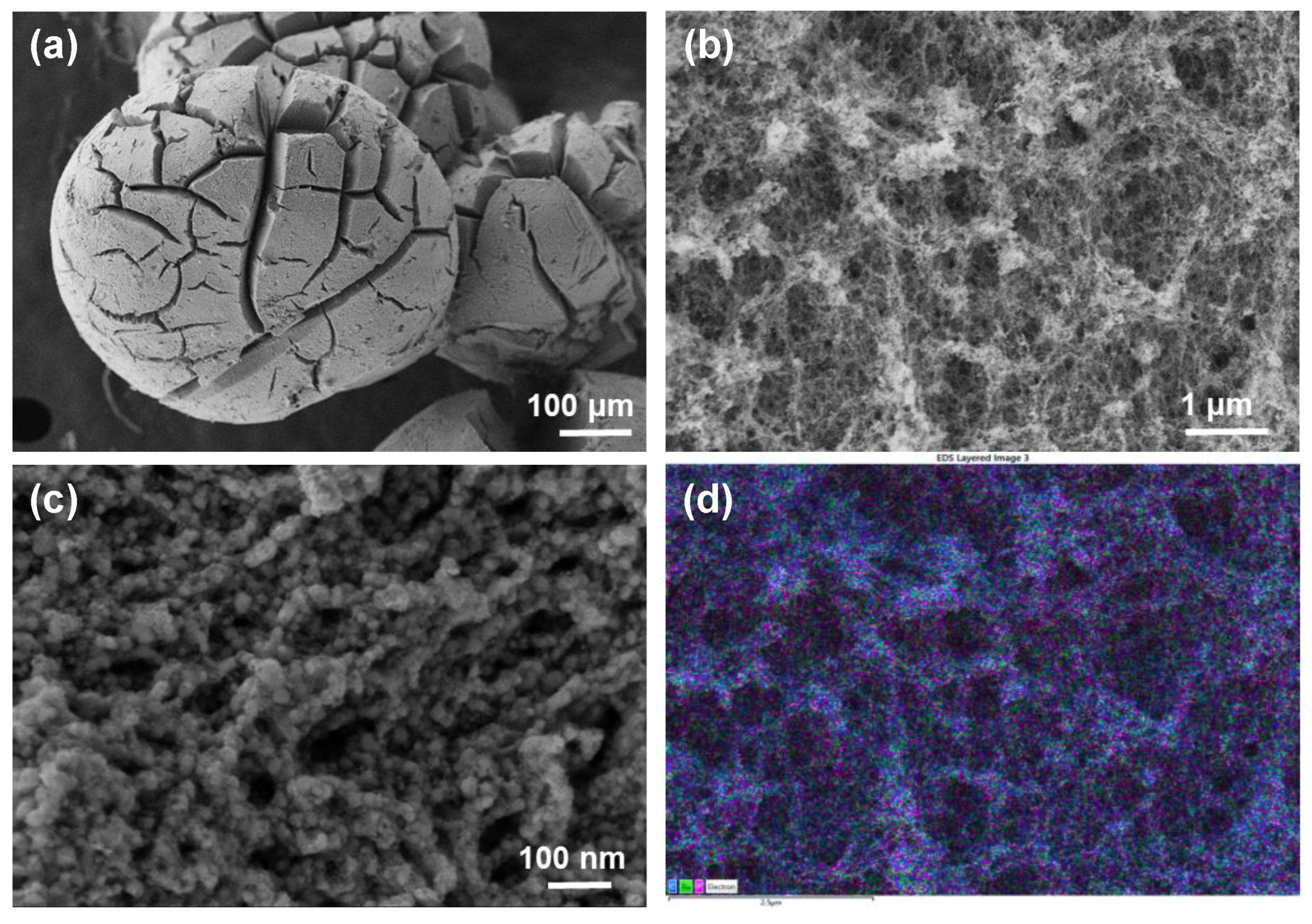
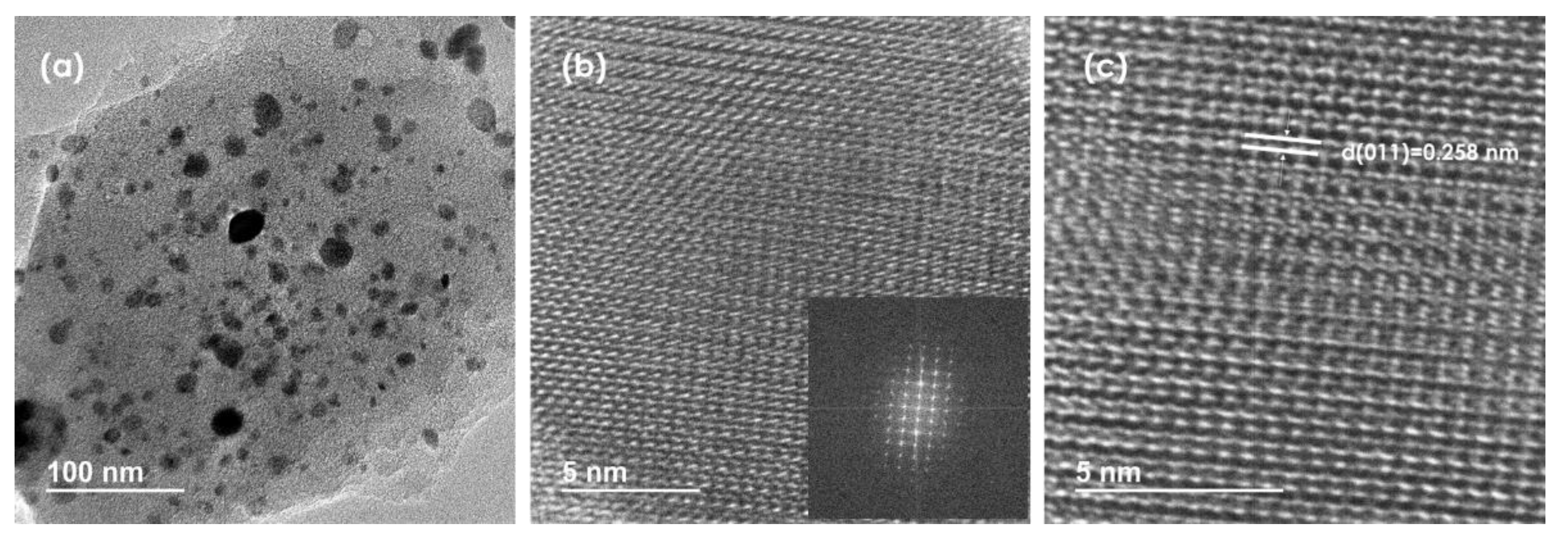
3. Results and Discussion
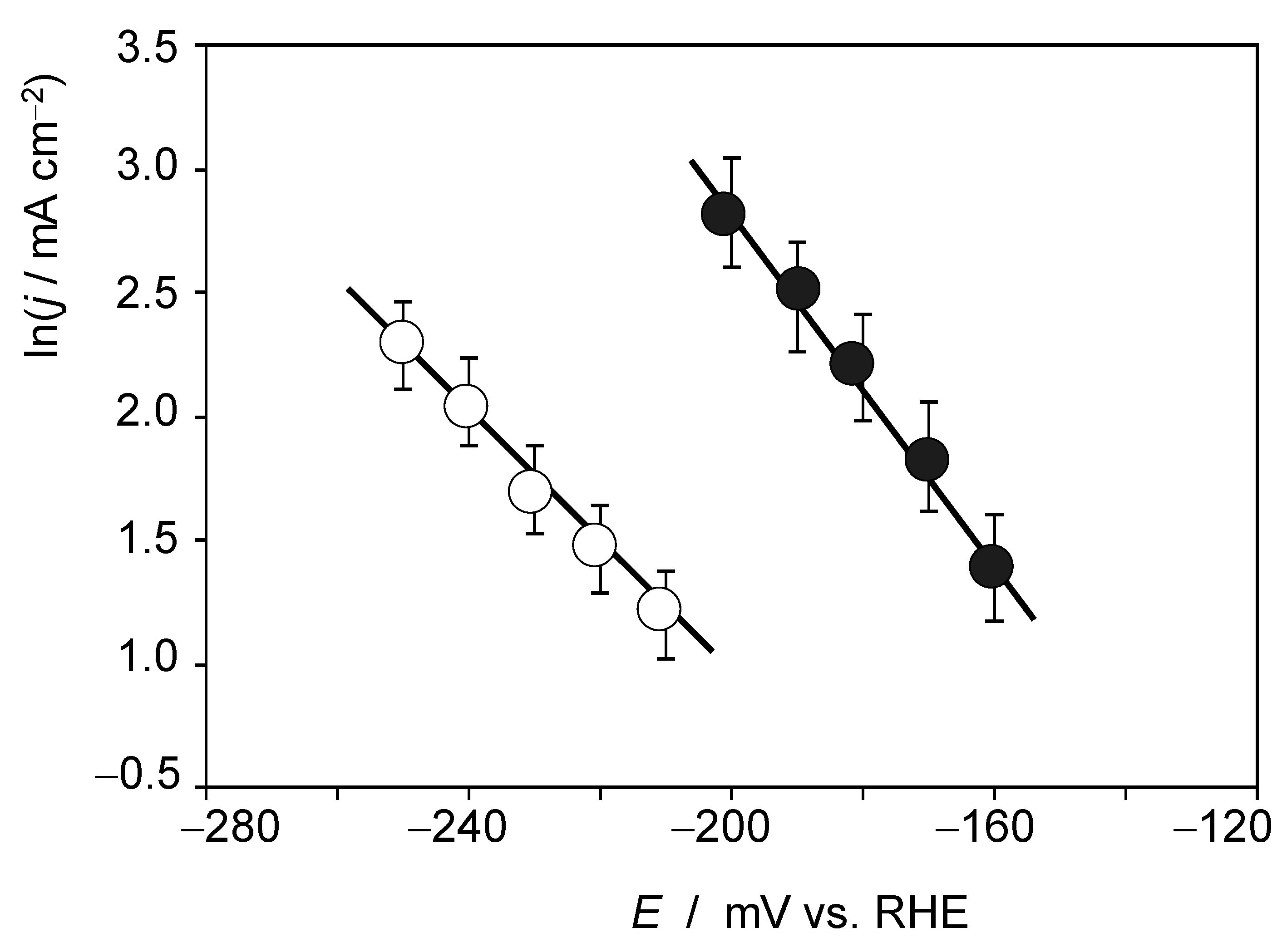
4. Electrochemistry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGlade, C.; Ekins, P. The geographical distribution of fossil fuels unused when limiting global warming to 2 °C. Nature 2015, 517, 187–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welsby, D.; Price, J.; Pye, S.; Ekins, P. Unextractable fossil fuels in a 1.5 °C world. Nature 2021, 597, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable Hydrogen Production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Boretti, A.; Banik, B.K. Advances in Hydrogen Production from Natural Gas Reforming. Adv. Sustain. Syst. 2021, 2, 2100097. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies—A review. Int. J. Hydrog. Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Sultan, S.; Myung, C.W.; Yoon, T.; Li, N.; Ha, M.; Harzandi, A.M.; Park, H.J.; Kim, D.Y.; Chandrasekaran, S.S.; et al. Multicomponent electrocatalyst with ultralow Pt loading and high hydrogen evolution activity. Nat. Energy 2018, 3, 773–782. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Mahmood, N.; Yao, Y.; Zhang, J.-W.; Pan, L.; Zhang, X.; Zou, J.-J. Electrocatalysts for Hydrogen Evolution in Alkaline Electrolytes: Mechanisms, Challenges, and Prospective Solutions. Adv. Sci. 2018, 5, 1700464. [Google Scholar] [CrossRef]
- Mahmood, J.; Li, F.; Jung, S.-M.; Okyay, M.S.; Ahmad, I.; Kim, S.-J.; Park, N.; Jeong, H.Y.; Baek, J.-B. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nanotechnol. 2017, 12, 441–446. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Harzandi, A.M.; Ha, M.; Sultan, S.; Myung, C.W.; Park, H.J.; Kim, D.Y.; Thangavel, P.; Singh, A.N.; Sharma, P.; et al. High-Performance Hydrogen Evolution by Ru Single Atoms and Nitrided-Ru Nanoparticles Implanted on N-Doped Graphitic Sheet. Adv. Energy Mater. 2019, 9, 1900931. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Dang, N.K.; Sultan, S.; Thangavel, P.; Jeong, H.Y.; Kim, K.S. Multi-heteroatom-doped carbon from waste-yeast biomass for sustained water splitting. Nat. Sustain. 2020, 3, 556–563. [Google Scholar] [CrossRef]
- Bae, S.-Y.; Mahmood, J.; Jeon, I.-Y.; Baek, J.-B. Recent advances in ruthenium-based electrocatalysts for the hydrogen evolution reaction. Nanoscale Horiz. 2020, 5, 43–56. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, D.; Yu, Y.; Li, J.; Rao, P.; Jia, C.; Liu, Z.; Chen, Q.; Huang, W.; Luo, J.; et al. Bridge the activity and durability of Ruthenium for hydrogen evolution reaction with the RuOC link. Chem. Eng. J. 2022, 433, 134421. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Dang, N.K.; Park, H.J.; Sultan, S.; Kim, M.G.; Haiyan, J.; Lee, Z.; Kim, K.S. Remarkably enhanced catalytic activity by the synergistic effect of palladium single atoms and palladium–cobalt phosphide nanoparticles. Nano Energy 2020, 78, 105166. [Google Scholar] [CrossRef]
- Yu, Z.; Wei, X.-K.; Xu, J.; Li, Y.; Araujo, A.; Faria, J.L.; Dunin-Borkowski, R.E.; Liu, L. Multifunctional Noble Metal Phosphide Electrocatalysts for Organic Molecule Electro-Oxidation. ACS Appl. Energy Mater. 2021, 4, 1593–1600. [Google Scholar] [CrossRef]
- Zhao, X.; Kong, X.; Liu, Z.; Li, Z.; Xie, Z.; Wu, Z.; He, F.; Chang, X.; Yang, P.; Zheng, J.; et al. The cutting-edge phosphorus-rich metal phosphides for energy storage and conversion. Nano Today 2021, 40, 101245. [Google Scholar] [CrossRef]
- Chang, Q.; Ma, J.; Zhu, Y.; Li, Z.; Xu, D.; Duan, X.; Peng, W.; Li, Y.; Zhang, G.; Zhang, F.; et al. Controllable Synthesis of Ruthenium Phosphides (RuP and RuP2) for pH-Universal Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2018, 6, 6388–6394. [Google Scholar] [CrossRef]
- Wang, J.; Kong, H.; Zhang, J.; Hao, Y.; Shao, Z.; Ciucci, F. Carbon-based electrocatalysts for sustainable energy applications. Prog. Mater. Sci. 2021, 116, 100717. [Google Scholar] [CrossRef]
- Kweon, D.H.; Okyay, M.S.; Kim, S.-J.; Jeon, J.-P.; Noh, H.-J.; Park, N.; Mahmood, J.; Baek, J.-B. Ruthenium anchored on carbon nanotube electrocatalyst for hydrogen production with enhanced Faradaic efficiency. Nat. Commun. 2020, 11, 1278. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Wang, S.; Zhang, Q.; Chen, L.; Hu, W.; Li, C.M. Ultrasmall Ru2P nanoparticles on graphene: A highly efficient hydrogen evolution reaction electrocatalyst in both acidic and alkaline media. Chem. Commun. 2018, 54, 3343–3346. [Google Scholar] [CrossRef] [PubMed]
- Teller, H.; Krichevski, O.; Gur, M.; Gedanken, A.; Schechter, A. Ruthenium Phosphide Synthesis and Electroactivity toward Oxygen Reduction in Acid Solutions. ACS Catal. 2015, 5, 4260–4267. [Google Scholar] [CrossRef]
- El Kadib, A. Chitosan as a Sustainable Organocatalyst: A Concise Overview. ChemSusChem 2015, 8, 217–244. [Google Scholar] [CrossRef] [PubMed]
- El Kadib, A. Green and Functional Aerogels by Macromolecular and Textural Engineering of Chitosan Microspheres. Chem. Rec. 2020, 20, 753–772. [Google Scholar] [CrossRef]
- Primo, A.; Sánchez, E.; Delgado, J.M.; García, H. High-yield production of N-doped graphitic platelets by aqueous exfoliation of pyrolyzed chitosan. Carbon 2014, 68, 777–783. [Google Scholar] [CrossRef]
- Anouar, A.; Ramírez Grau, R.; Katir, N.; Franconetti, A.; El Kadib, A.; Primo, A.; García, H. Nanometer-thick defective graphene films decorated with oriented ruthenium nanoparticles. Higher activity of 101 vs 002 plane for silane-alcohol coupling and hydrogen transfer reduction. J. Catal. 2022, 407, 342–352. [Google Scholar] [CrossRef]
- Wang, J.; Kim, J.; Choi, S.; Wang, H.; Lim, J. A Review of Carbon-Supported Nonprecious Metals as Energy-Related Electrocatalysts. Small Methods 2020, 4, 2000621. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L. Removal of phosphate anions using the modified chitosan beads: Adsorption kinetic, isotherm and mechanism studies. Powder Technol. 2015, 277, 112–119. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Omer, A.M.; El-Aqapa, H.G.; Gaber, N.M.; Attia, N.F.; El-Subruiti, G.M.; Mohy-Eldin, M.S.; Abd El-Monaem, E.M. Chitosan based adsorbents for the removal of phosphate and nitrate: A critical review. Carbohydr. Polym. 2021, 274, 118671. [Google Scholar] [CrossRef] [PubMed]
- Anouar, A.; Grirrane, A.; Álvarez, E.; Katir, N.; Primo, A.; Garcia, H.; El Kadib, A. Nanosized copper stabilized on ternary P, N, S-doped graphene from chitosan shellfish waste: Preparation and catalysis of single and double A3-type amine coupling. Mater. Today Sustain. 2022, 18, 100109. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Zhang, M.; Ju, H.; Zhu, J.; Qiao, Q.; Wang, M.; Yang, S. Noncovalent phosphorylation of graphene oxide with improved hole transport in high-efficiency polymer solar cells. Nanoscale 2018, 10, 14840–14846. [Google Scholar] [CrossRef]
- Gao, M.-R.; Liang, J.-X.; Zheng, Y.-R.; Xu, Y.-F.; Jiang, J.; Gao, Q.; Li, J.; Yu, S.-H. An efficient molybdenum disulfide/cobalt diselenide hybrid catalyst for electrochemical hydrogen generation. Nat. Commun. 2015, 6, 5982. [Google Scholar] [CrossRef] [Green Version]
- Ding, Q.; Song, B.; Xu, P.; Jin, S. Efficient Electrocatalytic and Photoelectrochemical Hydrogen Generation Using MoS2 and Related Compounds. Chem 2016, 1, 699–726. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Jiao, Y.; Vasileff, A.; Qiao, S.-Z. The Hydrogen Evolution Reaction in Alkaline Solution: From Theory, Single Crystal Models, to Practical Electrocatalysts. Angew. Chem. Int. Ed. 2018, 57, 7568–7579. [Google Scholar] [CrossRef] [PubMed]
- Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K.-C.; Uchimura, M.; Paulikas, A.P.; Stamenkovic, V.; Markovic, N.M. Enhancing Hydrogen Evolution Activity in Water Splitting by Tailoring Li+-Ni(OH)2-Pt Interfaces. Science 2011, 334, 1256–1260. [Google Scholar] [CrossRef]
- Dubouis, N.; Grimaud, A. The hydrogen evolution reaction: From material to interfacial descriptors. Chem. Sci. 2019, 10, 9165–9181. [Google Scholar] [CrossRef] [Green Version]
- Liu, E.; Li, J.; Jiao, L.; Doan, H.T.T.; Liu, Z.; Zhao, Z.; Huang, Y.; Abraham, K.M.; Mukerjee, S.; Jia, Q. Unifying the Hydrogen Evolution and Oxidation Reactions Kinetics in Base by Identifying the Catalytic Roles of Hydroxyl-Water-Cation Adducts. J. Am. Chem. Soc. 2019, 141, 3232–3239. [Google Scholar] [CrossRef]
- Weber, D.J.; Janssen, M.; Oezaslan, M. Effect of Monovalent Cations on the HOR/HER Activity for Pt in Alkaline Environment. J. Electrochem. Soc. 2019, 166, F66–F73. [Google Scholar] [CrossRef]
- Anouar, A.; Katir, N.; El Kadib, A.; Primo, A.; García, H. Palladium Supported on Porous Chitosan–Graphene Oxide Aerogels as Highly Efficient Catalysts for Hydrogen Generation from Formate. Molecules 2019, 24, 3290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Primo, A.; Forneli, A.; Corma, A.; García, H. From Biomass Wastes to Highly Efficient CO2 Adsorbents: Graphitisation of Chitosan and Alginate Biopolymers. ChemSusChem 2012, 5, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-P.; Liu, Y.; Liu, Y.-P.; Ren, T.-Z.; Chen, T.; Yuan, Z.-Y. Direct Synthesis of Phosphorus-Doped Mesoporous Carbon Materials for Efficient Electrocatalytic Oxygen Reduction. ChemCatChem 2015, 7, 2903–2909. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Z.; Li, J.; Zeng, M.; Li, Z.; He, L.; Li, F. Chitosan/phytic acid hydrogel as a platform for facile synthesis of heteroatom-doped porous carbon frameworks for electrocatalytic oxygen reduction. Carbon 2018, 137, 68–77. [Google Scholar] [CrossRef]
- Anouar, A.; Katir, N.; Mamede, A.-S.; Aboulaich, A.; Draoui, K.; Royer, S.; El Kadib, A. Synthesis and multifaceted use of phosphorylated graphene oxide: Growth of titanium dioxide clusters, interplay with gold nanoparticles and exfoliated sheets in bioplastics. Mater. Chem. Front. 2019, 3, 242–250. [Google Scholar] [CrossRef]
- Pu, Z.; Amiinu, I.S.; Kou, Z.; Li, W.; Mu, S. RuP2-Based Catalysts with Platinum-like Activity and Higher Durability for the Hydrogen Evolution Reaction at All pH Values. Angew. Chem. Int. Ed. 2017, 56, 11559–11564. [Google Scholar] [CrossRef]
- Guo, Z.; Li, J.; Qi, H.; Sun, X.; Li, H.; Tamirat, A.G.; Liu, J.; Wang, Y.; Wang, L. A Highly Reversible Long-Life Li–CO2 Battery with a RuP2-Based Catalytic Cathode. Small 2019, 15, 1803246. [Google Scholar] [CrossRef]
- He, J.; Anouar, A.; Primo, A.; García, H. Quality Improvement of Few-Layers Defective Graphene from Biomass and Application for H2 Generation. Nanomaterials 2019, 9, 895. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gao, Y.; You, T.L.; Ciucci, F. Bimetal-decorated nanocarbon as a superior electrocatalyst for overall water splitting. J. Power Sources 2018, 401, 312–321. [Google Scholar] [CrossRef]
- Wu, H.; Geng, J.; Ge, H.; Guo, Z.; Wang, Y.; Zheng, G. Egg-Derived Mesoporous Carbon Microspheres as Bifunctional Oxygen Evolution and Oxygen Reduction Electrocatalysts. Adv. Energy Mater. 2016, 6, 1600794. [Google Scholar] [CrossRef]
- Wang, J.; Ciucci, F. In-situ synthesis of bimetallic phosphide with carbon tubes as an active electrocatalyst for oxygen evolution reaction. Appl. Catal. B 2019, 254, 292–299. [Google Scholar] [CrossRef]
- Pu, Z.; Zhao, J.; Amiinu, I.S.; Li, W.; Wang, M.; He, D.; Mu, S. A universal synthesis strategy for P-rich noble metal diphosphide-based electrocatalysts for the hydrogen evolution reaction. Energy Environ. Sci. 2019, 12, 952–957. [Google Scholar] [CrossRef]
- Zhou, F.; Sa, R.; Zhang, X.; Zhang, S.; Wen, Z.; Wang, R. Robust ruthenium diphosphide nanoparticles for pH-universal hydrogen evolution reaction with platinum-like activity. Appl. Catal. B 2020, 274, 119092. [Google Scholar] [CrossRef]
- Luo, Q.; Xu, C.; Chen, Q.; Wu, J.; Wang, Y.; Zhang, Y.; Fan, G. Synthesis of ultrafine ruthenium phosphide nanoparticles and nitrogen/phosphorus dual-doped carbon hybrids as advanced electrocatalysts for all-pH hydrogen evolution reaction. Int. J. Hydrog. Energy 2019, 44, 25632–25641. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wang, Y.; Wang, J.; Liu, H.; Liu, X.; Wang, L.; Liu, X.; Xue, W.; Ma, N. Electrochemical synthesis of phosphorus-doped graphene quantum dots for free radical scavenging. Phys. Chem. Chem. Phys. 2017, 19, 11631–11638. [Google Scholar] [CrossRef]
- Nicholson, R.S.; Shain, I. Theory of Stationary Electrode Polarography -Single Scan and Cyclic Methods Applied to Reversible, Irreversible, and Kinetic Systems. Anal. Chem. 1964, 36, 706–723. [Google Scholar] [CrossRef]
- SavÉAnt, J.M.; Vianello, E. Recherches sur les courants catalytiques en polarographie—Oscillographique à balayage linéaire de tension. Etude Théorique. In Advances in Polarography; Longmuir, I.S., Ed.; Pergamon: Oxford, UK, 1960; pp. 367–374. [Google Scholar]
- Li, G.; Zhang, D.; Qiao, Q.; Yu, Y.; Peterson, D.; Zafar, A.; Kumar, R.; Curtarolo, S.; Hunte, F.; Shannon, S.; et al. All The Catalytic Active Sites of MoS2 for Hydrogen Evolution. J. Am. Chem. Soc. 2016, 138, 16632–16638. [Google Scholar] [CrossRef]
- Andrieux, C.P.; Hapiot, P.; Savéant, J.M. Electron-transfer coupling of diffusional pathways: Theory for potential step chronoamperometry and chronocoulometry. J. Electroanal. Chem. Interf. Electrochem. 1984, 172, 49–65. [Google Scholar] [CrossRef]
- Miller, C.J.; Majda, M. Microporous aluminum oxide films at electrodes part II. studies of electron transport in the Al2O3 matrix derivatized by adsorption of poly(4-vinylpyridine). J. Electroanal. Chem. Interf. Electrochem. 1986, 207, 49–72. [Google Scholar] [CrossRef]
- Miller, C.J.; Majda, M. Microporous aluminum oxide films at electrodes. Dynamics of ascorbic acid oxidation mediated by ferricyanide ions bound electrostatically in bilayer assemblies of octadecyltrichlorosilane and an octadecylviologen amphiphile. Anal. Chem. 1988, 60, 1168–1176. [Google Scholar] [CrossRef]
- Guidelli, R.; Cozzi, D. Homogeneous chemical equilibria in polarography. II. Examples. J. Phys. Chem. 1967, 71, 3027–3034. [Google Scholar] [CrossRef]
- De Jong, H.G.; Van Leeuwen, H.P.; Holub, K. Voltammetry of metal complex systems with different diffusion coefficients of the species involved: Part I. Analytical approaches to the limiting current for the general case including association/dissociation kinetics. J. Electroanal. Chem. Interf. Electrochem. 1987, 234, 1–16. [Google Scholar] [CrossRef]
- Evans, D.H. Multicomponent diffusion with chemical reactions and its effect in voltammetry. J. Electroanal. Chem. Interf. Electrochem. 1989, 258, 451–456. [Google Scholar] [CrossRef]
- Blauch, D.N.; Anson, F.C. Effects of interconversion and electron transfer on voltammetric responses for two-component systems with differing diffusion coefficients. J. Electroanal. Chem. Interf. Electrochem. 1991, 309, 313–318. [Google Scholar] [CrossRef]
- Oldham, K.B. Steady-state microelectrode voltammetry as a route to homogeneous kinetics. J. Electroanal. Chem. Interf. Electrochem. 1991, 313, 3–16. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Koshevoy, I.O.; Montoya, N.; Pakkanen, T.A.; Doménech-Carbó, M.T. Solvent-Independent Electrode Potentials of Solids Undergoing Insertion Electrochemical Reactions: Part II. Experimental Data for Alkynyl–diphosphine Dinuclear Au(I) Complexes Undergoing Electron Exchange Coupled to Anion Exchange. J. Phys. Chem. C 2012, 116, 25984–25992. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Scholz, F.; Montoya, N. Solvent-Independent Electrode Potentials of Solids Undergoing Insertion Electrochemical Reactions: Part III. Experimental Data for Prussian Blue Undergoing Electron Exchange Coupled to Cation Exchange. J. Phys. Chem. C 2012, 116, 25993–25999. [Google Scholar] [CrossRef]
- Doménech, A.; García-España, E.; Navarro, P.; Reviriego, F. Electrochemical determination of the stability of complexes formed by proton-ionizable ligands of 3,5-disubstituted 1H-pyrazole with phenethylamine. Talanta 2000, 51, 625–636. [Google Scholar] [CrossRef]
- van der Niet, M.J.T.C.; Garcia-Araez, N.; Hernández, J.; Feliu, J.M.; Koper, M.T.M. Water dissociation on well-defined platinum surfaces: The electrochemical perspective. Catal. Today 2013, 202, 105–113. [Google Scholar] [CrossRef]
- McCrum, I.T.; Janik, M.J. pH and Alkali Cation Effects on the Pt Cyclic Voltammogram Explained Using Density Functional Theory. J. Phys. Chem. C 2016, 120, 457–471. [Google Scholar] [CrossRef]
- Chen, X.; McCrum, I.T.; Schwarz, K.A.; Janik, M.J.; Koper, M.T.M. Co-adsorption of Cations as the Cause of the Apparent pH Dependence of Hydrogen Adsorption on a Stepped Platinum Single-Crystal Electrode. Angew. Chem. Int. Ed. 2017, 56, 15025–15029. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.; Shao, Z.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [CrossRef] [PubMed]





| Sample | C (%) | N (%) | P (%) | Ru (%) | H (%) | Surface Area (m2/g) |
|---|---|---|---|---|---|---|
| N-P-C | 59.6 | 1.9 | 3.1 | - | 0.5 | 817 |
| RuP2@N-P-C | 53.1 | 2 | 8.9 | 6.7 | 0.78 | 765 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anouar, A.; Doménech-Carbó, A.; Garcia, H. Phosphorus-Rich Ruthenium Phosphide Embedded on a 3D Porous Dual-Doped Graphitic Carbon for Hydrogen Evolution Reaction. Nanomaterials 2022, 12, 3597. https://doi.org/10.3390/nano12203597
Anouar A, Doménech-Carbó A, Garcia H. Phosphorus-Rich Ruthenium Phosphide Embedded on a 3D Porous Dual-Doped Graphitic Carbon for Hydrogen Evolution Reaction. Nanomaterials. 2022; 12(20):3597. https://doi.org/10.3390/nano12203597
Chicago/Turabian StyleAnouar, Aicha, Antonio Doménech-Carbó, and Hermenegildo Garcia. 2022. "Phosphorus-Rich Ruthenium Phosphide Embedded on a 3D Porous Dual-Doped Graphitic Carbon for Hydrogen Evolution Reaction" Nanomaterials 12, no. 20: 3597. https://doi.org/10.3390/nano12203597